Abstract
Direct and site-selective C-H functionalization of alkenes under environmentally benign conditions represents a useful and attractive yet challenging transformation to access value-added molecules. Herein, a unified protocol for a variety of intermolecular Heck-type functionalizations of Csp2-H bond of alkenes has been developed by thianthrenation. The reaction features metal-free and operationally simple conditions for exclusive cine-selective C-H functionalization of aliphatic and aryl alkenes to forge C-C, C-N, C-P, and C-S bonds at room temperature, providing a general protocol for intermolecular Heck-type reaction of alkenes with nucleophiles (Nu = sulfinates, cyanides, amines, amides). Alkenes undergo cine-sulfonylation, cyanation, amination to afford alkenyl sulfones, alkenyl nitriles and enamines.
Similar content being viewed by others
Introduction
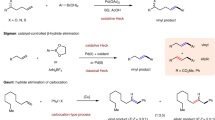
Alkenes represent one of the most useful functional groups due to their profound potential to a myriad of other functional groups as well their orthogonal reactivity over other polar functional groups1,2,3,4. Owing to the abundance, diversity, and easy-availability of alkenes, developing efficient and practical functionalizations of alkenes has been a long-term preoccupation in synthetic chemistry5,6. Among which, Heck reaction is one of the most straightforward and efficient means to functionalize alkenes7,8,9. Typically, Heck reaction gives access to ipso-substitution of alkenes where a leaving group is bonded to the olefinic carbon atom by nucleophilic species. Comparably, cine-substitution of Heck reaction are less investigated. Over the past decades, transition-metal-catalyzed Heck-type cine-arylation and vinylation of electron-deficient alkenes bearing a leaving group have been developed10,11,12,13,14,15,16,17,18. Straightforward and environmentally benign Heck-type methods that transform alkenes into versatile carbon electrophiles would be highly desirable19,20. In particular, alkenyl sulfones, alkenyl nitriles, enamines, and enamides are of importance in pharmaceuticals, biochemistry, and materials sciences21,22,23,24, providing a straightfoward opportunity in multistep organic synthesis or covalent modification of proteins in drug discovery to couple with different electrophiles. Therefore, direct and regioselective sulfonylation, cyanation, amination, amidation of alkenes would be an enabling synthetic tool to access such privileged structures. To date, metal-free intermolecular Heck-type reaction of alkenes to access alkenyl sulfones25, alkenyl nitriles26, enamines, and enamides remains underdeveloped27,28.
On the other hand, alkenyl thianthrenium salts29,30,31,32,33,34,35,36,37,38,39,40 have been considered as one umpolung strategy of alkenes for further chemical synthesis pioneered by Shine41,42. Recently, Ritter developed the practical and scalable synthesis of alkenyl thianthrenium salts43,44, creating new opportunities for derivatization of unactivated alkenes15,45,46. In particular, metal-free functionalization of alkenes represent an attractive aspect to functionalize alkenes under mild conditions. In 2021, elegant examples of electrochemical aziridination of alkenes with primary amines have been demonstrated with or without thianthrene47,48. The generation of dicationic intermediates also offers potential opportunities for ipso- and cine-substitution reactions. In 2022, Shu group developed a unified metal-free intermolecular aziridination and cyclopropanation of alkenes by thianthrenation (Fig. 1b, top)49. Sulfonamides, carbamates, amides, primary amines, and methylenes with acidic protons were all successfully employed as nucleophiles. In 2021, Wickens and Shu independently reported the allylic functionalizations of alkenes by thianthrenation to from C–N, C–C, C–O, and C–S bonds in the presence of nucleophiles (Fig. 1b, middle)50,51. Recently, Soós group developed an ene-type Kornblum-Ganem oxidation of alkenes by thianthrenation to access various α,β-unsaturated carbonyls (Fig. 1b, bottom)52. Interestingly, metal-free transformations of alkenes by thianthrenation basically led to bond-formation at ipso-carbon of alkenyl thianthrenium salts. We questioned the possibility of realizing a new bond-formation mode of alkenyl thianthrenium salts to functionalization cine-carbon of alkenes under metal-free conditions48. Herein, we report a unified protocol for metal-free cine-functionalizations of alkenes by thianthrenation (Fig. 1c). The reaction explores the new reactivity of alkenyl thianthrenium salts to form a new chemical bond at cine-position instead of ipso-position of vinyl thianthrenium salts. The mild condition allows for the site-selective C–H functionalization of alkenes to forge C–S, C–N, C–P and C–C bonds with diverse nucleophiles.
