Abstract
Objective
The objective of this study is to examine the relationship between neonatal risk factors and feeding difficulties (FDs) in the NICU and the impact of FD on neurodevelopmental (ND) outcome in ELGA infants.
Study design
Two hundred and eighteen ELGA infants (59 FDs and 159 no-FDs) were compared for neonatal morbidities, feeding milestones, and Bayley-III scores at 8 and 20 mo CA. Multiple regression analyses adjusted for the effect of risk factors on FD and ND outcome.
Results
Twenty-seven percent of infants had FD. Postmenstrual age (PMA) at start of oral feeds was the only predictor of FD. At 8 mo CA, FD was the strongest predictor of cognitive <85 (p = 0.018) and motor index <70 (p = 0.019). In linear regression, PMA at start of oral feeds was the only predictor of 8 mo cognitive and motor index (p = 0.006). FD did not predict ND outcome at 20 months CA.
Conclusions
FDs are common in ELGA infants and are associated with worse cognitive and motor outcomes in the first year of life.
Similar content being viewed by others
Background
Advancements in perinatal care have led to increasing survival rates for extremely low gestational age (ELGA; birth GA < 28 weeks) infants, a vulnerable population who remain at high risk for both neonatal morbidities and subsequent neurodevelopmental impairment after discharge from the neonatal intensive care unit (NICU) [1,2,3,4]. These infants are also at high risk for feeding difficulties (FDs), which are known to be associated with adverse neurodevelopmental outcome [5,6,7,8,9,10]. Neonatal morbidities including bronchopulmonary dysplasia (BPD), severe brain injury, and necrotizing enterocolitis (NEC) can adversely impact feeding endurance, swallowing abilities, and tolerance to enteral nutrition. Delayed attainment of full oral feedings can lead to prolongation of the NICU hospitalization. Recent studies have demonstrated an association between delayed oromotor skills and/or need for gastrostomy tube placement and worse neurodevelopmental outcome at age 2 years [7, 9]. Nevertheless, the literature is sparse with respect to the impact of feeding milestones and neonatal morbidities on the development of FDs and the impact of FDs on ND outcome in ELGA infants [5,6,7,8,9,10].
During the years 2008–2012, neonatologists at our institution considered infants to have FD if they were unable to take any oral feeding by 35 weeks postmenstrual age (PMA) and/or at least 100 ml/kg/day by mouth by 38 weeks PMA. Although there have been significant practice changes since that time, our criteria for what was considered “delayed attaintment of feedings milestones” was similar to that of other centers [11]. As ELGA infants have higher rates of neonatal morbidities associated both with FD and neurodevelopment impairments as compared with older preterm infants, we wanted to better delineate risk factors and outcome for a contemporary ELGA population. We sought to examine the (1) predictors of FD and (2) impact of FD on ND outcome at 8 and 20 months corrected age (CA) in ELGA infants.
Methods
Population and feeding data
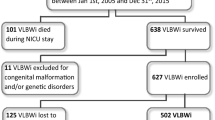
This is a retrospective chart review of all ELGA infants born at Rush University Medical Center (RUMC) from 2008 to 2012, who were discharged from the Rush NICU. Exclusion criteria included the presence of a major congenital malformation and/or genetic syndrome and infants who were transported to or from RUMC. Remaining infants were categorized according to FD, the diagnosis of which was multidisciplinary and made in conjunction with neonatology and nursing, and at times speech pathology and documented as such in the medical record. Although there was minor variation in practice, the majority of neonatologists during the study years at our institution considered infants to have “feeding difficulty” if they were unable to take any oral feeding by 35 weeks PMA and/or at least 100 ml/kg/day by mouth by 38 weeks PMA. This resulted in a cohort of 218 ELGA infants (59 FDs and 159 no-FDs; mean birth weight (BW) 852 ± 188 g, mean GA 26.2 ± 1.2 weeks), who were compared in terms of neonatal morbidity and feeding milestones in the NICU and ND outcome at 8 and 20 months CA after NICU discharge (Fig. 1). At 8 months CA, 50 (85%) infants in the FD and 116 (73%) infants in the no-FD group completed ND assessments. At 20 months CA, 39 (66%) of infants with FD and 106 (66%) of infants with no-FD completed ND assessments.
