Featured
-
-
Article
| Open Access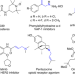 Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
The oxidative intermolecular amination of C-H bonds represents a straightforward method to construct aliphatic allylic amines. However, the utilization of widely available internal alkenes remains a synthetic challenge. Here, the authors present a regioselective Cu-catalyzed oxidative allylic C-H amination of internal olefins with azodiformates.
- Le Wang
- , Cheng-Long Wang
- & Shu-Yu Zhang
-
Article
| Open Access Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Enhancing the sp3-hybridized character of molecular structures in drug discovery is paramount. Here, the authors introduce a deoxygenative cross-electrophile coupling technique that pairs easily accessible carboxylic acid-derived redox-active esters with aldehyde sulfonyl hydrazones, employing Eosin Y as an organophotocatalyst under visible light irradiation.
- Stefano Bonciolini
- , Antonio Pulcinella
- & Timothy Noël
-
Article
| Open Access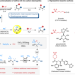 Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Stoichiometric amounts of metal reductant and the requirement of a directing group limit the application of transition metal-catalyzed reductive difunctionalization of alkenes with alkyl halides. Here, the authors report a reductive difunctionalization of alkenes via a radical-anion relay with Na2S2O4 as both reductant and sulfone-source.
- Xiaoya Hou
- , Hongchi Liu
- & Hanmin Huang
-
Review Article
| Open Access Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
α,α-Disubstituted α-amino acids (α-AAs) have improved properties compared to other amino acids. Here, the authors provide an overview of the recent advancements since 2015 and discuss existing challenges for their synthesis.
- Yu Zhang
- , Jaro Vanderghinste
- & Shoubhik Das
-
Article
| Open Access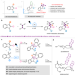 Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Conformationally rigid 3D molecules are of high interest. Here the authors develop intermolecular aza-Paternò-Büchi reactions to access ladder-shape azetidine-fused indoline pentacycles with excellent regiodivergency and stereoselectivity.
- Jianjian Huang
- , Tai-Ping Zhou
- & Fangrui Zhong
-
Article
| Open Access Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Photoexcitation of the electron-donor-acceptor (EDA) complexes are an effective approach to achieve radicals by triggering electron transfer but the catalytic version of EDA complex photoactivation remains underdeveloped. Here, the authors introduce 4-nitrophthalonitrile as an electron acceptor to facilitate π-stacking with electron-rich aromatics to form EDA complexes.
- Ting Xue
- , Cheng Ma
- & Rong Zeng
-
Article
| Open Access Cross-catenation between position-isomeric metallacages
Cross-catenation between position-isomeric metallacages
The study of cross-catenated metallacages could provide facile insights into achieving more precise control over low-symmetry/high-complexity hierarchical assembly systems but is currently lacking. Here, the authors report a cross-catenane formed between two position-isomeric Pt(II) metallacages in the solid state.
- Yiliang Wang
- , Taotao Liu
- & Jun Li
-
Article
| Open Access Water-stable boroxine structure with dynamic covalent bonds
Water-stable boroxine structure with dynamic covalent bonds
Despite the structural significance of boroxines in different classes of materials, their applicability in aqueous media is limited by their hydrolytic instability. Here, the authors discovered a water-stable boroxine structure with excellent pH stability and water-compatible dynamic covalent bonds.
- Xiaopei Li
- , Yongjie Zhang
- & Guangyan Qing
-
Article
| Open Access Carbene organic catalytic planar enantioselective macrolactonization
Carbene organic catalytic planar enantioselective macrolactonization
Macrolactones exhibit distinct conformational and configurational properties and are widely found in natural products but catalysts to govern both macrolactone formation and stereochemical control remain largely unexplored. Here, the authors disclose an N-heterocyclic carbene (NHC)-catalyzed enantioselective synthesis of chiral macrolactones varying in ring size from sixteen to twenty members that feature distinct configurationally stable planar stereogenicity.
- Xiaokang Lv
- , Fen Su
- & Yonggui Robin Chi
-
Article
| Open Access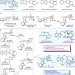 Collective total synthesis of stereoisomeric yohimbine alkaloids
Collective total synthesis of stereoisomeric yohimbine alkaloids
Nature generates the stereoisomeric yohimbine alkaloids using bioavailable monoterpene secolaganin as the building block. Here, the authors reset the stage by the development of a bioinspired coupling, in which the pentacyclic skeleton is constructed through enantioselective kinetic resolution of an achiral, easily accessible synthetic surrogate.