Results
Optimization of the reaction conditions
We started the investigation using sodium methanesulfinate (1a) and 4-phenylbut-1-enylthianthrenium salt (2a) as model substrates to evaluate the reaction conditions (Table 1). To our delight, cine-sulfonation of the C = C bond of the vinyl thianthrenium salt was exclusively formed, without the formation of formal allylic C-H sulfonation byproduct (3a’) as previously reported. After evaluation of the reaction parameters, we define the reaction in DCE (0.1 M) at room temperature without any additive as standard conditions, providing the desired product (3-(methylsulfonyl)but-3-en-1-yl)benzene 3a in 83% isolated yield (Table 1, entry 1). The use of other solvents instead of DCE could also mediate the desired transformation, albeit giving 3a in lower yields (Table 1, entries 2–9).
Scope of the reaction
With the optimized conditions in hand, the scope of alkenes and sodium sulfinates is examined and the results are summarized in Fig. 2. First, the scope of alkenes was evaluated. A wide range of alkenes with diverse electronic and steric properties are suitable for this reaction, allowing the corresponding cine-sulfonylation of alkenes by thianthrenation with sodium methanesulfinate in good yields (3a-3z). Aliphatic terminal alkene-based thianthrenium salts are all compatible in this reaction, producing alkenyl sulfones in 52–88% yields (3a-3m). Alkenes with pendant amides, bromides, esters were compatible in the reaction, giving the corresponding cine-substitution products (3c, 3d, 3g-3i) in 69–88% yields. It is noteworthy that amides with free N–H, free alcohols, and alkenes were all compatible in the reaction to furnish the desired alkenyl sulfones (3h, 3j, and 3k) in 70–75% yields, leaving chemical space for further elaboration. In addition, α-branched aliphatic alkene-based thianthrenium salts are also good substrates in the reaction, giving corresponding cine-sulfonylation products in 81% and 85% yields (3l and 3m). Moreover, styrenes could be efficiently involved in the cine-substitution process by thianthrenation, giving the desired sulfones in 61–92% yields (3n-3s). In addition, cyclic alkenes were easily converted to alkenylsulfones in 59–89% yields (3t-3w) under the reaction conditions. Notably, gaseous alkenes, such as ethylene and propene, could be successfully involved in this cine-substitution process by thianthrenation, giving 3e, 3x and 3y in 52–88% yields. Lithocholic acid drived alkene with molecular complexity underwent cine-substitution process smoothly, giving the desired product (3z) in 70% yield.
Standard conditions, see Table 1 for details. Isolated yield is shown. aReaction was run for 24 h. bReaction was conducted at 50 oC. cReaction was run at 50 oC for 36 h. dReaction was conducted on 4.0 mmol scale.
