Abstract
We aimed to determine motor, cognitive, and behavioral outcome at school age of children who had either necrotizing enterocolitis (NEC) or spontaneous intestinal perforation (SIP). This case-control study included infants with NEC Bell's stage IIA onward, infants with SIP, and matched controls (1996–2002). At school age, we assessed motor skills, intelligence, visual perception, visuomotor integration, verbal memory, attention, behavior, and executive functions. Of 93 infants with NEC or SIP, 28 (30%) died. We included 52 of 65 survivors for follow-up. At mean age of 9 y, we found that 68% of the children had borderline or abnormal scores on the Movement Assessment Battery for Children (versus 45% of controls). Their mean total intelligence quotient (IQ) was 86 ± 14 compared with 97 ± 9 in the controls. In addition, attention and visual perception were affected (p < 0.01 and p = 0.02). In comparison to controls, surgically treated children were at highest risk for adverse outcome. In conclusion, at school age, the motor functions and intelligence of many children with NEC or SIP were borderline or abnormal and, specifically, attention and visual perception were impaired. Children with NEC or SIP form a specific risk group for functional impairments at school age even though the majority does not have overt brain pathology.
Similar content being viewed by others
Main
Necrotizing enterocolitis (NEC) is a common, acute gastrointestinal disease in newborn, mostly preterm infants. The clinical manifestation and course of NEC can vary from nonspecific symptoms requiring conservative treatment to a fulminant disease with major abdominal and systemic symptoms requiring surgery.
Spontaneous intestinal perforation (SIP) is a less common gastrointestinal disorder. Its incidence has increased over time with the increasing rate of survival of very LBW infants. Infants with SIP have clinical and radiographic features that are often less pronounced than infants with NEC, but they always require surgical treatment (1). Mortality in infants with NEC or SIP is found to range between 15 and 30% (1,2).
Newborn infants with NEC or SIP often require prolonged ventilatory support and they are prone to develop sepsis, which can lead to white matter injury (3). In addition, they often have difficulty tolerating enteral feeding that subjects them to inadequate nutrition and growth impairment. All these factors contribute to the risk of neurodevelopmental disabilities. Earlier studies reported that ∼20% of the infants with NEC develop CP, while even more children have cognitive impairments (4,5). Most of these studies, however, only followed the children up to the age of 2 y. Furthermore, infants with SIP are frequently excluded from outcome studies because SIP is a different disease than NEC, while they have similar presenting signs. Consequently, the neurodevelopmental outcome at school age of children with NEC or SIP is unknown. As functional demands at school age are higher than at younger ages, more specific motor, cognitive, and behavioral deficits that were not detected previously may now become apparent.
The first aim of our study was to determine the motor, cognitive, and behavioral outcome at school age of children with the gastrointestinal diseases NEC or SIP compared with control children of similar GA. Our second aim was to identify disease-related risk factors for adverse outcome, such as type of treatment, Bell's stage, and presence of late-onset sepsis. We hypothesized that children with surgically treated NEC (SurgNEC) would be at highest risk for neurodevelopmental impairments compared with children with SIP and medically treated NEC (MedNEC), because they had often been severely ill for a significant period of time and their illness was frequently complicated with perforation.
METHODS
Patients.
