Abstract
Fear-related disorders are thought to reflect strong and persistent fear memories. The basolateral amygdala (BLA) and the medial prefrontal cortex (mPFC) form strong reciprocal synaptic connections that play a key role in acquisition and extinction of fear memories. While synaptic contacts of BLA cells onto mPFC neurons are likely to play a crucial role in this process, the BLA connects with several additional nuclei within the fear circuit that could relay fear-associated information to the mPFC, and the contribution of direct monosynaptic BLA–mPFC inputs is not yet clear. Here we establish an optogenetic stimulation protocol that induces synaptic depression in BLA–mPFC synapses. In behaving mice, optogenetic high-frequency stimulation of BLA inputs to mPFC interfered with retention of cued associations, attenuated previously acquired cue-associated responses in mPFC neurons and facilitated extinction. Our findings demonstrate the contribution of BLA inputs to mPFC in forming and maintaining cued fear associations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Maren, S. & Quirk, G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 5, 844–852 (2004).
Pape, H.C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Sierra-Mercado, D., Padilla-Coreano, N. & Quirk, G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011).
Vidal-Gonzalez, I., Vidal-Gonzalez, B., Rauch, S.L. & Quirk, G.J. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 13, 728–733 (2006).
Cho, J.H., Deisseroth, K. & Bolshakov, V.Y. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80, 1491–1507 (2013).
Arruda-Carvalho, M. & Clem, R.L. Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding. J. Neurosci. 34, 15601–15609 (2014).
Adhikari, A. et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179–185 (2015).
Do-Monte, F.H., Manzano-Nieves, G., Quiñones-Laracuente, K., Ramos-Medina, L. & Quirk, G.J. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 35, 3607–3615 (2015).
Courtin, J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014).
Felix-Ortiz, A.C., Burgos-Robles, A., Bhagat, N.D., Leppla, C.A. & Tye, K.M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209 (2016).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
Laviolette, S.R., Lipski, W.J. & Grace, A.A. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J. Neurosci. 25, 6066–6075 (2005).
Little, J.P. & Carter, A.G. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J. Neurosci. 33, 15333–15342 (2013).
Senn, V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G.J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012).
Garcia, R., Vouimba, R.M., Baudry, M. & Thompson, R.F. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402, 294–296 (1999).
Klavir, O., Genud-Gabai, R. & Paz, R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron 80, 1290–1300 (2013).
Hoover, W.B. & Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007).
Ray, J.P. & Price, J.L. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J. Comp. Neurol. 323, 167–197 (1992).
Sah, P., Faber, E.S., Lopez De Armentia, M. & Power, J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 83, 803–834 (2003).
Froc, D.J. & Racine, R.J. Interactions between LTP- and LTD-inducing stimulation in the sensorimotor cortex of the awake freely moving rat. J. Neurophysiol. 93, 548–556 (2005).
Maroun, M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur. J. Neurosci. 24, 2917–2922 (2006).
Klavir, O., Genud-Gabai, R. & Paz, R. Low-frequency stimulation depresses the primate anterior-cingulate-cortex and prevents spontaneous recovery of aversive memories. J. Neurosci. 32, 8589–8597 (2012).
Maroun, M. & Richter-Levin, G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J. Neurosci. 23, 4406–4409 (2003).
Histed, M.H., Bonin, V. & Reid, R.C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009).
Stuber, G.D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Spellman, T. et al. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314 (2015).
Raimondo, J.V., Kay, L., Ellender, T.J. & Akerman, C.J. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat. Neurosci. 15, 1102–1104 (2012).
Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
Nabavi, S. et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014).
Creed, M., Pascoli, V.J. & Lüscher, C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347, 659–664 (2015).
Zhang, Y.P. & Oertner, T.G. Optical induction of synaptic plasticity using a light-sensitive channel. Nat. Methods 4, 139–141 (2007).
Hass, C.A. & Glickfeld, L.L. High-fidelity optical excitation of cortico-cortical projections at physiological frequencies. J. Neurophysiol. 116, 2056–2066 (2016).
Bossert, J.M. et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J. Neurosci. 32, 4982–4991 (2012).
Milad, M.R. et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry 62, 1191–1194 (2007).
Fitzgerald, P.J. et al. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol. Learn. Mem. 113, 69–81 (2014).