- Meiyi Tang
- , Haigen Lu
- & Liansuo Zu
-
Article
| Open Access Location-agnostic site-specific protein bioconjugation via Baylis Hillman adducts
Location-agnostic site-specific protein bioconjugation via Baylis Hillman adducts
Proteins labelled site-specifically with small molecules are valuable assets for chemical biology and drug development. Here, the authors report Baylis Hillman orchestrated protein aminothiol labelling (BHoPAL), a bioconjugation strategy for specific labelling of the 1,2-aminothiol moiety and combine it with a lipoic acid ligase-based technology to achieve labelling at any desired site within proteins.
- Mudassir H. Mir
- , Sangeeta Parmar
- & Dimpy Kalia
-
Article
| Open Access Mechanochemical synthesis of organoselenium compounds
Mechanochemical synthesis of organoselenium compounds
While the reported methods for the synthesis of organoselenium compounds mainly employ preformed activated selenium reagents, the direct utilization of elemental selenium in the synthesis is a more attractive but challenging strategy. Here, the authors report the rapid synthesis of versatile organoselenium compounds via mechanical stimulation.
- Shanshan Chen
- , Chunying Fan
- & Xiaofeng Wei
-
Article
| Open Access C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
Sulfur fluoride exchange (SuFEx), a new type of linkage reaction, has great potential for application in functional molecule linkage to prepare pharmaceuticals, biomolecules, and polymers. Herein, the authors report the synthesis of a range of sulfoximines through SuFEx reaction between sulfonimidoyl fluorides and aryl/alkyl organotrifluoroborates.
- Suqin Zhao
- , Daming Zeng
- & Xuefeng Jiang
-
Article
| Open Access Enantioselective synthesis of [4]helicenes by organocatalyzed intermolecular C-H amination
Enantioselective synthesis of [4]helicenes by organocatalyzed intermolecular C-H amination
Current methods to construct helical chiral molecules for asymmetric catalysis almost entirely rely on catalytic enantiocontrolled fused-ring system extension. Herein, the authors report a direct terminal peri-functionalization strategy, which allows for efficient assembling of substituted carbohelicenes via an organocatalyzed enantioselective amination reaction.
- Xihong Liu
- , Boyan Zhu
- & Rui Wang
-
Article
| Open Access Total syntheses of Tetrodotoxin and 9-epiTetrodotoxin
Total syntheses of Tetrodotoxin and 9-epiTetrodotoxin
Tetrodotoxin and congeners are specific voltage-gated sodium channel blockers that exhibit remarkable anesthetic and analgesic effects but total synthesis procedures are often limited by the scale. Here, the authors present a scalable asymmetric syntheses of Tetrodotoxin and 9-epiTetrodotoxin from the abundant chemical feedstock furfuryl alcohol.
- Peihao Chen
- , Jing Wang
- & Xiangbing Qi
-
Article
| Open Access Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Electrophotocatalytic hydrogenation of imines and reductive functionalization of aryl halides
Open-shell catalytically active species are widely used in energy-consuming redox reactions, but their excited-state lifetimes are usually short. Here, the authors report a closed-shell thioxanthone-hydrogen anion species generated under electrochemical conditions, which can be photochemically converted to a potent and long-lived reductant.
- Wen-Jie Kang
- , Yanbin Zhang
- & Hao Guo
-
Article
| Open Access Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation
Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation
Direct and site-selective C–H functionalization of alkenes under environmentally benign conditions represents a useful and attractive yet challenging transformation to access value-added molecules. Here, the authors report a protocol for a variety of intermolecular Heck-type functionalization of C(sp2)–H bond of alkenes by thianthrenation.
- Ming-Shang Liu
- , Hai-Wu Du
- & Wei Shu
-
Article
| Open Access Synthesis of inter-[60]fullerene conjugates with inherent chirality
Synthesis of inter-[60]fullerene conjugates with inherent chirality
Inter-fullerene conjugates are non-naturally occurring carbon allotropes. Here the authors report on the chemical synthesis and solid-state structure of three inter-[60]fullerene hybrids with inherent chirality.