Next, the scope of sulfinates was tested. para-Substituted aryl sulfinates with electron-donating (4a-4d) or electron-withdrawing (4e-4i) groups were all well-tolerated in this reaction, giving corresponding cine-sulfonylation products in good yields (61–96%). Moreover, meta- and ortho-substituted aryl sulfinates were also good substrates for this reaction to give the desired products (4j and 4k) in 76% and 60% yields, respectively. Fused aryl and heteroaryl sulfinates underwent cine-substitution to give the desired products (4l and 4m) in 79% and 89% yields. Vinyl sulfinate proceeded smoothly to give the corresponding alkenyl sulfone 4n in 84% yield. Notably, allylic, acyclic and cyclic alkyl sulfinates were compatible in this metal-free cine-substitution process and afforded the corresponding sulfones in 65-90% yields (4o-4q). It is noteworthy that bulky sulfinates smoothyl underwent cine-substitution of C-H bond by thianthrenation to give the corresponding alkenyl sulfones in 75% and 86% yields (4r and 4s). In addition, the structure of the alkenyl sulfones was unambiguously confirmed by X-ray diffraction of 4k. The reaction could be scaled up to 4.0 mmol to afford 4a in 84% yield (1.16 g), rendering the reaction useful for large-scale synthesis.
Furthermore, the protocol for cine-functionalizations of alkenes by thianthrenation was further applied to cine-cyanation, cine-amination, and cine-amidation to forge C-C bonds and C-N bonds from C-H bonds (Fig. 3). With slight modification of the solvent and base, cine-cyanation was achieved with zinc cyanide in the presence of KF (3.0 equiv) in CH3CN (0.1 M) at room temperature, delivering the desired 2-methylene-4-phenylbutanenitrile (5a) in 70% isolated yield (Supplementary Tables 2 and 3) (for details see Supplementary Information)48. Then, the scope of cine-cyanation was evaluated. α-Branched terminal alkene derived thianthrenium salt is compatible with this cine-cyanation, delivering the corresponding cine-substitution product (5b) in 53% yield. Additionally, aliphatic alkenes tethered with esters, amides, alcohols were all compatible in this reaction, affording the desired acrylonitriles in 65-77% yields (5c-5e). Moreover, alkenes with bromides and alkenes were successfully transformed to the cine-cyanation products in 84% and 47% yields (5f and 5g). Notably, cyclic and acyclic internal aliphatic alkenes derived thianthrenium salts were amenable to this cine-cyanation, affording diverse acrylonitriles in 76% and 46% yields (5h and 5i). Interestingly, styrenes also worked well for this cine-cyanation to afford 5j in 70% yield.
The reaction was carried out using 1 (0.15 mmol), 2 (0.10 mmol), KF (3.0 equiv) in CH3CN (0.1 M) at room temperature for 10 h unless otherwise stated. Isolated yield is shown. aThe reaction was conducted at 50 oC. bThe reaction was conducted at 50 oC for 24 h. cThe reaction was conducted using K2CO3 (1.0 equiv). rr = ratio of regioisomers. dThe reaction was conducted on 5.0 mmol scale using K2CO3 (1.0 equiv). eThe reaction was conducted using Cs2CO3 (1.0 equiv).
Impressively, this operationally simple cine-substitution protocol could be successfully applied to cine-amination of C-H bonds using nitrogen nucleophiles (Fig. 3). The reaction of 2q with indole in the presence of K2CO3 (1.0 equiv) in CH3CN (0.1 M) at room temperature afforded the selective cine-substitution at nitrogen product 1-(1-(4-fluorophenyl)vinyl)-1H-indole 6a in 82% isolated yield (Supplementary Tables 4 and 5) (for details see Supplementary Information). Ketone-, aldehyde-, and bromo-substituted indoles were well-tolerated under this cine-amination conditions, delivering the corresponding N-vinyl indoles (6b-6d) in 76 − 81% yields. Additionally, carbazole and imidazole were all excellent substrates for this cine-substitution, yielding the desired N-vinyl carbazole (6e) and imidazole (6f) in 81% and 84% yields. Moreover, sulfonamides and amides were smoothly transformed into corresponding N-vinyl amides in 64% and 80% yields (6g and 6h). Furthermore, pyrazoles and 1,2,4-triazoles were remarkable substrates for this cine-substitution reaction, producing the desired N-vinyl pyrazole (6i) and triazole (6j) in 66% and 70% yields. 