Choi, D.C. et al. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc. Natl. Acad. Sci. USA 107, 2675–2680 (2010).
Price, J.L. Comparative aspects of amygdala connectivity. Ann. NY Acad. Sci. 985, 50–58 (2003).
Kirkwood, A., Dudek, S.M., Gold, J.T., Aizenman, C.D. & Bear, M.F. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science 260, 1518–1521 (1993).
Pascoli, V., Turiault, M. & Lüscher, C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481, 71–75 (2011).
Jackman, S.L., Beneduce, B.M., Drew, I.R. & Regehr, W.G. Achieving high-frequency optical control of synaptic transmission. J. Neurosci. 34, 7704–7714 (2014).
Herry, C., Vouimba, R.M. & Garcia, R. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J. Neurophysiol. 82, 2827–2832 (1999).
Habib, D. & Dringenberg, H.C. Low-frequency-induced synaptic potentiation: a paradigm shift in the field of memory-related plasticity mechanisms? Hippocampus 20, 29–35 (2010).
Calabresi, P., Pisani, A., Mercuri, N.B. & Bernardi, G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 19, 19–24 (1996).
Gerdeman, G.L., Ronesi, J. & Lovinger, D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 5, 446–451 (2002).
Phillips, R.G. & LeDoux, J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 (1992).
Wang, H. et al. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc. Natl. Acad. Sci. USA 100, 4287–4292 (2003).
Sparta, D.R. et al. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front. Behav. Neurosci. 8, 129 (2014).
Lilley, C.E. et al. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J. Virol. 75, 4343–4356 (2001).
Gunaydin, L.A. et al. Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 (2010).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2011).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2011).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sato, Y. et al. Three-dimensional multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. Med. Image Anal. 2, 143–168 (1998).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Acknowledgements
We thank R. Levy for help with viral vector production and R. Zwang for help with cloning. We thank I. Goshen, Y. Ziv and all Yizhar lab members for discussions and critical reading of the manuscript. This work was supported in part by grants to O.Y. from the Israel Ministry of Science Technology and Space, the Israel Science Foundation (ISF #1351-12), FP7 grants StG #337637 and CIG #321919, and the Human Frontier Science Program. R.P.'s contribution was supported by ISF #26613, Minerva and ERC-FP7-StG 281171. O.Y. is supported by the Gertrude and Philip Nollman Career Development Chair. M.P. was supported by a Minerva postdoctoral fellowship. Work in the Yizhar lab is supported by the Adelis Foundation, the Grodetsky Center for Higher Brain Functions, Jean-Charles Schwartz and Marc-Antoine Schwartz, the Appleton Family Trust and the Lord Sieff Brimpton Memorial Fund.
Author information
Authors and Affiliations
Contributions
O.K., M.P. and O.Y. designed and planned the experiments. O.K. carried out in vivo electrophysiology and behavioral experiments. M.P. conducted the in vitro electrophysiology and anatomical tracing experiments. A.S. helped with behavioral experiments. R.P. contributed to ideas and discussions. O.K., M.P. and O.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Quantification of spontaneous EPSPs and paired-pulse parameters
(a) Representative voltage responses to current injections (left) and light pulses of varying intensities (right) in a mPFC L2 pyramidal neuron from a mouse injected with rAAV5-CaMKIIα-ChETATC-eYFP in the ipsilateral BLA. (b) Average light dose-response curves of oEPSPs evoked in regular-spiking (blue) and fast-spiking (red) neurons by 445 nm light pulses of increasing irradiance. (c) Spontaneous EPSP rates before and after optical stimulation protocols (control, N = 10 cells; short oHFS, N = 11 cells, long oHFS, N = 19; individual points represent single recordings. Averages are shown in black, green and blue for control, short and long oHFS, respectively (P = 0.74 t(9) =0.4234, P = 0.85 t(10) = 1.9234 and P = 0.42 t(18) = 0.1795), respectively; paired two-tailed t-test). (d) Amplitude of first (left) and second (right) oEPSCs during the paired-pulse protocol, taken before and after oHFS (left and middle). Gray lines represents average amplitude of 60 successfully evoked oEPSCs in the individual cells before and after oHFS; red lines depict average over cells (N = 7 cells; oEPSC1: P = 0.0037, oEPSC2: P = 0.035; Paired two-tailed t-test). Paired Pulse Ratio is computed from 60 trials in which both light pulses successfully evoked an oEPSC (right; P = 0.43, unpaired two-tailed t-test). (e) Failure rates, calculated as the percentage of 60 consecutive traces in which no oEPSC was evoked within a 10 ms search window after light pulse onset (P = 0.03 and P = 0.12, respectively; unpaired one-tailed t-test). Error bars represent s.e.m; * P < 0.05.
