Abstract
Gastrointestinal complications during the neonatal period, i.e. necrotizing enterocolitis (NEC) and spontaneous intestinal perforation (SIP), are associated with adverse short-term outcome in very-low-birthweight infants (VLBWI, <1500 g birth weight). However, little is known about the neurological outcome of survivors at school age. We analysed data of 2241 infants followed-up at the age of 6 years. To determine the effect of NEC and SIP on cognitive outcome in consideration of other important confounding factors, we used multivariable logistic regression models. In addition, infants with surgical diagnosis of NEC (n = 43) or SIP (n = 41) were compared to NEC (n = 43) or SIP (n = 41) negative controls using Mahalanobis distance matching. Infants with a history for NEC had a three times increased risk (RR 3.0 [1.8–4.2], p < 0.001) to develop IQ scores <85 while history of surgical SIP did not increase the relative risk for lower IQs at school age (RR 1.0 [0.4–2.1], p = 1.000). In a matched-cohort analysis, we confirmed that infants with surgical NEC had lower mean IQ results than unaffected controls (±SD) (85±17 vs. 94±14, p = 0.023) while no differences were found for history of SIP. Our results reflect that the different aetiology and inflammatory extent of NEC and SIP may lead to disparate neurodevelopment trajectories. Hence, our data suggest a potential role of early gut-brain axis distortion in infants with NEC which needs to be further explored.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) and spontaneous intestinal perforation (SIP) are typical gastrointestinal complications in very-low-birthweight infants (VLBWI) and have a remarkable impact on mortality and long-term morbidity in this vulnerable group1,2,3,4. In VLBWI, NEC mainly occurs during day 14–28 of life5 and seems to have multifactorial facets including genetic predisposition, intestinal immaturity, inflammation, oxidative stress, ischemia, nutritional aspects and gut dysbiosis2,6,7. SIP usually occurs in the first 14 days of life8 and is mainly associated with extreme prematurity, use of non-steroidal and steroidal anti-inflammatory drugs and prolonged evacuation of meconium9,10. Recently, we noted an increased SIP risk in infants <25 weeks of gestation who were primarily managed with less invasive respiratory care3.
Epidemiological data of infants with NEC or SIP show delayed neurodevelopment in early childhood2,9,11, but little is known about outcome at school age. Roze et al. found an increased rate of reduced intelligence quotient (IQ) and motor testing scores among children at school age who suffered from NEC or SIP11 compared to controls without these complications. However, the authors did not adjust for confounding variables such as gestational age or maternal education level and did not differentiate between NEC and SIP.
Despite a potential clinical overlap between NEC and SIP, e.g. succus entericus-induced peritonitis, our study is based on the assumption that NEC and SIP are distinct entities with different pathophysiology and histopathological findings12,13,14,15. We here hypothesize that NEC - as a systemic “inflammatory disease” - and SIP - as a local gut disease – have different impact on long-term neurodevelopmental trajectories. The objective of our study was to evaluate the effect of NEC and SIP requiring surgery on intelligence and development of cerebral palsy at 6 years of age in a large cohort of very-low-birthweight infants from the German Neonatal Network.
Results
Cohort characteristics
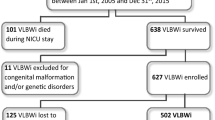
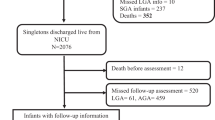
In total, 8022 VLBWI were born and discharged in the German Neonatal Network between 2009 and 2014 (Fig. 1). Infants who suffered from both NEC and SIP were excluded (n = 20). Due to aspects concerning study coordination and organization, 3333 infants were not invited for follow-up. In 123 cases, the diagnosis of NEC or SIP was unknown. The remaining 4546 infants were invited for follow-up. In this group, 177 (2.2%) had a history of NEC while 123 (1.5%) infants had previously suffered from SIP. Of the invited 4546 infants, 2482 infants were tested for intelligence and motor function at the age of 6 years (Fig. 1). Baseline characteristics of the cohort at 6 years are described in Table 1. 241/2482 infants were not capable to perform a Wechsler preschool and primary scale of intelligence test (WPSSI-III, German edition) due to (a) WPPSI or other cognitive test performed within the last 12 months (n = 161), (b) language problems (n = 24), (c) lack of motivation (n = 39), (d) serious disorder not related to prematurity (e.g. trisomy 21) (n = 7) and (e) other reasons (n = 10). These infants had a significant lower gestational age (28.4 vs. 28.9 weeks GA, p < 0.001), birth weight (917 vs. 1163 g, p < 0.001) and an increased rate of neonatal complications including SIP (4.1 vs. 1.7%, p = 0.003) as compared to infants who performed WPPSI (Supplementary Table 1). Final analysis was based on a cohort of 2241 infants including 43 VLBWI (1.9%) with a history of surgical NEC and 41 (1.8%) who had previously suffered from surgical SIP. 306 VLBWI (13.7%) had an IQ < 85 on follow-up at school age.