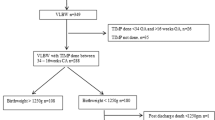
We selected all newborn infants that were admitted to the NICU of the University Medical Center Groningen between 1996 and 2002 and diagnosed with either NEC, from Bell's stage IIA onward, or SIP. We found the infants by searching the patient database on the diagnoses NEC and intestinal and gastric perforations. We also included control infants from our NICU that were born in the same period (1996–2002) and matched for gender and GA. We aimed at including 1.5–2 times as many control infants as cases in the subgroups of infants with gastrointestinal diseases [i.e. MedNEC (n = 15), SurgNEC (n = 17), SIP (n = 20)]. We therefore included 31 controls. We excluded patients with major chromosomal anomalies. The infants in the control group were comparable to our study group in all respects except that they did not have gastrointestinal diseases (Table 1) (6). The diagnosis of either NEC or SIP was made in a multidisciplinary setting, involving neonatologists, pediatric surgeons, and radiologists based on 1) clinical signs, including abdominal distention and discoloration, bloody stools, and circulatory and respiratory instability; 2) radiological signs consistent with pneumatosis intestinalis, pneumoperitoneum, or both; and 3) the extent of the affected bowel during surgery. All the infants with SIP and SurgNEC underwent laparotomy in the acute phase of the illness. We reviewed the medical charts for neonatal and disease-related characteristics. Disease severity in the infants with NEC was classified according to Bell's stages by using the clinical, radiographic, and laparotomy findings (7). We also recorded the number of disease-related reoperations in the first year of life.
Follow-up.
The children were invited prospectively to participate in an extension of the routine follow-up program which was supervised by a child neuropsychologist (K.N.J.A.B.). The program entailed the assessment of motor performance, cognition, and behavior at the age of 6 to 13 y. The follow-up took ∼2.5 h to complete including breaks. Parents gave their written informed consent to participate in the follow-up program. The study was approved by the Medical Ethical Committee of the University Medical Center Groningen.
Motor outcome.
We determined the presence or absence of CP following Bax' criteria (8). In case of CP, gross motor functioning was scored with the Gross Motor Function Classification System (GMFCS), a functional, five-level classification system for CP (9). Higher GMFCS levels indicate more severe functional impairments.
To assess the children's motor outcome, we administered the Movement Assessment Battery for Children (Movement-ABC), a standardized test of motor skills for children (10). This test yields a total motor performance score that is based on subscores for manual dexterity (fine motor skills), ball skills, and static and dynamic balance (coordination). The higher the score, the poorer the performance. We also measured the height and weight of the children which are expressed as z-scores.
Cognitive outcome.
Total, verbal, and performance intelligence were assessed using a shortened version of the Wechsler Intelligence Scale for Children, third edition, Dutch version (WISC-III-NL) (11). In addition, we assessed central visual perception and visuomotor integration with the subtests Geometric Puzzles and Design Copying of the NEPSY-II, a neuropsychological test battery for children (12). Visuomotor integration involves the integration of visual information with finger-hand movements. We assessed verbal memory with a standardized Dutch version of Rey's Auditory Verbal Learning Test (13).
We measured selective attention and attentional control with the subtests Map Mission and Opposite Worlds of the Test of Everyday Attention for Children (14). Selective attention refers to a child's ability to select target information from an array of distractors. Attentional control refers to the ability to shift attention flexibly and adaptively.
Behavioral outcome.
To obtain information on the children's behavioral and emotional competencies and problems, we asked the parents to complete the Child Behavior Checklist (15). It consists of two subscales, one for internalizing problems (withdrawn behavior, somatic complaints, and anxious/depressed scales) and the other for externalizing problems (delinquent and aggressive behavior scales), and a composite total scale.
The parents also filled out the Behavior Rating Inventory of Executive Function (16) to assess executive functioning involved in well-organized, purposeful, goal-directed, and problem-solving behavior. Test scores obtained when a child was too tired and/or uncooperative (as assessed by the trained experimenter), as well as incomplete questionnaires, were excluded. The experimenter was blinded to the presence or absence of gastrointestinal diseases in the children.
Statistical analyses.