1,2,3-Triazoles were also compatible in the reaction, delivering a mixture of 6k and 6k’ in 36% and 28% yields. Moreover, a variety of alkenes worked well the C-N bond-forming process from cine-C-H bond of alkenes by thianthrenation. Chloro-, bromo-substituted styrenes and internal styrenes were compatible with this cine-substitution reaction, generating cine-amination products (6l-6n) in 78-82% yields. Linear and α-branched aliphatic alkenes derived thianthrenium salts were all tolerated under the cine-amination conditions, delivering corresponding enamines (6o and 6p) in 60% and 74% yields. Isolated diene selectively underwent thianthrenation and sequential cine-amination on one alkene, giving corresponding product 6q in 65% yield. Unsymmetrical alkenes could be involved in the regioselective thianthrenation and sequential cine-amination to yield 6r in 73% yield with >15:1 rr. Cyclic alkenes with different ring size could be involved to this cine-substitution with imidazole to furnish enamines (6s and 6t) in 57% yield. Additionally, drug molecules have been derivatized. Metaxalone, and lansoprazole underwent selective cine-amination reaction of the C-H bond of alkenes with amides and benzoimidazoles to give N-acyl and N-aryl enamines (6u and 6v) in 82% and 70% yields, respectively. Interestingly, melatonin underwent chemoselective N-vinylation with of indoles instead of the amides at cine-position of alkenes to give N-aryl enamine derivative (6w) in 75% yield under standard conditions. Additionally, cine-phosphonylation product 7a was got in 69% yield from phosphoryl nucleophile and alkenylthianthrenium salt.
Mechanistic study
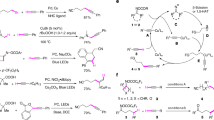
To enhance the practicality of this operationally simple protocol, a one-pot procedure was evaluated for cine-sulfonylation and amination (Fig. 4a). The one pot thianthrenation of 4-phenyl-1-butene using thianthrene S-oxide, followed by the reaction of sodium methanesulfinate or imidazole under corresponding conditions were conducted, affording the desired cine-substituted products in comparable yields (3a, 71%) or (6o, 65%) without any intermediate purification. Furthermore, the reactions of 1a and 2a in the presence of 2 equiv of radical scavenger (TEMPO or BHT) were carried out under otherwise identical to standard conditions, providing the desired alkenyl sulfone 3a in 72% and 83% yields (Fig. 4b). The result that the presence of TEMPO or BHT did not decrease the efficiency of this reaction, excluding the radical pathway of this cine-substitution reaction. To further detect the mechanism of this cine-substitution, the preformed alkyl thianthrenium salts (8a and 8b) were subjected to nucleophiles under standard conditions (Fig. 4c). Corresponding alkenyl sulfone 3a and alkenyl amine 6o were obtained in 63% and 57% yield, suggesting the primary alkyl thianthrenium salts may serve as the intermediate for the selective cine-substitution process. Additionally, the reaction of 1aa and deuterated alkenyl thianthrenium salt 2cc was conducted under standard conditions, affording the desired cine-amination product 8c in 65% yield. Interestingly, partial deuterium scrambling was observed (Fig. 4d), supporting the proton exchange of β-position of alkenyl thianthrenium salt with surroundings and protonation of α-position of alkenyl thianthrenium.
Based on the mechanistic experiments and literature48,49,50,51,52, a plausible mechanism of the metal-free cine-substitution of alkenyl thianthrenium salts is proposed and depicted in Fig. 5. First, the zwitterionic alkyl thianthrenium salt intermediate M1 could be formed by intermolecular addition of nucleophiles on distal position of alkenes to forge C-S/C-C/C-N/C-P bonds. After protonation of M1 to form a more stable intermediate M2, M2 underwent deprotonation on cine-site yield the zwitterion M3. M3 could undergo intramolecular elimination to afford alkenyl sulfones, acrylonitriles, enamines, and enamides by releasing thianthrene.
