Abstract
Background/Objectives:
Maternal obesity increases risk for childhood obesity, but molecular mechanisms are not well understood. We hypothesized that primary umbilical vein endothelial cells (HUVEC) from infants of overweight and obese mothers would harbor transcriptional patterns reflecting offspring obesity risk.
Subjects/Methods:
In this observational cohort study, we recruited 13 lean (pre-pregnancy body mass index (BMI) <25.0 kg m−2) and 24 overweight-obese (‘ov-ob’, BMI⩾25.0 kg m−2) women. We isolated primary HUVEC, and analyzed both gene expression (Primeview, Affymetrix) and cord blood levels of hormones and adipokines.
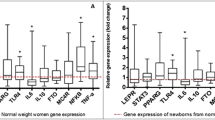
Results:
A total of 142 transcripts were differentially expressed in HUVEC from infants of overweight-obese mothers (false discovery rate, FDR<0.05). Pathway analysis revealed that genes involved in mitochondrial and lipid metabolism were negatively correlated with maternal BMI (FDR<0.05). To test whether these transcriptomic patterns were associated with distinct nutrient exposures in the setting of maternal obesity, we analyzed the cord blood lipidome and noted significant increases in the levels of total free fatty acids (lean: 95.5±37.1 μg ml−1, ov-ob: 124.1±46.0 μg ml−1, P=0.049), palmitate (lean: 34.5±12.7 μg ml−1, ov-ob: 46.3±18.4 μg ml−1, P=0.03) and stearate (lean: 20.8±8.2 μg ml−1, ov-ob: 29.7±17.2 μg ml−1, P=0.04), in infants of overweight-obese mothers.
Conclusions:
Prenatal exposure to maternal obesity alters HUVEC expression of genes involved in mitochondrial and lipid metabolism, potentially reflecting developmentally programmed differences in oxidative and lipid metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gluckman PD, Hanson MA, Cooper C, Thornburg KL . Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008; 359: 61–73.
Woo M, Patti ME . Diabetes risk begins in utero. Cell Metab 2008; 8: 5–7.
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS . Fetal nutrition and cardiovascular disease in adult life. Lancet 1993; 341: 938–941.
Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B . Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect 2013; 121: 359–366.
Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X . Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 2013; 8: e61627.
Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006; 118: e1644–e1649.
Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC . Differential methylation in glucoregulatory genes of offspring born before vs after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci USA 2013; 110: 11439–11444.
Catalano PM, Ehrenberg HM . The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006; 113: 1126–1133.
Isganaitis E, Woo M, Ma H, Chen M, Kong W, Lytras A et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 2014; 63: 688–700.
Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI et al. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 2014; 63: 2702–2713.
Koskinen A, Lehtoranta L, Laiho A, Laine J, Kaapa P, Soukka H . Maternal diabetes induces changes in the umbilical cord gene expression. Placenta 2015; 36: 767–774.
Lewis LJ, Hoak JC, Maca RD, Fry GL . Replication of human endothelial cells in culture. Science 1973; 181: 453–454.
Westermeier F, Salomon C, Gonzalez M, Puebla C, Guzman-Gutierrez E, Cifuentes F et al. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes 2011; 60: 1677–1687.
Giri H, Chandel S, Dwarakanath LS, Sreekumar S, Dixit M . Increased endothelial inflammation, sTie-2 and arginase activity in umbilical cords obtained from gestational diabetic mothers. PLoS One 2013; 8: e84546.
Floris I, Descamps B, Vardeu A, Mitic T, Posadino AM, Shantikumar S et al. Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microRNA-101 and histone methyltransferase enhancer of zester homolog-2. Arterioscler Thromb Vasc Biol 2015; 35: 664–674.
Casanello P, Krause B, Torres E, Gallardo V, Gonzalez M, Prieto C et al. Reduced L-arginine transport and nitric oxide synthesis in human umbilical vein endothelial cells from intrauterine growth restriction pregnancies is not further altered by hypoxia. Placenta 2009; 30: 625–633.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS . New intrauterine growth curves based on United States data. Pediatrics 2010; 125: e214–e224.
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP . Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 2003; 31: e15.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267–273.
Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 2007; 35 (Web Server issue): W169–W175.
Folch J, Lees M, Sloane Stanley GH . A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509.
Morrison WR, Smith LM . Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—methanol. J Lipid Res 1964; 5: 600–608.
Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD . Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler Thromb Vasc Biol 1998; 18: 1818–1827.
Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ et al. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 2002; 43: 1899–1907.
Rasmussen KM, Yaktine AL Institute of Medicine (U.S.) Committee to Reexamine IOM Pregnancy Weight Guidelines Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press: Washington, DC, USA, 2009.
Boschmann M, Engeli S, Moro C, Luedtke A, Adams F, Gorzelniak K et al. LMNA mutations, skeletal muscle lipid metabolism, and insulin resistance. J Clin Endocrinol Metab 2010; 95: 1634–1643.
Sukonina V, Lookene A, Olivecrona T, Olivecrona G . Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA 2006; 103: 17450–17455.
Lappas M . Effect of pre-existing maternal obesity, gestational diabetes and adipokines on the expression of genes involved in lipid metabolism in adipose tissue. Metabolism 2014; 63: 250–262.