We classified the intelligence quotients (IQs) as normal (IQ ≥ 85), borderline (mildly abnormal, IQ 70–85), moderately abnormal (IQ 69-55), and severely abnormal (IQ <55). We used the percentiles on the standardization samples of the Movement-ABC and cognitive tests to classify raw scores into normal (>P15), borderline (P5-P15), and abnormal (<P5). For the Child Behavior Checklist and Behavior Rating Inventory of Executive Function, we used a similar classification following the criteria in the manual. Visual inspection of the histograms and Q-Q plots were used to determine which outcome measures were normally distributed. We then used the t, Mann-Whitney U, and 4 χ2 test where appropriate to compare the outcome measures of the study group with the control group and to relate disease characteristics with outcome. We used backward logistic regression analysis to calculate the odds ratios (ORs) for worse outcome when comparing the children with gastrointestinal diseases to the controls and the children with SurgNEC to the groups of children with both MedNEC and SIP. We repeated the logistic regression analyses, adjusting for severe cerebral pathology. Cerebral pathology was detected by serial cranial ultrasound scans and defined as severe in case of grade III germinal matrix hemorrhage, posthemorrhagic ventricular dilatation, periventricular hemorrhagic infarction, and cystic periventricular leukomalacia.
Next, to identify additional disease-related risk factors for adverse outcome, we performed a univariate analysis to relate age at development of NEC, Bell's stage, localization of SIP (gastric or intestinal), presence of late-onset sepsis, age at surgery, and multiple surgeries to outcome within the group of children with gastrointestinal diseases. Throughout the analyses, p < 0.05 was considered to be statistically significant. SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL) was used for the analyses. The analyses were performed by E.R. and A.F.B. with support from a statistician.
RESULTS
Between 1996 and 2002, a total of 3947 patients were admitted to our NICU. After database search, 59 infants with NEC and 34 infants with SIP were included. Twenty (34%) of the infants with NEC and 8 (24%) of the infants with SIP died in the neonatal period. A total of 65 survivors with gastrointestinal diseases remained, of whom 52 (80%) participated in the follow-up program—8 sets of parents declined the invitation to participate, 4 could not be traced, and 1 child could not be assessed as a result of being deaf. We included 31 control infants from our NICU for follow-up.
Patient characteristics.
Table 1 gives an overview of the patient demographics of the children with gastrointestinal diseases and the controls. The demographics of the 13 children who did not participate were comparable to the children included in our study (n = 7 with NEC, n = 6 with SIP, median GA 28.9 wk, birth weight 1100 g). The child that could not be assessed as a result of being deaf had MedNEC.
Of the 15 infants with MedNEC, 13 had Bell's stage IIA and 2 stage IIB. One of these infants was surgically treated for an intestinal stenosis several weeks after recovering from NEC. Of the 17 infants with SurgNEC, 1 had Bell's stage IIA, 1 stage IIB, 3 stage IIIA, and 12 stage IIIB.
Two of the 20 infants with SIP had a stomach perforation and 18 had an intestinal perforation. All infants with SIP and SurgNEC underwent laparotomy in the acute phase of their gastrointestinal disease. One child with SIP received postnatal steroids from day 11 onward because of respiratory problems, before the development of SIP.
During childhood, three children required pediatric intensive care treatment for respiratory support, which was not related to their gastrointestinal disease from the neonatal period. One child with MedNEC had subglottic laryngitis, one child with SIP required a cardiac intervention for pulmonary artery stenosis, and one control child had a respiratory syncytial virus infection.
Follow-up.
The mean age at follow-up was 9.3 y (range 6.2–13.3 y). At school age, we found that 1 child had visual problems requiring prescription glasses, 4 children had hearing problems requiring hearing aids, and 4 children had epilepsy. These disabilities were not found in the control group. Regarding growth, we found that children with gastrointestinal diseases had a mean height z-score of −0.37 (SD, 1.17), weight of 0.09 (SD 1.34), and head circumference of −0.40 (SD 1.09). In the control children this was −0.40 (SD 0.95) for height, 0.11 (SD 1.57) for weight, and −0.43 (SD 1.45) for head circumference.
Motor outcome.
Of the 52 children with gastrointestinal diseases, 3 developed unilateral CP (6%) and 5 bilateral CP (9%). Their functional impairments were limited to GMFCS level I in 3 and level II in 4 children. One child with severe functional impairments had GMFCS level IV. In the control group, only one child developed bilateral CP (5%) with GMFCS level II. The increased incidence of CP in the children with gastrointestinal diseases was almost significant (p = 0.08).