Feeding data collected from the NICU hospitalization included the following: age (in days of life and PMA) at (1) start and attainment of full enteral feedings, (2) start and attainment of full oral feedings (if applicable), (3) occurrence of nil per os (no enteral feedings; NPO) status after initiation of feeding protocol (if applicable), (4) discontinuation of mechanical ventilation (any positive pressure ventilation administered through endotracheal tube, tracheostomy tube, nasal prongs, or mask). Feeding data also included, when applicable, age at speech consultation, Otolaryngology (ENT) evaluation, video fluoroscopic swallow study, gastrostomy tube placement, as well as the need for the use of the following: hydrolyzed cow’s milk protein formulas, anti-gastroesophageal reflux disease (GERD) medications, and the use of additives to formula or breastmilk to thicken consistency of feedings.
Neonatal and socio-demographic data
Socio-demographic and birth data collected included maternal age, health insurance status, in-utero drug exposure, antenatal steroid administration, mode of and reason for delivery, maternal chorioamnionitis, maternal preeclampsia, infant BW and GA, race/ethnicity, type of medical insurance, multiple gestation and small for GA status, defined as birth weight <10th percentile according to Fenton [12]. Neonatal morbidity information collected included presence of a patent ductus arteriosus treated with medication and/or surgery, treated hypotension (treatment at the attending physician discretion with fluids, inotropes, or hydrocortisone), the presence of BPD, defined as oxygen dependence at 36 weeks’ PMA, treatment with postnatal steroids, sepsis, defined as a positive blood or cerebrospinal fluid culture, NEC stage 2–3, defined according to Bells’ criteria, spontaneous intestinal perforation, any stage of retinopathy of prematurity (ROP), and head ultrasound (HUS) findings [13]. Finally, for the NICU stay, data were collected on home oxygen therapy, diet, and PMA at discharge.
Neurodevelopmental follow-up data
During the period of study it was the policy to evaluate all ELGA infants in the Neonatal High Risk Follow-up Clinic, a multidisciplinary clinic that monitors the growth, neurologic, and developmental status of infants cared for in the NICU. Outcome measures included neurologic exam and results of the Bayley Scales of Infant and Toddler Development-III (BSITD-III) at 8 and 20 months CA [14]. Outcome measures on the BSITD-III included the cognitive, language, and motor index scores, and the receptive and expressive language, and the fine and gross motor subscale scores. The mean for index scores is 100 ± 15 and for subscale scores is 10 ± 3 [14]. All scores >1 SD below the mean (<85 for index scores and <7 for subscale scores) were classified as subnormal. All scores >2 SDs below the mean (<70 for index and <4 for subscale) were classified as severely abnormal. Neurologic examination of muscle tone was performed according to Amiel-Tisonand Stewart [15]. Neurologic abnormalities were classified as hypertonia, hypotonia, and cerebral palsy.
Statistical analyses
A series of χ2- or ϕ, point-biserial and Pearson’s correlations were calculated to analyze the following bivariate associations between FD and socio-demographic, birth and neonatal morbidity variables, and FD and BSITD-III index and subscale scores at 8 and 20 months CA. In addition, χ2- or ϕ, point-biserial, and Pearson’s correlations were calculated to analyze bivariate associations between neonatal and socio-demographic data, and 8 and 20 month BSITD-III index and subscale scores.
Hierarchical, step-wise procedures were used for multiple linear and logistic regressions predicting the impact of FD on ND outcome after controlling for neonatal and social risk factors. Factors significantly associated with ND outcome in bivariate analyses (p < 0.05) were entered in the first step, FD was entered in the second step, and within each step variables with p < 0.2 were retained. The study was reviewed by and approved the Institutional Review Board of RUMC.
Results
Socio-demographic data and neonatal morbidities
Twenty-seven percent (n = 59) of the cohort had FD. Socio-demographic, birth, and neonatal morbidity data for the two groups is presented in Table 1. There were no differences in mean BW, GA, maternal, or infant socio-demographic characteristics between the two groups. Infants with FD were significantly more likely to have been treated with hydrocortisone (66% vs, 40%), have treated hypotension (51% vs, 35%), develop BPD (83% vs. 62%), ROP (49% vs. 31%) and, as expected, were discharged at an older PMA (45.7 ± 10 vs. 39.3 ± 3 weeks) compared with infants with no-FD.