- Yoshifumi Hashikawa
- , Shu Okamoto
- & Yasujiro Murata
-
Article
| Open Access Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles
Organocatalytic diastereo- and atroposelective construction of N–N axially chiral pyrroles and indoles
N–N axially chiral motifs are of synthetic interest due to their presence in natural products, pharmaceuticals, and chiral ligands. Here, the authors develop a direct catalytic synthesis of N–N atropisomers with simultaneous creation of contiguous axial and central chirality by oxidative NHC catalyzed (3 + 3) cycloaddition.
- Shao-Jie Wang
- , Xia Wang
- & Shenci Lu
-
Article
| Open Access Regio- and enantioselective synthesis of acyclic quaternary carbons via organocatalytic addition of organoborates to (Z)-Enediketones
Regio- and enantioselective synthesis of acyclic quaternary carbons via organocatalytic addition of organoborates to (Z)-Enediketones
All-carbon quaternary stereocenters are an important synthetic motif but are especially difficult to synthesize enantioselectively. Here, the authors demonstrate the organocatalytic regio- and enantioselective synthesis of valuable acyclic 1,4-dicarbonyl products with vinylated and arylated quaternary centers.
- Po-Kai Peng
- , Andrew Isho
- & Jeremy A. May
-
Article
| Open Access Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Amide C–N bonds activation by A new variant of bifunctional N-heterocyclic carbene
Developing efficient methods for the formation and cleavage of amide species is a primary research goal, but the amide C–N bond cleavage is exceptionally challenging. Here, the authors report the development of an organocatalyst that can effectively catalyze the atroposelective ring-opening of biaryl lactams via amide C–N bond cleavage.
- Yuxing Cai
- , Yuxin Zhao
- & Yong Huang
-
Article
| Open Access C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
C−F bond activation enables synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres
Bioisosteric design has become an essential approach during drug molecule discovery process. Here, the authors describe a strategy for defluorinative couplings of propellanes, enabling the synthesis of aryl difluoromethyl bicyclopentanes as benzophenone-type bioisosteres.
- Mingshuo Chen
- , Yuang Cui
- & Xiaheng Zhang
-
Article
| Open Access Unlocking regioselective meta-alkylation with epoxides and oxetanes via dynamic kinetic catalyst control
Unlocking regioselective meta-alkylation with epoxides and oxetanes via dynamic kinetic catalyst control
Regioselective arene C−H bond alkylation is a powerful tool in synthetic chemistry, yet subject to many challenges. Here, the authors report the meta-C−H bond alkylation of aromatics bearing N-directing groups using (hetero)aromatic epoxides as alkylating agents.
- Peng-Bo Bai
- , Alastair Durie
- & Igor Larrosa
-
Article
| Open Access Atomic-resolution structure analysis inside an adaptable porous framework
Atomic-resolution structure analysis inside an adaptable porous framework
Single crystal X-ray diffraction is one of the most powerful structure elucidation tools, but it’s challenging to determine complex structures. Here the authors report a metal-organic framework for encapsulation and immobilization of various guests using highly ordered internal water network, obtaining high quality atomic-resolution data.
- Yuki Wada
- , Pavel M. Usov
- & Masaki Kawano
-
Article
| Open Access Modular access to chiral bridged piperidine-γ-butyrolactones via catalytic asymmetric allylation/aza-Prins cyclization/lactonization sequences
Modular access to chiral bridged piperidine-γ-butyrolactones via catalytic asymmetric allylation/aza-Prins cyclization/lactonization sequences
N/O-Heterocycles are widely spread in natural products and drug candidates. Here, the authors report a modular access to bridged piperidine-γ-butyrolactones via Cu/Ir-catalyzed asymmetric allyation and aza-Prins cyclization/lactonization sequences.
- Cong Fu
- , Ling He
- & Chun-Jiang Wang
-
Article
| Open Access Atroposelective hydroarylation of biaryl phosphines directed by phosphorus centres
Atroposelective hydroarylation of biaryl phosphines directed by phosphorus centres
Directing group strategies have been used extensively in C–H activation reactions but current methods rely heavily on coordination with nitrogen or oxygen atoms in molecules and have therefore been found to exhibit limited generality in asymmetric syntheses. Here, the authors report enantioselective C–H activation with unsaturated hydrocarbons directed by phosphorus centres to rapidly construct libraries of axially chiral phosphines through dynamic kinetic resolution.