The median scores on the Movement-ABC are shown in Table 2. The child with severe CP (GMFCS-IV) was not assessed with the Movement-ABC. The children with gastrointestinal diseases scored significantly worse than the controls on the total Movement-ABC score and on the subtests fine motor skills and coordination. In Table 3, we classified the outcome into the categories normal, borderline, and abnormal and present the ORs for worse outcome after correction for cerebral pathology. Sixty-eight percent of the children with gastrointestinal diseases obtained borderline or abnormal scores on the Movement-ABC with an OR of 2.27 compared with controls. Before correction for cerebral pathology, ORs for borderline/abnormal outcome on the total score of the Movement-ABC and abnormal outcome on coordination were slightly higher (OR 2.66, CI 1.06–6.68, p = 0.04 and OR 3.64, CI 1.20–11.02, p = 0.02, respectively). All other ORs were the same.
Cognitive and behavioral outcome.
Of the 52 children with gastrointestinal diseases, 15 (28%) attended special education classes and 14 (27%) had to repeat classes. In the control group (n = 31), one child (3%) attended a special education class and eight (26%) had to repeat classes.
Table 2 shows the mean and median scores, where appropriate, on the cognitive and behavioral measures. For three children with gastrointestinal diseases, the neuropsychological tests were too difficult because of very low IQ scores. In comparison to the controls, the children with gastrointestinal diseases scored significantly lower on intelligence (total, verbal, and performance), visual perception, and attention. There was a trend toward lower scores on visuomotor integration. On the delayed recall of verbal memory, they scored slightly better than the controls. The incidence of behavioral problems in the groups was comparable.
In Table 3, we classified the scores into the categories normal, borderline, and abnormal including the ORs for worse outcome after correction for cerebral pathology. The children whose neuropsychological functions could not be assessed were included in the category abnormal. Regarding IQ, we found that n = 3 children had moderately abnormal total IQs, while n = 2 had severely abnormal total IQs; for verbal IQ, this was n = 5 and n = 2 and for performance IQ n = 5 and n = 2, respectively. The ORs confirmed the analyses of the mean scores except for visual perception in which case the OR was not significant. The better scores on verbal memory were not confirmed by the ORs. Analyses without correction for cerebral pathology revealed similar results with slightly different ORs, but no differences in level of significance (data not shown). Only ORs for borderline/abnormal verbal IQ and visuomotor integration were higher before correction (OR 3.28, CI 0.99–10.88, p = 0.05 and OR 4.15, CI 1.10–15.66, p = 0.03, respectively).
Disease characteristics in relation to outcome.
Subsequently, we determined whether the disease characteristics of the children with gastrointestinal diseases were related to outcome at school age. Between the subgroups of children with gastrointestinal diseases (MedNEC, SurgNEC, and SIP), no differences in outcome were found. Next, we analyzed the outcomes of the subgroups in comparison to the controls. In Figure 1, we provide the Movement-ABC and IQ scores. The children with SIP had the highest Movement-ABC scores, which indicates adverse outcome. The IQ scores of the children with MedNEC, SurgNEC, and SIP were lower than those of the controls. We found the biggest differences for children with SurgNEC or SIP.
Movement-ABC scores (A) and IQs (B) in children with gastrointestinal diseases compared with controls. In (A), the Movement-ABC total score is shown on the left y axis; scores on the subtests are shown on the right y axis. In (B), IQ scores are shown on the y axis. *p < 0.05 in comparison to controls.  MedNEC,
MedNEC,  SurgNEC, ▪ SIP, □ Controls
SurgNEC, ▪ SIP, □ Controls
In Table 4, we present the ORs for borderline/abnormal outcome of the children with MedNEC, SurgNEC, or SIP in comparison to the control group. The children with SIP had a significant increased risk for worse motor outcome compared with the controls. The ORs for lower intelligence reached significance in the children with SurgNEC and SIP and showed a trend toward significance in children with MedNEC. The children with SurgNEC were at risk for worse visuomotor integration. Attention was worse in all three groups, although only the ORs of the children with SurgNEC and SIP reached significance. When calculating the ORs for abnormal outcome only, the domains in which the children had increased ORs for worse outcome were similar, apart from some empty fields (data not shown).