Details of feeding milestones and feeding history
Details of feeding milestones and feeding history between the two groups are presented in Table 2. There were no differences in mean age at which enteral feeds were initiated between the two groups. Infants with FD were significantly more likely to have been made NPO for gastrointestinal symptoms prior to achieving full enteral feeds as compared to infants with no-FD (28% vs. 12%, p = 0.031). Infants with FD were on mechanical ventilation significantly longer and until an older PMA (34.5 vs. 32.8 weeks, p < 0.001) and started oral feeds at a significantly later PMA (36.9 vs. 34.9 weeks, p < 0.001) as compared with infants with no-FD. Twenty-four percent, 55%, and 20% of infants with FD were treated with hydrolyzed protein formula, medication for GERD, and feeding thickeners, respectively. Only 83% of infants with FD achieved full oral feedings at the time of NICU discharge and 27% of infants underwent placement of a gastrostomy tube (mean PMA 43.7 ± 2.3 weeks).
Unadjusted analyses of neurodevelopmental outcome at 8 and 20 months
Fifty (85%) infants with FD and 116 (73%) infants with no-FD completed ND assessments at 8 months. Infants who completed follow-up at 8 months had lower rates of severely abnormal HUS (13% vs. 25%, p = 0.043) but higher rates of BPD (72% vs. 53%, p = 0.011) compared with infants who did not attend the 8-month visit. At 20 months CA, 39 (66%) infants with FD and 106 (66%) infants with no-FD completed ND assessments. Infants who completed 20-month follow-up were significantly less likely to have had intrauterine drug exposure (2% vs. 8%, p = 0.03) and severe HUS abnormalities (12% vs. 22%, p = 0.045) but more likely to be Hispanic (28% vs. 13%, p = 0.026), have had BPD (73% vs. 57%, p = 0.018), sepsis (24% vs.13%, p = 0.042), and be on a diet of human milk at NICU discharge (44% vs. 29%, p = 0.03). Mean Bayley Index Scores at 8 and 20 months are presented in Table 3. Patients in the FD group had a significantly lower mean cognitive and motor index score at 8 months compared with those in the no-FD group. There were no differences in mean Bayley Index Scores at 20 months between the FD and no-FD group. Infants with FD also had higher rates of severely abnormal motor index <70 (26% vs. 7%, p < 0.001) and gross motor subscale scores <4 (30% vs. 9%, p < 0.001), as well as higher rates of subnormal cognitive index <85 (18% vs. 3%, p = 0.001), motor index <85 (46% vs. 30%, p = 0.045), and fine motor subscale <7 (25% vs. 10%, p = 0.013) at 8 months CA as compared with infants with no-FD.
Multivariate analyses of neurodevelopmental outcome at 8 and 20 months
The results of multiple logistic regression analyses of subnormal and severely abnormal Bayley Index and Subscale Scores at 8 months are shown in Table 4. Infants with FD had 5.7 times the odds of having a subnormal cognitive index, and 3.4 and 3.9 times the odds of having severely abnormal motor index and gross motor subscale at 8 months CA, respectively, compared with those with no-FD. Furthermore, age at start of oral feeds was an independent predictor of subnormal cognitive index, motor index, and fine motor outcome at 8 months CA. There were no other neonatal predictors of subnormal outcome in the first year of life. In multiple linear regression analyses, age at the start of oral feeds was the only significant predictor of both cognitive and motor index (p = 0.006) at 8 months CA. FDs did not predict ND outcome at 20 months CA.
Discussion
FDs, although acknowledged to be common, are understudied in the preterm infant population, particularly for the most immature infants in whom delayed attainment of full oral feeds is very high. We found that ELGA infants with FDs had a higher number of ventilator days and were more likely to have been made NPO for gastrointestinal issues and subsequently started oral feeds at a significantly older age as compared to infants with no-FD. In our ELGA population age at the start of oral feedings was an independent predictor of worse neurodevelopmental outcome. Furthermore, infants identified as having FD had significantly worse cognitive and motor outcome in the first year of life even after adjusting for neonatal morbidities. Although other studies published in the past decade have examined the relationship between neonatal morbidities and feeding abilities [5, 7], and the relationship between FD and ND outcome [6, 9], to our knowledge no study has examined both in a contemporary cohort of ELGA infants.