Discussion
In conclusion, a unified metal-free protocol for diverse intermolecular cine-functionalizations of the C–H bond of alkenes by thianthrenation has been achieved. The reaction features metal-free C–H functionalizations of alkenes under mild conditions to forge C–S, C–C, C–N, and C–P bonds from C–H bonds via cine-sulfonylation, cine-cyanation, cine-amination, cine-amidation, and cine-phosphonylation. The reaction represents new metal-free reaction mode to functionalize cine-carbon of alkenyl thianthrenium salts, which is complementary to previous functionalization at ipso-carbon. Mechanistic investigations revealed the reaction undergo site-selective nucleophilic addition followed by regioselective elimination to afford the formal Heck reaction of alkenes, affording synthetic useful synthons which are difficult to access from readily accessible starting materials.
Methods
General procedure A for intermolecular Heck-type sulfonylation of alkenes by thianthrenation
Sodium sulfinate (0.15 mmol) and vinyl thianthrenium salt (0.1 mmol) were placed in a 10.0 mL Schlenk tube which equipped with a magnetic stir bar. After back-filled with nitrogen (this process was repeated three times), DCE (1.0 mL) was added. The vial was sealed and at room temperature (for the large hindrance substrates its require at 50 °C) with stirring until TLC indicated the complete consumption of thianthrene (typically 10 h or 36 h). The reaction mixture was evaporated and purified directly by column chromatography to afford the product.
General procedure B for intermolecular Heck-type cyanation of alkenes by thianthrenation
Zn(CN)2 (17.6 mg), KF (17.4 mg) and vinyl thianthrenium salt (0.1 mmol) were placed in a 10.0 mL Schlenk tube which equipped with a magnetic stir bar. After back-filled with nitrogen (this process was repeated three times), CH3CN (1.0 mL) was added. The vial was sealed and at room temperature (for the large hindrance substrates it require at 50 °C) with stirring until TLC indicated the complete consumption of thianthrene (typically 10 h or 24 h). The reaction mixture was evaporated and purified directly by column chromatography to afford the product.
General procedure C for intermolecular Heck-type amination and amidation of styrenes by thianthrenation
Nucleophile (0.15 mmol), K2CO3 (13.8 mg) and vinyl thianthrenium salt (0.1 mmol) were placed in a 10.0 mL Schlenk tube which equipped with a magnetic stir bar. After back-filled with nitrogen (this process was repeated three times), CH3CN (1.0 mL) was added. The vial was sealed and at room temperature with stirring until TLC indicated the complete consumption of thianthrene (typically 10 h). The reaction mixture was evaporated and purified directly by column chromatography to afford the product.
General procedure D for intermolecular Heck-type amination and amidation of aliphatic alkenes by thianthrenation
Nucleophile (0.15 mmol), Cs2CO3 (32.6 mg) and vinyl thianthrenium salt (0.1 mmol) were placed in a 10.0 mL Schlenk tube which equipped with a magnetic stir bar. After back-filled with nitrogen (this process was repeated three times), CH3CN (1.0 mL) was added. The vial was sealed and at room temperature with stirring until TLC indicated the complete consumption of thianthrene (typically 10 h). The reaction mixture was evaporated and purified directly by column chromatography to afford the product.
Data availability
The X-ray crystallographic coordinates for structures that support the findings of this study have been deposited at the Cambridge Crystallographic Data Center (CCDC) with the accession codes CCDC 2241239 (4k) and CCDC 2241240 (6g) via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request.
References
Rychnovsky, S. D. Oxo polyene macrolide antibiotics. Chem. Rev. 95, 2021–2040 (1995).
Oger, C., Balas, L., Durand, T. & Galano, J.-M. Are alkyne reductions chemo-, regio-, and stereoselective enough to provide pure (Z)-olefins in polyfunctionalized bioactive molecules? Chem. Rev. 113, 1313–1350 (2013).
Tejedor, D., Méndez-Abt, G., Cotos, L. & García-Tellado, F. Propargyl claisen rearrangement: Allene synthesis and beyond. Chem. Soc. Rev. 42, 458–471 (2013).