Analyses without correction for cerebral pathology showed that visual perception tended to be worse in children with SIP compared with controls (OR 4.93, CI 0.87–27.88, p = 0.07). All other ORs were the same before and after correction.
None of the additional disease-related risk factors (the age at development of NEC, Bell's stage, localization of SIP (gastric or intestinal), and presence of late-onset sepsis, age at surgery, and multiple surgeries) were related to adverse outcome at school age.
DISCUSSION
This study showed that at school age, 68% of newborn infants with gastrointestinal diseases (NEC or SIP) had borderline or abnormal motor outcomes. On average, their intelligence was 11 IQ points lower than matched control children without gastrointestinal diseases. In addition, their attention and visual perception were impaired. Visuomotor integration was also affected, albeit to a lesser extent. Behavioral problems and executive functions were comparable to the control group. Surgically treated children were at highest risk for abnormal outcome at school age when compared with the controls. These findings remained present after correction for severe cerebral pathology of the children.
We found that at a mean age of 9 y, 15% of the children with NEC or SIP had developed CP and as many as 40% had abnormal motor skills. These results were similar to the findings of previous studies on the neurodevelopmental outcome of survivors with NEC at 2 to 3 y of age. These studies reported that ∼20% of children develop CP and that 31% of children with NEC have an abnormal (<70) Psychomotor Developmental Index (4,5).
With regard to cognitive outcome, we found that the mean IQ of children with NEC or SIP was 86, which was considerably lower than that of the control children. Although we found that at school age only 10% of the children had an abnormal total IQ (<70), 43% had IQ scores below 85, which is the approximate cutoff point for being able to attend regular education in The Netherlands. Hintz et al. (5) found that at 2 y of age, children with NEC have a Mental Developmental Index that is 6 points lower than controls and that 41% of the children obtain an abnormal score (<70). Thus, although the percentage of children with abnormal intelligence at school age seems smaller in our study, the difference in mean scores with matched control children seems to be higher in our population compared with Hintz' study. At school age, cognition can be determined more reliably because behavioral aspects fit the testing situation better and test validity is higher.
In addition to lower intelligence, we also found poorer selective attention, attentional control, visual perception, and visuomotor integration in children with NEC or SIP. These specific cognitive deficits, which may only come to light when the children reach school age, may hamper school performance even further. A case in point is poor attention that may affect learning.
With regards to the incidence of behavioral problems and executive functions, the study group was comparable to the controls, even though in both groups the incidence of behavioral problems was increased compared with the reference population. It is known that ∼20% of preterm infants develop behavioral problems, which is comparable to our findings (17,18). Apparently, the presence of gastrointestinal disease does not increase this risk still further.
It is likely that the neurodevelopmental impairments we found in the survivors with NEC or SIP are the result of a combination of factors the infants were subjected to during the neonatal period. NEC and associated tissue injury initially leads to an inflammatory response. The subsequent release of proinflammatory cytokines by the activation of microglial cells in the brain leads to injury to the preoligodendrocytes and axons in the white matter. These glial cells, which play an important role in myelination of the immature brain, are highly vulnerable to damage (19). The increased permeability of the blood-brain barrier during inflammation further increases the susceptibility of the brain to this inflammatory response (20). Both NEC and SIP can be complicated by sepsis, which is associated with an increased risk for overt, and also more subtle and diffuse, white matter abnormalities (3,21,22). Furthermore, infants with gastrointestinal diseases are at risk for respiratory and circulatory insufficiency which contributes to cerebral hypoxemia. Indeed, in this study we found that the children with NEC or SIP had been ventilated significantly more frequently and had required more inotropes compared with the controls. Finally, nutritional difficulties and the presence of short bowel syndrome after surgery are other factors that could contribute to impaired growth and neurodevelopment (23,24).