In a study of 186 preterm infants <36 weeks’ GA born in 1998–1999, Jadcherla et al. [5] found that PMA age at full oral feedings was significantly increased for infants with lower GA, hypotension, gastroesophageal reflux disease, and mechanical ventilation, and that infants with apnea of prematurity, sepsis, and/or on mechanical ventilation had significantly lower odds of achieving full oral feedings by NICU discharge. Contrary to their findings, we did not see a relationship between any neonatal morbidity on our feeding outcome measure. However, our study examined only infants of birth GA < 28 weeks and the relationship between neonatal morbidities and feeding milestones on an institutional definition of FD. Also, our ELGA infant population had significantly higher rates of BPD (67% vs. 31%) and lower rates of sepsis (20% vs. 49%), hypotension (40% vs. 74%), postnatal steroid exposure (47% vs. 63%), and treatment for GERD (15% vs. 31%) as compared with the subgroup of ELGA infants in the prior study, and likely a reflection of differences in institutional practices as well as changes in neonatal care since 2000 [5]. Interestingly, although our ELGA infants achieved full enteral gavage feedings at an earlier PMA (30.4 vs. 33.6 weeks), our cohort achieved full oral feedings at a significantly later PMA (38.9 vs. 36.6 weeks) compared with the ELGA infants in the prior study [5]. We speculate that this may be due to the higher rates of BPD in our cohort, or more likely relates to the fact that during these study years our practice did not include discharging infants home on gavage feeds, which was seen in almost 50% of the ELGA infants in the prior study [5]. Hence, our infants were kept in-patient longer to work on oral feeding skills and mean age at gastrostomy tube placement 125 ± 18 days. Furthermore, 55% of the infants with FDs (16% of all ELGA infants) had been treated with either H2 receptor antagonists and/or proton pump inbibitors, the use of which has been associated with FD, prolongation of NICU stay, and worse ND outcome in extremely low birth weight infants [16, 17].
Gianni et al. [7] studied a contemporary cohort of 84 infants born at <32 weeks’ GA in 2012 and found that infants with lower birth weight, BPD, and any gastrointestinal surgery achieved full oral feedings at a significantly later PMA. Unlike our population, however, these infants were of larger BW and had significantly lower rates of BPD (15%) and postnatal steroid use (13%). This healthier group of infants started oral feeds (33.7 vs. 35.5 weeks) and achieved full oral feeds (36.7 vs. 38.9 weeks) at a younger PMA as compared with our cohort, with only one infant being fed gavage at discharge. Their study, however, highlights once again the vulnerability of the extremely preterm infant who must undergo a longer period of physiologic, neurodevelopmental, and gastrointestinal maturation prior to introduction of oral feeds, and who remains at higher risk for respiratory morbidities that preclude safe introduction of oral feeds. As such, a strength of our cohort is that it included only ELGA infants, thus allowing us to focus on external factors such as neonatal morbidities and feedings practices on the development of FDs.
It is difficult, however, to ascertain whether FDs are an independent predictor of adverse neurodevelopmental outcome or rather a marker for existing neuropathology. FDs may serve as a proxy for neuropathology in infants with comorbid conditions that (1) directly result in reduced vigor and altered suck/swallow coordination (i.e., intraventricular hemorrhage) and/or (2) indirectly preclude introduction of oral feedings (i.e., BPD). For example, competency in oral feedings requires a complex coordination of suck, swallow, and respirations, in order to feed safely without cardiorespiratory events [18,19,20]. In particular, infants with BPD have frequent derangements in respiratory control during the swallowing phase of oral feeding [18]. These infants in turn require more frequent pacing, closer monitoring, and are often not afforded the opportunity to consistently orally feed until an older PMA. Similarly, Lau et al. [19] demonstrated that sucking and swallowing frequency, as well as feeding bolus size increased with increasing age, but preterm infants swallowed at different points in the respiratory cycle as compared with term infants. Interestingly, we found that older PMA at first oral feeding was an independent predictor of FD and was associated with worse developmental outcomes in the first year of life. It is possible that ELGA infants with younger PMA at first oral feeding had the least neuropathology and thus had better developmental outcomes in the first year of life. Alternatively, it is possible that the process of orally feeding at an earlier GA provides neurosensory and oral motor stimulation, and allows for early acquisition of feeding milestones [20, 21]. The process of feeding a preterm infant requires a high degree of caregiver interaction and communication, both of which can improve ND outcome. Infants who are discharged from the NICU on tube feedings may be at further neurodevelopmental risk if caregivers do not spend the additional time working on oral feedings or interacting with their infants who are not medically cleared for oral feeds. As such, attainment of oral feeds may be a proxy for developmental outcome and could explain why our findings of subnormal cognitive and motor scores did not persist in the second year of life, when the majority of our infants had attained these goals.