Austin MA, Hutter CM, Zimmern RL, Humphries SE . Familial hypercholesterolemia and coronary heart disease: a HuGE association review. Am J Epidemiol 2004; 160: 421–429.
Zobel DP, Andreasen CH, Burgdorf KS, Andersson EA, Sandbaek A, Lauritzen T et al. Variation in the gene encoding Kruppel-like factor 7 influences body fat: studies of 14 818 Danes. Eur J Endocrinol 2009; 160: 603–609.
Aulchenko YS, Pullen J, Kloosterman WP, Yazdanpanah M, Hofman A, Vaessen N et al. LPIN2 is associated with type 2 diabetes, glucose metabolism, and body composition. Diabetes 2007; 56: 3020–3026.
Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000; 26: 76–80.
Liu X, Chen Q, Tsai HJ, Wang G, Hong X, Zhou Y et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 2014; 55: 223–230.
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003; 100: 8466–8471.
Peterside IE, Selak MA, Simmons RA . Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab 2003; 285: E1258–E1266.
Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015; 142: 681–691.
Lassance L, Haghiac M, Minium J, Catalano P, Hauguel-de Mouzon S . Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J Clin Endocrinol Metab 2015; 100: E11–E18.
Mele J, Muralimanoharan S, Maloyan A, Myatt L . Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab 2014; 307: E419–E425.
Ambra R, Manca S, Palumbo MC, Leoni G, Natarelli L, De Marco A et al. Transcriptome analysis of human primary endothelial cells (HUVEC) from umbilical cords of gestational diabetic mothers reveals candidate sites for an epigenetic modulation of specific gene expression. Genomics 2014; 103: 337–348.
Di Camillo B, Sanavia T, Iori E, Bronte V, Roncaglia E, Maran A et al. The transcriptional response in human umbilical vein endothelial cells exposed to insulin: a dynamic gene expression approach. PLoS One 2010; 5: e14390.
Thakali KM, Saben J, Faske JB, Lindsey F, Gomez-Acevedo H, Lowery CL Jr et al. Maternal pregravid obesity changes gene expression profiles toward greater inflammation and reduced insulin sensitivity in umbilical cord. Pediatr Res 2014; 76: 202–210.
Ozanne SE, Dorling MW, Wang CL, Nave BT . Impaired PI 3-kinase activation in adipocytes from early growth-restricted male rats. Am J Physiol Endocrinol Metab 2001; 280: E534–E539.
Ozanne SE, Nave BT, Wang CL, Shepherd PR, Prins J, Smith GD . Poor fetal nutrition causes long-term changes in expression of insulin signaling components in adipocytes. Am J Physiol 1997; 273 (1 Pt 1): E46–E51.
Shelley P, Martin-Gronert MS, Rowlerson A, Poston L, Heales SJ, Hargreaves IP et al. Altered skeletal muscle insulin signaling and mitochondrial complex II-III linked activity in adult offspring of obese mice. Am J Physiol Regul Integr Comp Physiol 2009; 297: R675–R681.
Yee JK, Mao CS, Ross MG, Lee WN, Desai M, Toda A et al. High oleic/stearic fatty-acid desaturation index in cord plasma from infants of mothers with gestational diabetes. J Perinatol 2014; 34: 357–363.
Fekete K, Gyorei E, Lohner S, Verduci E, Agostoni C, Decsi T . Long-chain polyunsaturated fatty acid status in obesity: a systematic review and meta-analysis. Obes Rev 2015; 16: 488–497.
de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: the MEFAB cohort. Prostaglandins Leukot Essent Fatty Acids 2014; 91: 81–85.
Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T et al. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. J Atheroscler Thromb 2009; 16: 480–489.
Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 2015; 520: 192–197.
Scifres CM, Catov JM, Simhan HN . The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity (Silver Spring) 2014; 22: 932–938.
Olmos PR, Rigotti A, Busso D, Berkowitz L, Santos JL, Borzone GR et al. Maternal hypertriglyceridemia: a link between maternal overweight-obesity and macrosomia in gestational diabetes. Obesity (Silver Spring) 2014; 22: 2156–2163.
Acknowledgements
We are thankful for our research support. SMRC was supported by a Fullbright Fellowship. EI was supported by NICHD K99/R00 Award HD064793. JMD and the Joslin Bioinformatics Core are supported by a Diabetes Research Center (DRC) grant (DK036836). GAPdS was supported by a grant from the National Council of Technological and Scientific Development of Brazil (CNPq 472031/2010). We are grateful for the services of the Vanderbilt Lipid Core.
Author contributions
SMRC designed the study, collected/analyzed the data and reviewed the manuscript; SMRC is the guarantor of the clinical data. EI analyzed the data and wrote the manuscript; EI is the guarantor of transcriptomics and lipidomics analyses. TJM, KH, GD and JMD assisted with the data analysis. GAPdS supervised the clinical study design. M-EP supervised the data analysis and reviewed/edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Costa, S., Isganaitis, E., Matthews, T. et al. Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int J Obes 40, 1627–1634 (2016). https://doi.org/10.1038/ijo.2016.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.142
This article is cited by
-
Developmental Programming of Body Composition: Update on Evidence and Mechanisms
Current Diabetes Reports (2019)
-
Mitochondrial inefficiency in infants born to overweight African-American mothers
International Journal of Obesity (2018)