Enrollment, in- and exclusion for analysis of neurologic and motor development at the age of 6 years. In- and exclusion for motor function and intelligence testing in 6 year old children born as VLBWI; *Reasons for not participating the follow-up assessment despite selection were: no current contact data available n = 1349, parents declined invitation for follow-up n = 519, parents were interested to participate but were not available at suggested follow-up dates n = 131, no-show despite arranged follow-up assessment n = 65; **Reasons for no WPPSI assessment included: WPPSI or other cognitive test within 12 months n = 161, language problems n = 24, child not motivated n = 39, serious disorder not related to prematurity (e.g. trisomy 21) n = 7, other reasons n = 10.
NEC and SIP
Compared to infants without history of NEC, infants with surgical NEC in our cohort were born at lower gestational ages (26.3 vs. 29.1 weeks GA), lower birth weight (810 g vs. 1124 g) and received less antenatal steroids (83.1 vs. 89.8%) (see Table 1). VLBWI with NEC showed increased frequencies for intracerebral haemorrhage (ICH) (40.7 vs. 15.4%), periventricular leukomalacia (PVL) (11.9 vs. 2.7%), neurosurgery (4.0 vs. 1.6%) and bronchopulmonary dysplasia (BPD) (53.7 vs. 14.1%). VLBWI with surgical SIP were also born at lower gestational ages (25.6 vs. 29.1 weeks GA) and with lower birth weight (749 g vs. 1124 g) compared to non-SIP infants. SIP patients were more often male than female (66.7 vs. 33.3%) and of non-European ethnicity (23.0 vs. 14.7%). ICH (43.9 vs. 15.4%), PVL (5.7 vs. 2.7%), BPD (41.0 vs. 14.1%) were also increased in the SIP group.
Neurodevelopmental outcome
To account for several confounding risk factors leading to adverse neurodevelopmental outcome as for example gestational age or other short-term complications, we performed multivariable logistic regression models and multidimensional Mahalanobis distance matching.
The multivariable logistic regression analysis revealed that infants with a history for NEC were associated with IQ scores <85 (OR 4.3 [2.1–8.8], p < 0.001) while history of surgical SIP was not associated with lower IQs at school age (OR 1.0 [0.4–2.6], p = 1.000) (see Supplementary Table 3). We calculated the relative risks from logistic regression by using the method described by Zhang et al.16. Here, infants with NEC showed up with a three times increased risk for IQ results < 85 (RR 3.0 [1.8–4.2], p < 0.001) (see Table 2). We matched history of NEC (n = 43) vs. controls (n = 43) and history of SIP (n = 41) vs. controls (n = 41) for different clinical parameters (gestational age, ICH, PVL, European ethnicity, BPD, female gender, antenatal administration of steroids, and highest maternal education level) using Mahalanobis distance. Baseline data about matched groups are given in Supplementary Table 2. SIP infants were born at significantly lower GA (25.4 ± 1.9 vs. 27.7 ± 2.2 weeks GA, p < 0.001) with lower birth weight (749 ± 260 vs 917 ± 309 g, p < 0.001), length (32.5 ± 3.8 vs. 34.7 ± 4.4, p = 0.021) and head circumference (23.0 ± 2.5 vs. 24.8 ± 2.6 cm, p = 0.003) than controls.
In this matched model we confirmed that infants with surgical NEC had lower mean IQ results than unaffected controls (±SD) (85 ± 17 vs. 94 ± 14, p = 0.023) (Table 3, Fig. 2a). There were no differences between SIP patients and controls regarding cognitive impairments (93 ± 17 vs. 91 ± 21, p = 0.831) (Fig. 2b).