The literature is sparse with respect to the impact of FD on neurodevelopmental outcome in preterm infants [6, 9]. In a prospective study of 52 preterm infants born in 2004–2006, Wolthuis-Stigter et al. [9] examined the relationship between achievement of sucking behaviors at varying PMA on ND outcome at age 2 years. The authors found that 25% (n = 13) of the cohort had abnormal development, and that lack of mature sucking pattern at 44 weeks PMA and inability to sustain mature sucking by 46 weeks PMA were significantly associated with abnormal ND outcome at age 2 years[9]. They noted that immature or aberrant sucking behaviors specifically between 44 and 46 weeks PMA were associated with abnormal ND outcome and thus potentially represented a particularly vulnerable period of development [9]. A significant limitation of our study is the lack of an objective measure of FD, which was a multidisciplinary diagnosis made in conjunction with speech/feeding therapists and the medical team, and documented as such with an outlined treatment plan in the medical record. Although our follow-up rate was high at 8 months CA, we had only 66% of infant’s complete follow-up at age 2 years, thus making our 20-month outcomes less reliable. Finally, it is difficult to compare our studies as we utilized the 3rd Edition of the Bayley Scales of Infant and Toddler Development, which not only has an additional language index but has also been shown to have higher scores than earlier versions [22].
Other studies have examined the relationship between gastrostomy tube placements in the NICU on later ND outcome [6]. Jadcherla et al. [6] studied 194 infants <37 weeks’ GA, who were referred as inpatients in the NICU to a specialized feeding disorders program for their FD. After enrollment in the program, only 40% of infants needed gastrostomy tube placement prior to NICU discharge. The study reported that infants with a G-tube had lower GA, higher rates of BPD and intraventricular hemorrhage, and had lower cognitive, language, and motor scores on the BSITD-III at 18–24 months CA as compared with infants who did not require a G-tube [6]. In multiple regression analyses, G-tube remained an independent predictor of cognitive and motor delay in the second year of life. Although we found an association between FD in the NICU and worse cognitive, language, and motor outcome in the first year of life, these findings did not persist at 20 months CA. We did not, however, examine infants with G-tubes separately due to our small number. These infants would presumably have had more significant FD for a variety of reasons and as the authors acknowledge may already have been at high risk for neurologic impairments. Interestingly, we found that PMA at first oral feed was an independent predictor of worse ND outcome, even after adjusting for neonatal morbidities such as BPD, which may have influenced this milestone. We speculate that this was likely due to need for prolonged CPAP/severity of BPD as well as practice variation in determining a safe clinical status to allow oral feeds.
There are several limitations of our study, namely that it is retrospective in nature and as such, one cannot determine an exact causal relationship between FD and neurodevelopmental outcome. Furthermore, the diagnosis of FD, although made by a multidisciplinary team, was not standardized and there was great heterogeneity with respect to the etiology of FD in the population, ranging from infants with severe BPD, who were on mechanical ventilation and/or had low endurance for oral feeds, to infants with NEC, presumed decreased intestinal motility and feeding intolerance, who had prolonged NPO status. During the study years there was also heterogeneity with respect to management of FD and threshold for G-tube placement. Furthermore, our ND follow-up rate declined from 76% at 8 months CA to 66% 20 months CA and there were significant differences between children who did and did not complete follow-up. Although ELGA infants who completed 20-month follow-up were significantly more likely to have had BPD and neonatal sepsis, they were less likely to have had intrauterine drug exposure, have had severe HUS abnormalities, and were more likely to be on a diet of breastmilk at the time of NICU discharge as compared with infants who did not complete follow-up. Given the association between breast milk and positive ND outcome, it is possible that our findings at 20 months may have underestimated the long-term impact of neonatal FD on early childhood ND outcome [23]. Strengths of our study include the inclusion of all ELGA infants in the NICU rather than just those referred for feeding therapy at the time of discharge and detailed data on hospital morbidities, and feeding milestones on ND outcome at two time points. Although 8-month ND outcome is not typically reported in most outcome studies, early identification of developmental delays with subsequent referral to Early Intervention, private therapies, and parenting recommendations may have impacted our ND outcome favorably in the second year of life.