- Zexian Li
- , Minyan Wang
- & Zhuangzhi Shi
-
Article
| Open Access C5 methylation confers accessibility, stability and selectivity to picrotoxinin
C5 methylation confers accessibility, stability and selectivity to picrotoxinin
Minor changes to complex structures can exert major influences on synthesis strategy and functional properties but synthetic difficulties can obstruct the exploration of natural product function. Here the authors explore two parallel series of picrotoxinin analogs and identify leads with selectivity between mammalian and insect ion channels.
- Guanghu Tong
- , Samantha Griffin
- & Ryan A. Shenvi
-
Article
| Open Access Photoinduced copper-catalyzed C–N coupling with trifluoromethylated arenes
Photoinduced copper-catalyzed C–N coupling with trifluoromethylated arenes
Selective defluorinative functionalization is a synthetic route to pharmaceutically important fluorine-containing compounds but activation of inert C–F bonds remains challenging. Here the authors report activation of di-or trifluoromethylated arenes for radical C–N coupling with carbazoles and aromatic amines using photoexcited copper catalysis.
- Jun Huang
- , Qi Gao
- & Jin Xie
-
Article
| Open Access Accessing gold p-acid reactivity under electrochemical anode oxidation (EAO) through oxidation relay
Accessing gold p-acid reactivity under electrochemical anode oxidation (EAO) through oxidation relay
The combination of gold redox chemistry with electrochemistry is of interest but has limited examples. Here, the authors report an approach for the efficient pi-activation of alkene/alkyne substrates via room temperature gold catalysis under electrochemical conditions.
- Shuyao Zhang
- , Jingwen Wei
- & Xiaodong Shi
-
Article
| Open Access Late-stage synthesis of heterobifunctional molecules for PROTAC applications via ruthenium-catalysed C‒H amidation
Late-stage synthesis of heterobifunctional molecules for PROTAC applications via ruthenium-catalysed C‒H amidation
PROTACs are uniquely powerful therapeutic agents, but their synthetic tractability significantly limit drug discovery programs. Here, the authors developed a single step synthesis of PROTAC conjugates via late stage ruthenium-catalysed C–H amidation.
- Daniele Antermite
- , Stig D. Friis
- & Magnus J. Johansson
-
Article
| Open Access Highly scalable photoinduced synthesis of silanols via untraversed pathway for chlorine radical (Cl•) generation
Highly scalable photoinduced synthesis of silanols via untraversed pathway for chlorine radical (Cl•) generation
Contrary to photocatalysis, the use of photochemical energy solely to initiate reactions is underexplored. Here, the authors demonstrate light initiated synthesis of functionalized organosilanols, silanediols, and polymeric siloxanol under ambient reaction conditions.
- Argha Saha
- , Wajid Ali
- & Debabrata Maiti
-
Article
| Open Access Catalytic stereodivergent allylic alkylation of 2-acylimidazoles for natural product synthesis
Catalytic stereodivergent allylic alkylation of 2-acylimidazoles for natural product synthesis
The simultaneous control of regio-, diastereo-, and enantioselectivity poses a significant synthetic challenge in contemporary organic synthesis. Here, the authors describe a stereodivergent allylation of 2-acylimidazoles using nickel and iridium catalysts to provide completely isomeric allylated compounds.
- Ruimin Lu
- , Qinglin Zhang
- & Chang Guo
-
Article
| Open Access Near-infrared light-triggered prodrug photolysis by one-step energy transfer
Near-infrared light-triggered prodrug photolysis by one-step energy transfer
Prodrug photolysis enables spatiotemporal control of drug release at the desired lesions, but most of the photocleavable groups cannot be directly activated by near-infrared (NIR) light that features deep penetration and low phototoxicity. Here, the authors report an upconversion-like process via only one step of energy transfer for NIR light-triggered prodrug photolysis.
- Kaiqi Long
- , Wen Lv
- & Weiping Wang
-
Article
| Open Access Chiral, air stable, and reliable Pd(0) precatalysts applicable to asymmetric allylic alkylation chemistry
Chiral, air stable, and reliable Pd(0) precatalysts applicable to asymmetric allylic alkylation chemistry
Stereoselective carbon-carbon bond formation via palladium-catalyzed asymmetric allylic alkylation is important to access chiral natural products but commonly used catalyst systems often require high loadings or specific preactivation protocols. Here, the authors report several chiral single-component Pd(0) precatalysts that are active at low loadings for a variety of asymmetric allylic alkylation reactions.