Supplementary Figure 2 High-frequency optogenetic stimulation using ChETATC leads to rapid adaptation of synaptic release in BLA–mPFC terminals.
(a) Representative whole cell recordings of a layer 2/3 pyramidal neuron in mPFC acute slices during application of a single test light pulse (top) and 100 Hz, 3 ms light pulse train for 9 s (long oHFS, bottom). Traces indicate that synaptic release rapidly adapts during oHFS, indicating that depletion is unlikely to be a major cause of oHFS-induced synaptic depression. (b) Individual recording traces (gray) and across-cell average (red) showing baseline-corrected Vm responses during oHFS (N = 10 cells), demonstrating similar post-synaptic response to ChETATC-mediated stimulation of BLA-mPFC terminals using a single 3 ms light pulse stimulation (left) and a long oHFS protocol (right; 900 3 ms pulses delivered at 100 Hz).
Supplementary Figure 3 Optrode placement during recording experiments.
(a) Representative slice depicting electrode placement in the mPFC (scale bar: 500 μm). (b) Schematic representation of optrode placement for the fear-conditioning and recording experiment described in figure 3 (scale bar: 100 μm). (c) Schematic representation of the final positions of optrode drives following recording experiments with movable optrode. Electrode locations were estimated based on a dorso-ventral distance of 200-500 μm from the tip of the optical fiber.
Supplementary Figure 4 mPFC unit firing rates during oHFS trains and recovery of evoked responses.
(a) Within-train activity: average FR during a 9-second baseline period, followed by a 9-second oHFS train and a subsequent no-light period. PSTH (Top) and raster plot (Center) depicting the spiking of all 57 responsive units before, during and after the first of 15 oHFS trains. (Bottom) Average FR of the responsive units before, during and after the first oHFS train (ANOVA F(2,54)=2.531, P = 0.084; post-hoc tests: P = 0.553 and P = 0.068, respectively.). Error bars represent s.e.m. (b) Histograms depicting change in evoked FR for each responsive unit, at each of the measured time points, from T0 (top) to T90 (bottom). Change in evoked FR is measured for each unit against the evoked FR prior to oHFS. Binomial tests: T0, 0.000002; T30, 0.00025; T60, 0.003; T90, 0.124; corrected α=0.0125. (c) Left: Scatter plot depicting the correlation between the dorso-ventral (D/V) location of the recording electrode (mm ventral from bregma) and the oHFS-induced change in evoked response (N = 57; R = 0.0029; P = 0.9836). The change is calculated as (FR(T0)-FR(Tpre))/(FR(T0)+FR(Tpre)). Right: Mean change in light-evoked spiking in units within the PL (D/V < 2.6 mm) and IL (D/V > 2.6 mm) regions (N(PL) = 24; N(IL) = 33; T(52) = -0.8433; P = 0.4029). Error bars in all panels indicate s.e.m.
Supplementary Figure 5 oHFS effects do not back-propagate from mPFC to the BLA.