Conclusion
We have found that recently surviving ELGA infants with FDs in the NICU have significantly worse cognitive and motor outcome in the first year of life, and that PMA at the start of oral feeds is an independent predictor of adverse ND outcome. These findings persist after adjusting for demographic and neonatal risk factors. Early identification of infants at risk for FD may allow for targeted feeding therapies in the NICU, including earlier introduction of oral feeds, to help reduce the risk of adverse ND in the first year of life. There is an urgent need to further study favorable periods of intervention both during and after the NICU hospitalization to help maximize feeding abilities and neurodevelopment in this vulnerable population.
References
Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams 2000 to 2009. Pediatrics. 2012;129:1019–26.
Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376:617–28.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends incare practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–51.
Kutz P, Horsch S, Kühn L, Roll C. Single‐centre vs. population‐based outcome data of extremely preterm infants at the limits of viability. Acta Paediatr. 2009;98:1451–5.
Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and co-morbidities on feeding milestones in neonates: a retrospective study. J Perinatol. 2010;30:201–8.
Jadcherla SR, Khot T, Moore R, Malkar M, Gulati IK, Slaughter JL. Feeding methods at discharge predict long-term feeding and neurodevelopmental outcomes in preterm infants referred for gastrostomy evaluation. J Pediatr. 2017;181:125–30.
Giannì ML, Sannino P, Bezze E, Plevani L, di Cugno N, Roggero P, et al. Effect of co-morbidities on the development of oral feeding ability in pre-term infants: a retrospective study. Sci Rep. 2015;5:16603. Published 2015 Nov 12.
Jadcherla SR. Dysphagia in the high-risk infant: potential factors and mechanisms. Am J Clin Nutr. 2016;103:622S–8S.
Wolthius-Stigter MI, Luinge MR, da Costa SP, Krijnen WP, van der Schans CP, Bos AF. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J Pediatr. 2015;166:26–30.
Rommel N, DeMeyer AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003;37:75–84.
Jadcherla SR, Dail J, Malkar MB, McClead R, Kelleher K, Nelin L. Impact of process optimization and quality improvement measures on neonatal feeding outcomes at an all-referral neonatal intensive care unit. J Parent Enter Nutr. 2015;40:646–55.
Fenton T. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and new format. BMC Pedia. 2003;3:13.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1979;187:1–7.
Bayley N. Bayley Scales of Infant Development. Third edition. San Antonio, TX: The Psychological Corporation; 2006.
Amiel-Tison C, Stewart AL. Follow-up studies in the first five years of life: a pervasive assessment of neurologic function. Arch Dis Child. 1989;64:496–502.
Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotton CM. NICHD Neonatal Research Network. Pediatrics. 2008;121:22–7.
Slaughter JL, Stenger MR, Reagan PB, Jadherla SR. Neonatal histamine-2 receptor antagonist and proton pump inhibitor treatment at United States Children’s Hospitals. J Pediatr. 2016;174:63–70.
Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurol. 2006;48:595–9.
Lau C, Smit EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr. 2003;92:721–7.
Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. J Pedia. 2002;141:230–6.
Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandez S, et al. Impact of personalized feeding program in 100 NICU infants: pathophysiology-based approach for better outcomes. J Pedia Gastroenterol Nutr. 2012;54:62–70.
Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pedia. 2012;161:222–8.
Patra K, Hamilton M, Johnson TJ, Greene M, Dabrowski E, Meier PP, et al. NICU human milk dose and 20-month neurodevelopmental outcome in very low birth weight infants. Neonatology. 2017;112:330–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patra, K., Greene, M.M. Impact of feeding difficulties in the NICU on neurodevelopmental outcomes at 8 and 20 months corrected age in extremely low gestational age infants. J Perinatol 39, 1241–1248 (2019). https://doi.org/10.1038/s41372-019-0428-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0428-4