(a) Total, verbal and performance intelligence quotient scores in infants with and without NEC. Boxplot of IQ scores (total, verbal, performance) for matched infants with history of NEC and without a history of NEC. p-values are derived from T-Test. (b) Total, verbal and performance intelligence quotient scores in infants with and without SIP. Boxplot of IQ scores (total, verbal, performance) for matched infants with history of SIP and without a history of SIP. p-values are derived from T-Test.
Growth parameters
In addition, head circumference at the age of 6 years is significantly lower in infants with NEC (48.8 ± 2.0 vs. 50.1 ± 2.1 cm, p = 0.004) and SIP (49.2 ± 2.4 vs. 50.4 ± 2.3 cm, p = 0.011). Infants with NEC history at the age of 6 years present with significant reduced body length as their matched controls (110.0 ± 6.4 vs. 113.0 ± 5.5 cm, p = 0.024).
Discussion
In a large cohort of VLBWI from the German Neonatal Network, we found that history of NEC but not SIP is associated with an increased risk of impaired neurological development, in particular reduced IQ scores at 6 years of age. Infants with NEC had a three times increased relative risk to develop IQ results <85. So far, most studies on the neurodevelopment of VLBWI reported outcome at the age of 18–36 months17 or used data of cohorts from the 1990s18 which are not necessarily comparable with recent cohorts who have a decreased short-term morbidity including NEC19,20,21,22. In one study, the authors found a reduced IQ score in children with a history of gastrointestinal disease (NEC or SIP)11. In our study, we made a clear distinction between surgical NEC and surgical SIP based on pathophysiological and macroscopical (as defined by attending surgeon) criteria. The pathophysiology of NEC is based on the immaturity of the gastrointestinal tract and understood as a multifactorial interplay leading to inflammatory processes23. Distinct characteristics of bacterial colonization and inappropriate colonization of the premature intestine predispose infants to NEC suggesting a causal relationship between gut bacteria and NEC24,25. Together with an inadequate anti-inflammatory response observed in the immature intestine, dysbiosis triggers an inflammatory cascade leading to NEC. Intestinal permeability is observed in NEC and modulated through the expression of Toll-like receptors (TLR4)12. Nino et al. found a link between NEC and brain injury through activation of TLR4 on microglial cells in the brain. TLR4 stimulation by gut-derived mediators impacts brain injury since TLR4-deficient mice were protected from NEC-induced brain injury. In the setting of prematurity, NEC is associated with a “proinflammatory regulatory protein profile”15 which suggests a link between sustained inflammation and adverse neurodevelopmental outcomes2,14.
In our matched cohort, we found decreased head circumferences in the NEC and SIP group. Some studies report substantial growth failure (<10th percentile) for weight, length, and head circumference in infants with NEC26, but results were conflicting11. One study examining head biometrics in magnet resonance imaging (MRI) after NEC found associations with reduced biparietal width27. Another study comparing white matter abnormalities (WMA) on brain MRI in NEC and SIP infants showed that infants with NEC had higher WMA scores than those with SIP28 assuming a higher vulnerability of oligodendroglial precursors after systemic inflammatory processes such as NEC. In our study, we cannot exactly give an explanation for our findings and can only speculate about causes. It is unclear if our findings are the result of sustained inflammation originating in the neonatal period, the result of chronic malnutrition in this vulnerable population or statistical biased observations. For example, the reduced head circumference in the SIP cohort could be explained by significant reduced birth weight and reduced lower gestational age in the matched SIP group leading to a reduced head growth. We therefore think that our findings concerning growth parameters should be interpreted with caution and further scientific efforts should clarify the role of inflammation or malnutrition in these infants, as interventions here could improve neurologic outcome.
NEC and SIP are both entities with low incidences in a very unique patient cohort. Here, our study has a powerful setting as a large multi-centre study with prospective collection of data and uniform follow-up assessment by the same study team. However, our study also has limitations. We decided to use stringent criteria for NEC (NEC requiring surgery) and SIP (requiring surgery) as primary outcome measure. Hence, the diagnosis of “medical NEC”, i.e. NEC not requiring surgery, which might be difficult to distinguish from other entities, was not considered. Second, our NEC or SIP definition is based on the clinical evaluation of the attending surgeon and neonatologist and not necessarily based on histopathology. Third, our follow-up cohort has a risk of selection bias. For the follow-up, we chose a random invitation practice, but were not able to reduce some differences in both groups. Some factors that were associated with better neurological outcomes were more common in children who were followed-up compared to those who were not including higher birth weight and higher exposure rate with antenatal steroids. On the other hand, BPD and multiple birth were overrepresented in the followed-up cohort. For these differences we accounted with logistic regression models and according matching strategies. Fourth, our study is a post-hoc analysis of an observational population-based design. Hence, a causal relationship cannot be made and mechanistic modelling is necessary. Furthermore, we are not able to rule out the possibility of unrecognized confounders. Preterm birth is associated with several postnatal complications that might impact neurologic development29. Potential factors such as antibiotic use, gut dysbiosis, nutrition, use of several drugs or unrecognized systemic reactions might have an impact on neurodevelopmental outcome which we cannot adjust for. Additionally, we were not able to control our analyses for short bowel syndrome as known risk factor for neurological impairments18 as we did not record this disease in our follow-up examinations. To account for a variety of known confounders we used Mahalanobis distance matching as it is proved to be a valid approach for adjustments for multiple outcomes30. The matched cohort showed a homogenous distribution of factors for NEC which suggest that the model fits adequately. In the SIP group, gestational age and birth weight were significantly lower than in the control group. However, both groups do not differ concerning outcome characteristics, strengthening our conclusion that SIP even at lower GA has no impact on intelligence quotient.
In conclusion, our results reflect that the different aetiology and inflammatory extent of NEC and SIP may lead to disparate neurodevelopment trajectories. Hence our data suggest a potential role of an early gut-brain axis distortion in infants with NEC.
Future longitudinal studies, specifically in cohorts with interventions on the preterm infants microbiome such as PRIMAL31, along with detailed mechanistic models are needed to disentangle the impact of gut dysbiosis and sustained inflammation on adverse neurodevelopmental outcome after prematurity.
Methods
Ethics
Approval by the local ethic committee for research in human subjects of the University of Lübeck (file number 08–022) and by the local ethic committees of all participating centres has been given. Written informed parental consent was given for the research and publication of the results of each infant included in the study. The German Neonatal Network is funded by the German Ministry for Education and Research (BMBF-grant-No: 01ER0805 and 01ER1501).
Cohort and definitions
The German Neonatal Network (GNN) is an ongoing multicentre population-based cohort study enrolling VLBWI with <1500 g birth weight in Germany. From 2009 until 2014, 43 tertiary German tertiary level NICUs contributed to the GNN (www.vlbw.de)32. Data were collected prospectively by neonatologists or trained study personal. Infants <1500 g birth weight and with a gestational age below 37 weeks were enrolled. A predefined clinical data set including antenatal and postnatal treatment and outcome data was recorded prospectively. The proper assessment of clinical data was ensured by yearly on-site-monitoring by a study nurse or paediatrician experienced in neonatology. NEC requiring surgery is defined as clinical NEC classified as Bell Stage II or Bell Stage III with the need for laparotomy with or without resection of necrotic gut, and the macroscopic diagnosis of NEC33. Clinical NEC without surgical treatment was excluded from our analysis. SIP diagnosis was defined as the occurrence of spontaneous intestinal perforation with the need for laparotomy and the macroscopic diagnosis of isolated SIP as described by the attending surgeon. Small for gestational age (SGA) was defined as birth weight <10th percentile according to population based birth weight reference values34.
Six-year follow-up
At the age of 5–6 years, VLBW children were invited for standardized follow-up examination by a study team consisting of a physician and nurses. During the invitation procedure, the study team contacted the clinic of birth for possible follow-up examination dates. The contact database was randomly searched for possible candidates with focus set on infants born <28 weeks gestational age, but infants born >28 weeks were not necessarily excluded. Infants who were born in the clinic of interest, contactable via telephone or postal letter and who could present at the follow-up examination appointment received an invitation letter.
Motor development
Cerebral palsy (CP) was defined as disorder of the central nervous system characterized by abnormal muscle tone in at least one extremity and abnormal motor development assessed with the Gross Motor Function Classification System (GMFCS ≥ level 1)35 and Bimanual Fine Motor Function (BFMF ≥ level 1)36. VLBW children without functional limitations were scored as “Level 0”.
Cognitive development
Total, verbal, and performance intelligence were assessed using the Wechsler Preschool and Primary Scale of Intelligence – Third Edition [WPPSI I-III, German]37.
Statistical analysis
Cohort characteristics
To describe the characteristics of the whole cohort of VLBWI, we present differences of infants with NEC, SIP or without one of these complications. Data are presented as numbers, frequencies and 95% confidence intervals (CI) of column percentages.
Neurological outcome analysis
For neurological outcome analysis at 6 years of age, we included infants who were capable to perform a Wechsler preschool and primary scale of intelligence test (WPSSI-III, German edition). Logistic regression analysis and Mahalanobis distance matching were used to calculate a potential impact of NEC or SIP on outcome at six years with simultaneous controlling for several potential confounding factors. Mahalanobis’ method is expected to be very successful in reducing bias in multivariate matching31 and was used to compare NEC or SIP affected individuals with unaffected controls with similar risk profile.
Logistic regression analysis
Odds ratio and corresponding 95% confidence interval deriving from a logistic regression model was calculated to characterize an association of intelligence quotient <85 at 6 years of age and CP with neonatal complications as potential confounders: birth weight, gestational age (GA), female gender, NEC, SIP, intracerebral haemorrhage (ICH) grade ≥3 and periventricular leukomalacia (PVL), neurosurgery for ventriculoperitoneal shunting (post-haemorrhagic hydrocephalus), European ethnicity, bronchopulmonary dysplasia (BPD), born small for gestational age (SGA), surfactant application, and maternal education level. We calculated the relative risks (RR) derived from the logistic regression by using the proposed method from Zhang et al.16.
For multiple testing, p-values were adjusted using Bonferroni-Holm method and regarded significant when p < 0.05. Infants who were not capable for WPSSI testing are analysed separately using descriptive statistics.
Mahalanobis distance matching
To analyse the effects on intelligence quotient and motor outcome, we matched the participants into two groups for each complication: NEC positive and NEC negative or SIP positive and SIP negative clinical courses. We matched the groups via Mahalanobis distance multi-dimensional modelling38. Matching was based on the calculated Mahalanobis distance, including gestational age, ICH, PVL, European ethnicity, BPD, female gender, antenatal administration of steroids, and maternal education for NEC and SIP analysis. For each index case with NEC or SIP, matches were chosen by the best fitting non-affected nearest partner using calculated Chi-squares of two distances.
All statistical analyses were performed with SPSS 22.0 software (IBM SPSS Statistics for Windows, Version 22.0. Munich, Germany). Graphics were created using GraphPad Prism Version 7.00 for Mac (GraphPad Software, La Jolla California USA, www.graphpad.com).
Ethics approval and consent to participate
Written informed consent was obtained from parents on behalf of the infants enrolled in our study. The study parts were approved by the local committee on research in human subjects of the University of Lübeck (08–022; 03.12.2010) and the local ethical committees at the other study centres.
Specifically: Ethical Board of the Medical Chamber of the North Rhine region, Ethical Board of the University of Aachen, Ethical Board of the University of Bonn, Ethical Board of the Medical Chamber of the federal state of Mecklenburg-Vorpommern, Ethical Board of the Medical Chamber of Berlin, Ethical Board of the University of Magdeburg, Ethical Board of the University of Halle, Ethical Board of the University of Tübingen, Ethical Board of the Medical School Hannover, Ethical Board of the University of Cologne, Ethical Board of the University of Essen, Ethical Board of the Medical Chamber of the Westphalia-Lippe region, Ethical Board of the Medical Chamber of Hamburg, Ethical Board of the Medical Chamber of the federal state of Hessen, Ethical Board of the Medical Chamber of the federal state of Baden-Württemberg, Ethical Board of the Medical Chamber of the federal state of Bavaria, Ethical Board of the Saar University.
Consent for publication
Consent was given by the parents or legal guardians.
Data availability
The datasets generated and analyzed during the current study are not publicly available but can be reviewed on reasonable request.