Our second aim was to identify risk factors for adverse outcome in children with gastrointestinal diseases. Because infants with SurgNEC had often been severely ill for a significant length of time—an illness that was frequently complicated with perforation—we hypothesized that their outcome would be worse than infants with SIP and MedNEC. In this light, it was striking that we did not find any significant differences in outcome at school age within the group of children with gastrointestinal diseases, although we did find a 10% higher mortality rate in children with overall NEC compared with SIP. Perhaps the pathophysiological mechanisms mainly responsible for worse outcome were similar among these children irrespective of mode of treatment and additional disease-related factors. The size of the study group may also have limited our ability to detect significant differences in development between groups.
When compared with controls, however, we did find that surgically treated children (both NEC and SIP) were at higher risk for worse outcome. Previous studies reported that 2-yr-old children with SurgNEC were at higher risk for abnormal motor and cognitive outcome than children with MedNEC compared with controls (5,25,26). The few studies that compared the outcome of children with SurgNEC with SIP found worse outcomes for children with NEC (1,27). In contrast, we found particularly high ORs for worse motor outcome in children with SIP. We were puzzled by this finding. One could suggest that the slightly higher rate of severe cerebral pathology in the children with SIP of our cohort played a role; however, after correction for cerebral pathology, our results did not change. Perhaps it was a chance finding due to the low number of children in the subgroups.
To the best of our knowledge, this is the first study that determines the functional outcome at school age of children with NEC or SIP. The strength of this study is that we examined in great detail a broad range of motor and cognitive skills and behavioral aspects that might limit functional abilities at school age. Moreover, we included control children who were matched for gender, GA, and birth year. A possible limitation is that this was a single-center study. Moreover, one child could not be assessed as a result of being deaf. Outcome of the children with gastrointestinal diseases may thus have been slightly worse than we reported.
Our study showed that survivors of either NEC or SIP form a specific high-risk group for functional impairments at school age, in the majority in the absence of overt brain pathology as can be detected on cranial ultrasonography. Not only their motor outcome but also their cognitive outcome was significantly impaired. Their intelligence, for example, was barely better compared with preterm children with severe brain lesions (28,29). In our opinion, therefore, these children deserve to be followed up to school age because it is likely that they are coping with impairments that require intervention. This study also touches on the question of the pathophysiological mechanisms responsible for these long-term functional impairments. We believe that MRI in the neonatal period may help to clarify these pathophysiological mechanisms and should be part of the neurodevelopmental assessment in these children.
Abbreviations
- GMFCS:
-
Gross Motor Function Classification System
- IQ:
-
intelligence quotient
- MedNEC:
-
medically treated NEC
- Movement-ABC:
-
Movement Assessment Battery for Children
- NEC:
-
necrotizing enterocolitis
- SIP:
-
spontaneous intestinal perforation
- SurgNEC:
-
surgically treated NEC
References
Adesanya OA, O'Shea TM, Turner CS, Amoroso RM, Morgan TM, Aschner JL 2005 Intestinal perforation in very low birth weight infants: growth and neurodevelopment at 1 year of age. J Perinatol 25: 583–589
Lin PW, Stoll BJ 2006 Necrotising enterocolitis. Lancet 368: 1271–1283
Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE 2008 Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 153: 170–175
Soraisham AS, Amin HJ, Al Hindi MY, Singhal N, Sauve RS 2006 Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight < or =1250 g?. J Paediatr Child Health 42: 499–504
Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R 2005 Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115: 696–703
Evans WA Jr, 1942 An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatry 47: 931–937
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T 1978 Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187: 1–7
Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D 2005 Proposed definition and classification of cerebral palsy, April. Dev Med Child Neurol 47: 571–576, 2005
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B 1997 Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39: 214–223
Smits-Engelsman BC 1998 Movement Assessment Battery for Children. Swets & Zeitlinger, Lisse, The Netherlands
Kort W, Compaan EL, Bleichrodt N, Resing WC, Schittekatte M, Bosmans M, Vermeir G, Verhaeghe P 2002 WISC-III NL Manual. NIP dienstencentrum, Amsterdam, The Netherlands
Korkman M, Kirk U, Kemp SL 2007 NEPSY II. Clinical and Interpretative Scoring Manual. Psychological Corporation, San Antonio, TX
van den Burg W, Kingma A 1999 Performance of 225 Dutch school children on Rey's Auditory Verbal Learning Test (AVLT): parallel test-retest reliabilities with an interval of 3 months and normative data. Arch Clin Neuropsychol 14: 545–559
Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH 2001 The differential assessment of children's attention: the Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry 42: 1065–1081
Achenbach TM 1991 Manual for the Child Behavior Checklist: 4–18 and 1991 profile. University of Vermont, Burlington, VT
Gioia GA, Isquith PK, Guy SC, Kenworthy L 2000 BRIEF—Behavior Rating Inventory of Executive Function: Professional Manual. Psychological Assessment Resources, Odessa, FL
Reijneveld SA, de Kleine MJ, van Baar AL, Kollee LA, Verhaak CM, Verhulst FC, Verloove-Vanhorick SP 2006 Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Arch Dis Child Fetal Neonatal Ed 91: F423–F428
Gray RF, Indurkhya A, McCormick MC 2004 Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics 114: 736–743
Volpe JJ 2008 Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr 153: 160–163
Abbott NJ 2000 Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 20: 131–147
Volpe JJ 2001 Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50: 553–562
Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, Ferriero DM, Miller SP 2008 Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 122: 299–305
Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ 2008 Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 122: e573–e582
Lucas A, Morley R, Cole TJ, Gore SM 1994 A randomised multicentre study of human milk versus formula and later development in preterm infants. Arch Dis Child Fetal Neonatal Ed 70: F141–F146
Tobiansky R, Lui K, Roberts S, Veddovi M 1995 Neurodevelopmental outcome in very low birthweight infants with necrotizing enterocolitis requiring surgery. J Paediatr Child Health 31: 233–236
Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, Leviton A 2010 Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr 157: 751–756
Blakely ML, Tyson JE, Lally KP, McDonald S, Stoll BJ, Stevenson DK, Poole WK, Jobe AH, Wright LL, Higgins RD 2006 Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 117: e680–e687
Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF 2009 Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics 123: 1493–1500
Downie AL, Frisk V, Jakobson LS 2005 The impact of periventricular brain injury on reading and spelling abilities in the late elementary and adolescent years. Child Neuropsychol 11: 479–495
Acknowledgements
We greatly acknowledge the help of Dr. T. Brantsma van Wulfften Palthe for correcting the English manuscript and Dr. V. Fidler for support in the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Roze, E., Ta, B., van der Ree, M. et al. Functional Impairments at School Age of Children With Necrotizing Enterocolitis or Spontaneous Intestinal Perforation. Pediatr Res 70, 619–625 (2011). https://doi.org/10.1203/PDR.0b013e31823279b1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31823279b1
This article is cited by
-
Necrotizing enterocolitis: current understanding of the prevention and management
Pediatric Surgery International (2024)
-
Neurodevelopmental outcomes of preterm with necrotizing enterocolitis: a systematic review and meta-analysis
European Journal of Pediatrics (2024)
-
Evidence-Based Approaches to Minimize the Risk of Developing Necrotizing Enterocolitis in Premature Infants
Current Treatment Options in Pediatrics (2022)
-
Surgical necrotizing enterocolitis but not spontaneous intestinal perforation is associated with adverse neurological outcome at school age
Scientific Reports (2020)
-
An economic analysis of human milk supplementation for very low birth weight babies in the USA
BMC Pediatrics (2019)