Zhou, F., Li, M., Jiang, H. & Wu, W. Recent advances in transformations involving electron-rich alkenes: Functionalization, cyclization, and cross-metathesis reactions. Adv. Syn. Catal. 363, 4841–4855 (2021).
Mandal, D., Roychowdhury, S., Biswas, J. P., Maiti, S. & Maiti, D. Transition bond alkylation using olefins: Recent advances and mechanistic aspects. Chem. Soc. Rev. 51, 7358–7426 (2022).
Luo, M.-J., Xiao, Q. & Li, J.-H. Electro-/photocatalytic alkene-derived radical cation chemistry: Recent advances in synthetic applications. Chem. Soc. Rev. 51, 7206–7237 (2022).
Heck, R. F. Palladium Reagents in Organic Syntheses (Academic Press, 1985).
Heck, R. F. Arylation, methylation, and carboxyalkylation of olefins by group viii metal derivatives. J. Am. Chem. Soc. 90, 5518–5526 (1968).
Crisp, G. T. Variations on a theme-recent developments on the mechanism of the Heck reaction and their implications for synthesis. Chem. Soc. Rev. 27, 427–436 (1998).
Suwiński, J. & Świerczek, K. cine- and tele-substitution reactions. Tetrahedron 57, 1639–1662 (2001).
Peng, Y. & Li, W.-D. Z. cine-Substitution and the Cu effect in Stille cross-coupling reactions: Mechanistic perspectives and synthetic utility. Eur. J. Org. Chem. 2010, 6703–6718 (2010).
Stork, G. & Isaacs, R. C. A. cine-Substitution in vinylstannane cross-coupling reactions. J. Am. Chem. Soc. 112, 7399–7400 (1990).
Yoshida, K. & Hayashi, T. A new cine-substitution of alkenyl sulfones with aryltitanium reagents catalyzed by rhodium: Mechanistic studies and catalytic asymmetric synthesis of allylarenes. J. Am. Chem. Soc. 125, 2872–2873 (2003).
Yu, J.-Y., Shimizu, R. & Kuwano, R. Selective cine substitution of 1-arylethenyl acetates with arylboron reagents and a diene/rhodium catalyst. Angew. Chem. Int. Ed. 49, 6396–6399 (2010).
Ye, Y., Zhu, J., Xie, H. & Huang, Y. Rhodium-catalyzed divergent arylation of alkenylsulfonium salts with arylboroxines. Angew. Chem. Int. Ed. 61, e202212522 (2022).
Kanoh, N., Ohno, Y., Itagaki, T., Fukuda, H. & Iwabuchi, Y. On the origin of cine-substitution in the stille coupling of trisubstituted iodoalkene and trans-vinylstannane. Synlett 24, 2660–2664 (2013).
Berger, F. et al. cine-Substitutions at five-membered hetarenes enabled by sulfonium salts. Org. Lett. 22, 5671–5674 (2020).
Zou, L.-H. et al. Selective synthesis of alkyl amines and N-vinylazoles from vinyl sulfonium salts with N-nucleophiles. Org. Chem. Front. 9, 3231–3236 (2022).
Mo, J., Xu, L., Ruan, J., Liu, S. & Xiao, J. Regioselective Heck arylation of unsaturated alcohols by palladium catalysis in ionic liquid. Chem. Commun. 3591–3593 (2006).
Qin, L., Ren, X., Lu, Y., Li, Y. & Zhou, J. Intermolecular Mizoroki-Heck reaction of aliphatic olefins with high selectivity for substitution at the internal position. Angew. Chem. Int. Ed. 51, 5915–5919 (2012).
Simpkins, N. S. The chemistry of vinyl sulphones. Tetrahedron 20, 6951–6984 (1990).
Fleming, F. F., Yao, L., Ravikumar, P. C., Funk, L. & Shook, B. C. Nitrile-containing pharmaceuticals: Efficacious roles of the nitrile pharmacophore. J. Med. Chem. 53, 7902–7917 (2010).
March, J. Advanced Organic Chemistry 4th edn, 728 (Wiley, 1992).
Beller, M. et al. Advances and adventures in amination reactions of olefins and alkynes. Synlett 10, 1579–1594 (2002).
Inomata, K., Kobayashi, T., Sasaoka, S.-I., Kinoshita, H. & Kotake, H. Convenient methods for the preparation of vinylic from alkenes. Chem. Lett. 15, 289–292 (1986).
Gao, D.-W. et al. Direct access to versatile electrophiles via catalytic oxidative cyanation of alkenes. J. Am. Chem. Soc. 140, 8069–8073 (2018).
Brice, J. L., Harang, J. E., Timokhin, V. I., Anastasi, N. R. & Stahl, S. S. Aerobic oxidative amination of unactivated alkenes catalyzed by palladium. J. Am. Chem. Soc. 127, 2868–2869 (2005).
Rogers, M. M., Kotov, V., Chatwichien, J. & Stahl, S. S. Palladium-catalyzed oxidative amination of alkenes: Improved catalyst reoxidation enables the use of alkene as the limiting reagent. Org. Lett. 9, 4331–4334 (2007).
Berger, F. & Ritter, T. Site-selective late-stage C–H functionalization via thianthrenium salts. Synlett 33, 339–345 (2021).
Chen, X.-Y., Wu, Y. & Wang, P. Recent advances in thianthrenation/phenoxathiination enabled site-selective functionalization of arenes. Synthesis 54, 3928–3940 (2022).
Meng, H., Liu, M.-S. & Shu, W. Organothianthrenium salts: Synthesis and utilization. Chem. Sci. 13, 13690–13707 (2022).
Berger, F. et al. Site-selective and versatile aromatic C-H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Engl, P. S. et al. C-N cross-couplings for site-selective late-stage diversification via aryl sulfonium salts. J. Am. Chem. Soc. 141, 13346–13351 (2019).
Jia, H., Häring, A. P., Berger, F., Zhang, L. & Ritter, T. Trifluoromethyl thianthrenium triflate: A readily available trifluoromethylating reagent with formal CF3+, CF3·, and CF3- reactivity. J. Am. Chem. Soc. 143, 7623–7628 (2021).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Chen, C., Wang, M., Lu, H., Zhao, B. & Shi, Z. Enabling the use of alkyl thianthrenium salts in cross-coupling reactions by copper catalysis. Angew. Chem. Int. Ed. 60, 21756–21760 (2021).
Chen, C., Wang, Z.-J., Lu, H., Zhao, Y. & Shi, Z. Generation of non-stabilized alkyl radicals from thianthrenium salts for C-B and C-C bond formation. Nat. Commun. 12, 4526 (2021).
Cabrera-Afonso, M. J., Granados, A. & Molander, G. A. Sustainable thioetherification via electron donor-acceptor photoactivation using thianthrenium salts. Angew. Chem. Int. Ed. 61, e202202706 (2022).
Granados, A., Cabrera-Afonso, M. J., Escolano, M., Badir, S. O. & Molander, G. A. Thianthrenium-enabled sulfonylation via electron donor-acceptor complex photoactivation. Chem. Catal. 2, 898–907 (2022).
Zhu, J., Ye, Y., Yan, Y., Sun, J. & Huang, Y. Highly regioselective dichalcogenation of alkenyl sulfonium salts to access 1,1-dichalcogenalkenes. Org. Lett. 25, 5324–5328 (2023).
Silber, J. J. & Shine, H. J. Ion radicals. XXII. Reaction of thianthrenium perchlorate (C12H8S2·+ClO4-) with aromatics. J. Org. Chem. 36, 2923–2926 (1971).
Qian, D.-Q., Shine, H. J., Guzman-Jimenez, I. Y., Thurston, J. H. & Whitmire, K. H. Mono- and bisadducts from the addition of thianthrene cation radical salts to cycloalkenes and alkenes. J. Org. Chem. 67, 4030–4039 (2002).