References
Battersby, C., Santhalingam, T., Costeloe, K. & Modi, N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch. Dis. Child. - Fetal Neonatal Ed. 103, F182–F189 (2018).
Martin, C. R. et al. Neurodevelopment of Extremely Preterm Infants who had Necrotizing Enterocolitis with or without Late Bacteremia. J. Pediatr. 157, 751–756.e1 (2010).
Härtel, C. et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 8, 8333 (2018).
Berry, M. J., Port, L. J., Gately, C. & Stringer, M. D. Outcomes of infants born at 23 and 24 weeks’ gestation with gut perforation. J. Pediatr. Surg. 54, 2092–2098 (2019).
Yee, W. H. et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 129, e298–304 (2012).
Moschopoulos, C. et al. The Neurodevelopmental Perspective of Surgical Necrotizing Enterocolitis: The Role of the Gut-Brain Axis. Mediators Inflamm. 2018, 1–8 (2018).
Warner, B. B. et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387, 1928–36 (2016).
Houben, C. et al. Spontaneous Intestinal Perforation: The Long-Term Outcome. Eur. J. Pediatr. Surg. 27, 346–351 (2017).
Wadhawan, R. et al. Spontaneous intestinal perforation in extremely low birth weight infants: association with indometacin therapy and effects on neurodevelopmental outcomes at 18–22 months corrected age. Arch. Dis. Child. - Fetal Neonatal Ed. 98, F127–F132 (2013).
Koshinaga, T. et al. Spontaneous localized intestinal perforation and intestinal dilatation in very-low-birthweight infants. Acta Paediatr. 95, 1381–1388 (2006).
Roze, E. et al. Functional Impairments at School Age of Children With Necrotizing Enterocolitis or Spontaneous Intestinal Perforation. Pediatr. Res. 70, 619–625 (2011).
Mihi, B. & Good, M. Impact of Toll-Like Receptor 4 Signaling in Necrotizing Enterocolitis. Clin. Perinatol. 46, 145–157 (2019).
Niño, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 10, eaan0237 (2018).
O’Shea, T. M. et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain. Behav. Immun. 29, 104–12 (2013).
Chan, K. Y. Y. et al. Immunoregulatory protein profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in preterm infants. PLoS One 7, e36977 (2012).
Zhang, J. & Yu, K. F. What’s the Relative Risk? JAMA 280, 1690 (1998).
Wadhawan, R. et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J. Perinatol. 34, 64–70 (2014).
Arnold, M., Moore, S. W., Sidler, D. & Kirsten, G. F. Long-term outcome of surgically managed necrotizing enterocolitis in a developing country. Pediatr. Surg. Int. 26, 355–360 (2010).
Salhab, W. A., Perlman, J. M., Silver, L. & Sue Broyles, R. Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants. J. Perinatol. 24, 534–40 (2004).
Härtel, C. et al. Lactobacillus acidophilus/Bifidobacterium infantis probiotics are associated with increased growth of VLBWI among those exposed to antibiotics. Sci. Rep. 7, 5633 (2017).
Härtel, C. et al. NOD2 Loss-of-Function Mutations and Risks of Necrotizing Enterocolitis or Focal Intestinal Perforation in Very Low-birth-weight Infants. Inflamm. Bowel Dis. 22, 249–256 (2016).
Härtel, C. et al. Prophylactic Use of Lactobacillus acidophilus/Bifidobacterium infantis Probiotics and Outcome in Very Low Birth Weight Infants. J. Pediatr. 165, 285–289.e1 (2014).
Kubota, A. et al. Focal intestinal perforation in extremely-low-birth-weight neonates: etiological consideration from histological findings. Pediatr. Surg. Int. 23, 997–1000 (2007).
Mizrahi, A., Barlow, O., Berdon, W., Blanc, W. A. & Silverman, W. A. Necrotizing enterocolitis in premature infants. J. Pediatr. 66, 697–705 (1965).
Kuppala, V. S., Meinzen-Derr, J., Morrow, A. L. & Schibler, K. R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 159, 720–5 (2011).
Hintz, S. R. Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants After Necrotizing Enterocolitis. Pediatrics 115, 696–703 (2005).
Kidokoro, H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134, e444–53 (2014).