- Jingjun Huang
- , Thomas Keenan
- & David C. Leitch
-
Article
| Open Access Electrosynthesis of buckyballs with fused-ring systems from PCBM and its analogue
Electrosynthesis of buckyballs with fused-ring systems from PCBM and its analogue
[6,6]-Phenyl-C61-butyric acid methyl ester (PCBM), a star molecule in the fullerene field, has found wide applications in materials science. Here, the authors demonstrate the synthesis of buckyballs with fused-ring systems via electrosynthesis through radical α-C−H functionalization of the side-chain ester for both PCBM and its analogue.
- Wei-Feng Wang
- , Kai-Qing Liu
- & Guan-Wu Wang
-
Article
| Open Access Catalyst control over pentavalent stereocentres
Catalyst control over pentavalent stereocentres
Pentavalent stereocentres encode an expanded stereochemical space, but their catalytic tractability remained unprecedented. In this report, the authors report that catalyst control over pentavalent stereocentres is feasible.
- Anton Budeev
- , Jianyang Dong
- & Christof Sparr
-
Article
| Open Access Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors
Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors
Visible light-induced glycosylation reactions are achieved by either photoactivating a photosensitizer or using a stoichiometric activator, while glycosylation via a photoactive glycosyl donor was so far not reported. In this study, the authors develop a photosensitizer free visible-light-mediated glycosylation approach using photoactive 2-glycosyloxy tropone as the donor, obtaining a wide range of O-glycosides or oligosaccharides.
- Jing Zhang
- , Zhao-Xiang Luo
- & De-Cai Xiong
-
Article
| Open Access Selective C(sp3)–H arylation/alkylation of alkanes enabled by paired electrocatalysis
Selective C(sp3)–H arylation/alkylation of alkanes enabled by paired electrocatalysis
The combination of electrochemistry and photochemistry in the context of organic synthesis is in its infancy. Here, the authors report selective C(sp3)–H arylation/alkylation of alkanes, using Earth-abundant bimetallic transition metal catalysis, under photo- and electrochemical conditions.
- Long Zou
- , Siqi Xiang
- & Qingquan Lu
-
Article
| Open Access Selective ring expansion and C−H functionalization of azulenes
Selective ring expansion and C−H functionalization of azulenes
Fused carbocycles are key structural elements of molecules in nature and they are often found in drugs and organic materials, but bicyclic systems containing six and seven-membered rings are difficult to prepare. Here, the authors functionalize an azulene skeleton that consists of fused five and seven-membered rings and carry out a ring expansion reaction to afford the desired bicycles.
- Sangjune Park
- , Cheol-Eui Kim
- & Phil Ho Lee
-
Article
| Open Access Nickel/photoredox dual catalyzed arylalkylation of nonactivated alkenes
Nickel/photoredox dual catalyzed arylalkylation of nonactivated alkenes
Dicarbofunctionalization of nonactivated alkenes is a simple way to rapidly build molecular complexity, but these transformations have not been extensively explore under metallaphotoredox conditions. Here, the authors develop a nickel/photoredox dual catalytic system for arylalkylation of alkenes.
- Yuxi Gao
- , Lijuan Gao
- & Chengfeng Xia
-
Article
| Open Access Ligand-modulated nickel-catalyzed regioselective silylalkylation of alkenes
Ligand-modulated nickel-catalyzed regioselective silylalkylation of alkenes
Multicomponent cross-coupling of alkenes with silicon reagents is used to yield complex silicon-containing compounds from readily accessible feedstock chemicals but the reaction with simple alkenes remains challenging. Here, the authors report a regioselective silylalkylation of simple alkenes, which is enabled by using a stable Ni(II) salt and an inexpensive trans-1,2-diaminocyclohexane ligand as a catalyst.
- Chao Ding
- , Yaoyu Ren
- & Guoyin Yin
-
Article
| Open Access Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis
Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis
Despite the rich coordination chemistry, hydroxylamines are rarely used as ligands for transition metal coordination compounds. Here the authors designed macrocyclic poly-N-hydroxylamines that form complexes containing a d-metal ion and coordinate by up to six hydroxylamine fragments.