Chen, J., Li, J., Plutschack, M. B., Berger, F. & Ritter, T. Regio- and stereoselective thianthrenation of olefins to access versatile alkenyl electrophiles. Angew. Chem. Int. Ed. 59, 5616–5620 (2020).
Juliá, F., Yan, J., Paulus, F. & Ritter, T. Vinyl thianthrenium tetrafluoroborate: A practical and versatile vinylating reagent made from ethylene. J. Am. Chem. Soc. 143, 12992–12998 (2021).
Xie, R., Zhu, J. & Huang, Y. Cu-catalyzed highly selective silylation and borylation of alkenylsulfonium salts. Org. Chem. Front. 8, 5699–5704 (2021).
Zhu, J., Ye, Y. & Huang, Y. Palladacycle-catalyzed olefinic C–P cross-coupling of alkenylsulfonium salts with diarylphosphines to access alkenylphosphines. Organometallics 41, 2342–2348 (2022).
Ošeka, M. et al. Electrochemical aziridination of internal alkenes with primary amines. Chem 7, 255–266 (2021).
Holst, D. E., Wang, D. J., Kim, M. J., Guzei, I. A. & Wickens, Z. K. Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication. Nature 596, 74–79 (2021).
Liu, M.-S., Du, H.-W., Cui, J.-F. & Shu, W. Intermolecular metal-free cyclopropanation and aziridination of alkenes with XH2 (X=N, C) by thianthrenation. Angew. Chem. Int. Ed. 61, e202209929 (2022).
Wang, D. J., Targos, K. & Wickens, Z. K. Electrochemical synthesis of allylic amines from terminal alkenes and secondary amines. J. Am. Chem. Soc. 143, 21503–21510 (2021).
Liu, M.-S., Du, H.-W. & Shu, W. Metal-free allylic C-H nitrogenation, oxygenation, and carbonation of alkenes by thianthrenation. Chem. Sci. 13, 1003–1008 (2022).
Angyal, P., Kotschy, A. M., Dudás, A., Varga, S. & Soós, T. Intertwining olefin thianthrenation with kornblum/ganem oxidations: Ene-type oxidation to furnish α,β-unsaturated carbonyls. Angew. Chem. Int. Ed. 62, e202214096 (2023).
Acknowledgements
Financial support from NSFC (21971101, 22171127, 22371115), Guangdong Basic and Applied Basic Research Foundation (2022A1515011806), Department of Education of Guangdong Province (2021KTSCX106, 2022JGXM054), Shenzhen Science and Technology Innovation Committee (JCYJ20220530114606013, JCYJ20230807093522044), The Pearl River Talent Recruitment Program (2019QN01Y261), Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002) is sincerely acknowledged. We thank Prof. Wickens (UWM) for insightful suggestions and comments. We acknowledge the assistance of SUSTech Core Research Facilities. We thank Dr. Xiaoyong Chang (SUSTech) for assistance with the X-ray crystallographic analysis of 4k (CCDC 2241239), 6g (CCDC 2241240), and Dr. Quan-Xing Zi (SUSTech) for reproducing the results of 3h, 4k, 5g and 6m.
Author information
Authors and Affiliations
Contributions
M.S.L. discovered and developed the reaction. W.S. conceived and directed the project. M.S.L. performed the experiments, M.S.L. and H.W.D. collected the data. M.S.L. and H.W.D. synthesized the substrate materials. H.M. and Y.X. discussed the project with W.S. and helped prepare the manuscript. All authors discussed and analyzed the data. W.S and M.S.L. wrote the manuscript with contributions from other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chengrong Ding, Yinhua Huang, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, MS., Du, HW., Meng, H. et al. Unified metal-free intermolecular Heck-type sulfonylation, cyanation, amination, amidation of alkenes by thianthrenation. Nat Commun 15, 529 (2024). https://doi.org/10.1038/s41467-024-44746-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-44746-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.