Shin, S. H. et al. Surgical Necrotizing Enterocolitis versus Spontaneous Intestinal Perforation in White Matter Injury on Brain Magnetic Resonance Imaging. Neonatology 110, 148–54 (2016).
Bouyssi-Kobar, M. et al. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics 138, e20161640 (2016).
Desai, R. J. et al. Extension of Disease Risk Score-Based Confounding Adjustments for Multiple Outcomes of Interest: An Empirical Evaluation. Am. J. Epidemiol. 187, 2439–2448 (2018).
Marißen, J. et al. Efficacy of Bifidobacterium longum, B. infantis and Lactobacillus acidophilus probiotics to prevent gut dysbiosis in preterm infants of 28+0–32+6 weeks of gestation: a randomised, placebo-controlled, double-blind, multicentre trial: the PRIMAL Clinical Study protocol. BMJ Open 9, e032617 (2019).
Humberg, A. et al. Active perinatal care of preterm infants in the German Neonatal Network. Arch. Dis. Child. - Fetal Neonatal Ed., fetalneonatal-2018-316770, https://doi.org/10.1136/archdischild-2018-316770 (2019).
Bell, M. J. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Voigt, M. et al. New percentile values for the anthropometric dimensions of twin neonates: analysis of perinatal survey data of 2007-2011 from all 16 states of Germany. Z. Geburtshilfe Neonatol. 218, 254–260 (2014).
Reid, S. M., Carlin, J. B. & Reddihough, D. S. Using the Gross Motor Function Classification System to describe patterns of motor severity in cerebral palsy. Dev. Med. Child Neurol. 53, 1007–1012 (2011).
Elvrum, A.-K. G. et al. Bimanual Fine Motor Function (BFMF) Classification in Children with Cerebral Palsy: Aspects of Construct and Content Validity. Phys. Occup. Ther. Pediatr. 36, 1–16 (2016).
Fortmann, I. et al. Antifungal Treatment and Outcome in Very Low Birth Weight Infants. Pediatr. Infect. Dis. J. 37, 1165–1171 (2018).
Diamond, A. & Sekhon, J. S. Genetic Matching for Estimating Causal Effects: A General Multivariate Matching Method for Achieving Balance in Observational. Studies. Rev. Econ. Stat. 95, 932–945 (2013).
Acknowledgements
The authors would like to thank all nurses, doctors and participating NICUs for their support and especially all participating infants and their parents. The German Neonatal Network is funded by the German Ministry for Education and Research (BMBF-grant-No: 01ER0805 and 01ER1501). The sponsor had no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
The authors have given final approval of the version to be published. Each author participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the authors have read the manuscript and agreed to its being submitted for publication. All individuals listed as authors meet the appropriate authorship criteria, nobody who qualifies for authorship has been omitted from the list. Authors and contributors have approved the acknowledgement of their contributions. All authors had complete access to the study data that support the publication. Designed study Alexander Humberg, Wolfgang Göpel, Christoph Härtel. Collected and analyzed the data: Alexander Humberg, Egbert Herting, Michael Zemlin, Christoph Härtel, Tanja K. Rausch, Wolfgang Göpel, Juliane Spiegler, Janina Marissen, Isabelle Swoboda. Wrote the first draft of the paper: Alexander Humberg, Christoph Härtel, Wolfgang Göpel, Egbert Herting, Juliane Spiegler, Mats Ingmar Fortmann.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Humberg, A., Spiegler, J., Fortmann, M.I. et al. Surgical necrotizing enterocolitis but not spontaneous intestinal perforation is associated with adverse neurological outcome at school age. Sci Rep 10, 2373 (2020). https://doi.org/10.1038/s41598-020-58761-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58761-6
This article is cited by
-
Necrotizing enterocolitis: current understanding of the prevention and management
Pediatric Surgery International (2024)
-
Factors affecting neurodevelopmental outcome following surgical necrotising enterocolitis: a systematic review
Pediatric Surgery International (2024)
-
Bench to bedside — new insights into the pathogenesis of necrotizing enterocolitis
Nature Reviews Gastroenterology & Hepatology (2022)
-
Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice
Journal of Neuroinflammation (2021)
-
Preterm birth and sustained inflammation: consequences for the neonate
Seminars in Immunopathology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.