(a) Schematic representation of experimental setup. Mice were injected unilaterally in the BLA with rAAV5-CaMKIIα-ChETATC-eYFP and implanted above the ipsilateral PL with an optical fiber. Eight weeks later, mice were anesthetized and an electrode array was lowered into the BLA. (b) Raster plots overlaid with PSTHs showing the spiking of representative light-responsive BLA units (n = 48 light responsive units, out of 112 BLA units recorded), before and immediately after oHFS. Test pulses were delivered to the mPFC at 5 Hz. Top: Representative short-latency response, showing low jitter and minimal oHFS-induced attenuation. Bottom: Representative unit with longer latency response, showing larger jitter and stronger oHFS-induced attenuation. (c) Within-train activity: average PSTH of FR of all light-responsive units (n = 48) during the first 9-second oHFS train (bin size: 10 ms). (d) Bar plot depicting the mean FR of all responsive units before, during and after the first of the three oHFS trains. (e) Average PSTHs for all 48 BLA units that showed responses to PFC light stimulation. Top: Average PSTH for responses to 5 Hz light stimuli (triggered on light pulse onset, 300 individual light pulses); Bottom: Average PSTH for responses of the same 48 units to 100 Hz oHFS light stimuli (triggered on light pulse onset, 300 first light pulses out of 900 during the first oHFS train). (f) A histogram presenting response latencies of all responses. (g) oHFS-induced attenuation in evoked response plotted against the response latency for individual BLA units (N = 48). Population data (gray circles) overlaid with mean and s.e.m. (black). Shading or error bars in all panels indicate s.e.m.
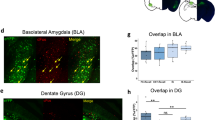
Supplementary Figure 6 mPFC-projecting BLA cells predominantly target the mPFC.
(a) Representative CLARITY image from a mouse injected bilaterally with rAAV5-CaMKIIα-eYFP in the BLA. Scale bar, 1 mm. (b) Top: Representative confocal images of coronal slices from a mouse injected in the BLA with rAAV5- CaMKIIα-eYFP. Slices are taken from anteroposterior positions -1.8, +2.0, +1.5 and -1.2 (from left to right; distance in mm from bregma). Overlays indicate anatomical landmarks based on the mouse stereotaxic atlas. Bottom: Representative confocal images of coronal slices from a mouse injected with HSV-EF1α-mCherry-IRES-Cre into the mPFC and rAAV5-EF1α-DIO-eYFP into the BLA. Anatomical locations of slices are as indicated above. Scale bars: mPFC, 100 μm; all others 500 μm. (c) Quantification of eYFP-labeled BLA afferents in mice injected with CaMKIIα-eYFP (blue, N = 5 mice) and HSV-Cre/DIO-eYFP (red, N = 6 mice). Fluorescence in each region was normalized to the average fluorescence of BLA axons in all measured slices. Two-way ANOVA revealed a significant experiment X region interaction (F(8,112)=2.47 p<0.05). A post hoc test showed that in HSV-Cre/DIO-eYFP mice the only significant differences were between the mPFC and all other measured regions (all p<0.01), while in the CaMKIIα-eYFP controls, mPFC fluorescence differed significantly only from three regions. Abbreviations: amPFC: anterior mPFC; pmPFC: posterior mPFC; LO: lateral orbitofrontal cortex; AcbSh: nucleus accumbens shell; MDL: mediodorsal thalamus. LSI: Lateral Septal Nucleus. Hbn: Habenula. SNC: Substantia Nigra. PV: Paraventricular thalamus. Error bars in all panels indicate s.e.m; * p < 0.05.
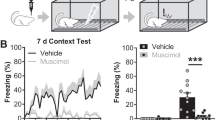
Supplementary Figure 7 Unilateral BLA–PL synaptic depression has no behavioral effect during extinction of cued fear.
Behavioral data are collected from mice in which electrophysiological recordings were performed for Figure 3. Bottom: Schematic representation of experimental setup. Recordings were performed 24 hours following cued fear acquisition. The baseline period consisted of 5 CS presentations. oHFS trains were interleaved with 15 subsequent CS presentations. Left: Both groups no oHFS (N = 5) and oHFS (N = 5) pooled to show freezing extinction main effect. Center: Unilateral BLA-PL synaptic depression during fear extinction (binned to four 5-tone bins). Right: Bar plot showing freezing (%) to the tone in both groups during extinction. Shading or error bars in all panels indicate s.e.m.
Supplementary Figure 8 Effects of BLA–PL short oHFS on conditioning and extinction of cued fear.
Bottom: Behavioral paradigm. Short-oHFS was immediately followed by cued fear conditioning. Extinction training was performed the next day in context B and an extinction-retrieval test in context B was performed the next day. Far-Left: Freezing (%) of control (black; N = 10) and ChETATC (blue; N = 8) mice measured during acquisition. Left: Freezing (%) of the two groups during extinction training shows extinction of freezing to the tone. Center: Freezing (%) of the two groups during early (first 10 trials) and late (last 10 trials) extinction training (Two-way ANOVA group X time bin interaction F(1,16)=6.6238 p<0.05, post hocs p=0.35, p<0.001 respectively). Right: Freezing (%) of the two groups during an extinction-retrieval test, 24 hours following extinction training. Far-right: Freezing (%) of the two groups during early (first 8 trials) and late (last 7 trials) extinction-retrieval (Two-way ANOVA main effect for group F(1,16)=2.9748, p=0.1038; group X time bin interaction F(1,16)=0.2748 p=0.6073 both N.S). Shading or error bars in all panels indicate s.e.m; * p < 0.05.
Supplementary Figure 9 Contextual fear memories acquired with cue and general anxiety are not affected by oHFS of BLA–mPFC projections.
(a) Freezing to the context after CS extinction - Bottom: Behavioral paradigm. oHFS was immediately followed by cued fear conditioning. Extinction training in context B was conducted on the next day. Seven days later, mice were reintroduced to the fear conditioning context (context A). Left: Freezing (%) during acquisition shows that mice in both groups increased freezing from first to last trials. Right: Following extinction of cued fear, freezing (%) of the two groups to the fear-conditioned context shows no difference between the groups. (b) No effect of BLA-PL synaptic depression on contextual fear response. Left: Schematic representation of surgical setup. Mice were injected bilaterally in the BLA with rAAV5-CaMKIIα-ChETATC-eYFP and implanted above the PL with an optic fiber. Bottom: Behavioral paradigm; oHFS of BLA-PL axons was immediately followed by contextual fear conditioning. Freezing was measured on the following day in the same context. Center: Freezing (%) of ChETATC (blue, N = 10) and control mice (black, N = 9) during acquisition (ANOVA revealed main effect of trial F(5,85)=20.13 P < 0.0001, both group effect and group × trial interaction N.S.). Right: Freezing responses of both groups to the fear-conditioning context were similar on the following day (t(17) = 1.34, p=0.199 N.S). (c) Bottom: Behavioral paradigm; oHFS was followed by an open field test. Left: Locomotion velocity of mice that underwent oHFS of BLA-PL projections (N = 10) and control mice (N = 10; t(18) = 0.064; P = 0.949). Right: Mean % time in center is not altered in the same two groups of mice (t(18) = 0.718; P = 0.4918). Error bars represent s.e.m. (d) Bottom: Behavioral paradigm. oHFS was followed by elevated plus maze test. Left: Control (N = 9) and ChETATC (N = 10) mice showed similar preference to the closed arms. A two-way ANOVA with stimulation and zone as factors yielded a main effect for zone (F(2,34)=12.92 P < 0.0001). Post hoc analysis showed no effect of stimulation (F(1,17) = 0.038 P = 0.16). Right: Mean velocity in the elevated plus maze was similar in control and ChETATC mice (t(17) = -1.581; P = 0.132). Error bars in all panels indicate s.e.m.
Supplementary Figure 10 Fiber-placement schemes for mice in all behavioral experiments.
Attenuation of blue light from the average location of the fiber (top right, based on Stujenske et al. 2015, Cell Rep. Jul 21;12(3):525-34). Line plot depicts the light irradiance profile through a vertical projection below the fiber center. Anatomical diagrams depict the fiber-placement schemes for each of the behavioral experiments conducted in this study.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 2415 kb)
Rights and permissions
About this article
Cite this article
Klavir, O., Prigge, M., Sarel, A. et al. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci 20, 836–844 (2017). https://doi.org/10.1038/nn.4523
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4523
This article is cited by
-
Pre-scan state anxiety is associated with greater right amygdala-hippocampal response to fearful versus happy faces among trait-anxious Latina girls
BMC Psychiatry (2024)
-
Prefrontal control of superior colliculus modulates innate escape behavior following adversity
Nature Communications (2024)
-
Memory consolidation drives the enhancement of remote cocaine memory via prefrontal circuit
Molecular Psychiatry (2024)
-
Activity-dependent organization of prefrontal hub-networks for associative learning and signal transformation
Nature Communications (2023)
-
Impaired learning, memory, and extinction in posttraumatic stress disorder: translational meta-analysis of clinical and preclinical studies
Translational Psychiatry (2023)