- Vladislav K. Lesnikov
- , Ivan S. Golovanov
- & Alexey Yu. Sukhorukov
-
Article
| Open Access Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
While carbon-introducing difunctionalization of C-C double bonds is well established, the analogous difunctionalization for introducing germanium group and other functionalities remains elusive. Here, the authors describe a nickel-catalyzed germylative alkylation of activated olefins with easily accessible primary, secondary and tertiary alkyl bromides and chlorogermanes as the electrophiles to form C-Ge and C-Calkyl bonds simultaneously.
- Rui Gu
- , Xiujuan Feng
- & Xuan Zhang
-
Article
| Open Access Mechanistic study on the side arm effect in a palladium/Xu-Phos-catalyzed enantioselective alkoxyalkenylation of γ-hydroxyalkenes
Mechanistic study on the side arm effect in a palladium/Xu-Phos-catalyzed enantioselective alkoxyalkenylation of γ-hydroxyalkenes
Recently, the asymmetric bifunctionalization of alkenes has received much attention but the development of enantioselective alkoxyalkenylation has posed a considerable challenge and has lagged largely behind. Here, the authors report a palladium-catalyzed enantioselective alkoxyalkenylation reaction, using a range of primary, secondary, and tertiary γ-hydroxyalkenes with alkenyl halides.
- Shuai Zhu
- , Zihao Ye
- & Junliang Zhang
-
Article
| Open Access Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes
Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes
Low-valent nickel(0) complexes that promote α-functionalization of carbonyls leveraging its pro-nucleophilic character in conjunction with olefin acceptors are very scarce despite their attractivity. Here, the authors report a Ni(0)NHC catalyst which converts ketones and non-conjugated dienes to synthetically highly valuable α-allylated products.
- Yi-Xuan Cao
- , Matthew D. Wodrich
- & Nicolai Cramer
-
Article
| Open Access Stereoselectivity control in Rh-catalyzed β-OH elimination for chiral allene formation
Stereoselectivity control in Rh-catalyzed β-OH elimination for chiral allene formation
The chiral allene formation reactions catalyzed by palladium occur via a syn elimination mechanism, but there is a lack in understanding of the stereoselectivity in rhodium catalyzed reactions, specifically the β-OH elimination step. Here, the authors report the sterically controlled Rh-catalyzed SN2’-type substitution reactions of tertiary propargylic alcohols with arylmetallic species affording the non-readily available enantioenriched tetrasubstituted allenes via either exclusive syn- or anti-β-OH elimination.
- Jie Wang
- , Wei-Feng Zheng
- & Shengming Ma
-
Article
| Open Access Facile access to benzofuran derivatives through radical reactions with heteroatom-centered super-electron-donors
Facile access to benzofuran derivatives through radical reactions with heteroatom-centered super-electron-donors
The use of heteroatom-centered anions as super-electron-donors (SEDs) for direct single-electron-transfer reactions has rarely been studied. Here, the authors show that heteroatom anions can be applied as SEDs to initiate radical reactions for synthesis of 3-substituted benzofurans, which have applications in both organic synthesis and pesticide development.
- Shichun Jiang
- , Wei Wang
- & Yonggui Robin Chi
-
Article
| Open Access Synthesis of dienes from pyrrolidines using skeletal modification
Synthesis of dienes from pyrrolidines using skeletal modification
Saturated N-heterocyclic pyrrolidines are common in natural products and medicinal compounds, but reconstruction of their skeletal structures to access new chemical space is a challenging. Here, the authors report a skeletal modification strategy for conversion of polar cyclic pyrrolidines into nonpolar linear dienes through a N-atom removal and deconstruction process.
- Haitao Qin
- , Ting Guo
- & Hongjian Lu
-
Article
| Open Access Thermo-responsive chiral micelles as recyclable organocatalyst for asymmetric Rauhut-Currier reaction in water
Thermo-responsive chiral micelles as recyclable organocatalyst for asymmetric Rauhut-Currier reaction in water
Developing eco-friendly chiral organocatalysts with the combined advantages of homogeneous catalysis and heterogeneous processes is challenging. Here, the authors present, a family of amphiphilic one handed helical polyisocyanides bearing phosphine pendants which self-assembled into well-defined chiral micelles in water and show thermo-responsiveness
- Lei Xu
- , Li Zhou
- & Zong-Quan Wu

 Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins