Article
|
Open Access
Featured
-
-
Article
| Open Access Dehydroxylative radical N-glycosylation of heterocycles with 1-hydroxycarbohydrates enabled by copper metallaphotoredox catalysis
Dehydroxylative radical N-glycosylation of heterocycles with 1-hydroxycarbohydrates enabled by copper metallaphotoredox catalysis
N-Glycosylated heterocycles play important roles in biological systems and drug development, but the synthesis heavily relies on ionic N-glycosylation. Herein, the authors report a dehydroxylative radical method for synthesizing N-glycosides by leveraging copper metallaphotoredox catalysis.
- Da-Peng Liu
- , Xiao-Sen Zhang
- & Xiang-Guo Hu
-
Article
| Open Access Polarizability matters in enantio-selection
Polarizability matters in enantio-selection
Polarizability, a property that is closely related to softness in the classic theory of Hard and Soft Acids and Bases (HSAB), has been largely overlooked in connecting with enantio-selection in the past. Here, the authors show local polarizability-based electronic effects can rationalize a wide range of stereochemical outcomes in widely-known asymmetric catalytic reactions.
- Fumin Chen
- , Yu Chen
- & Xiangyou Xing
-
Article
| Open Access Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
The synthesis of sequence-regulated oligosulfates has not yet been established due to the difficulties in precise reactivity control. Here, the authors report a multi-directional divergent iterative method to furnish oligosulfates based on a chain homologation approach, in which the fluorosulfate unit is regenerated.
- Min Pyeong Kim
- , Swatilekha Kayal
- & Sung You Hong
-
Article
| Open Access Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes
Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes
Developing facile and direct synthesis routes for enantioselective construction of cyclic π-conjugated molecules is crucial but the chirality orginiating from the distorted structure around heptagon-containing polyarenes is largely overlooked. Herein the authors present a highly enantioselective synthesis for fabrication of all carbon heptagon-containing polyarenes via palladium-catalyzed carbene-based cross–coupling of benzyl bromides and N-arylsulfonylhydrazones.
- Huan Zhang
- , Chuan-Jun Lu
- & Ren-Rong Liu
-
Article
| Open Access Noncovalent interaction with a spirobipyridine ligand enables efficient iridium-catalyzed C–H activation
Noncovalent interaction with a spirobipyridine ligand enables efficient iridium-catalyzed C–H activation
Exploitation of noncovalent interactions has received much attention for the design of metal catalysts. However, because of the weak nature, CH-π interactions have been less utilized for the control of organic reactions. Here, the authors report that the CH-π interaction can be used to kinetically accelerate catalytic C-H activation of arenes.
- Yushu Jin
- , Boobalan Ramadoss
- & Laurean Ilies
-
Article
| Open Access Selenium catalysis enables negative feedback organic oscillators
Selenium catalysis enables negative feedback organic oscillators
Multiple autocatalytic reactions producing thiols are known, but negative feedback loop motifs are unavailable for thiol chemistry. Here, the authors develop a negative feedback loop based on the selenocarbonates, in which thiols induce the release of aromatic selenols that catalyze the oxidation of thiols by organic peroxides.
- Xiuxiu Li
- , Polina Fomitskaya
- & Sergey N. Semenov
-
Article
| Open Access Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
The stereospecific Z/E isomerization of tetrasubstituted alkenes remains underdeveloped, thus lacking in a stereodivergent synthesis of axially chiral alkenes. Here, authors report the atroposelective synthesis of tetrasubstituted alkene analogues by asymmetric allylic substitution-isomerization, followed by the photocatalyzed Z/E isomerization.
- Jie Wang
- , Jun Gu
- & Ying He
-
Article
| Open Access Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes
Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes
Planar chiral [2.2]paracyclophanes have a wide range of applications in asymmetric synthesis and materials science. However, they are accessed via time-consuming chiral separations or kinetic resolution approaches. Here, the authors report a simple, metal-free protocol for organocatalytic desymmetrization of prochiral diformyl[2.2]paracyclophanes.
- Vojtěch Dočekal
- , Filip Koucký
- & Jan Veselý
-
Article
| Open Access Nickel-catalyzed switchable arylative/endo-cyclization of 1,6-enynes
Nickel-catalyzed switchable arylative/endo-cyclization of 1,6-enynes
Divergent functionalizations of pi bonds allow for synthetic chemists to move from simplicity to complexity. Here, the authors report a nickel-catalyzed switchable arylation/cyclization of 1,6-enynes in which the nature of the ligand dictates the regioselectivity of alkyne arylation, while the electrophilic trapping reagents determine the selectivity of the cyclization mode.
- Wenfeng Liu
- , Wei Li
- & Wangqing Kong
-
Article
| Open Access Paired electrocatalysis unlocks cross-dehydrogenative coupling of C(sp3)-H bonds using a pentacoordinated cobalt-salen catalyst
Paired electrocatalysis unlocks cross-dehydrogenative coupling of C(sp3)-H bonds using a pentacoordinated cobalt-salen catalyst
Cross-dehydrogenative coupling (CDC) of C-H bonds is an ideal approach for C-C bond construction but suffers from low selectivity of similar C-H bonds. Here, the authors describe a highly selective paired electrocatalysis strategy towards CDC combining hydrogen evolution reaction catalysis with hydride transfer catalysis.
- Ke Liu
- , Mengna Lei
- & Sheng Zhang
-
Article
| Open Access Umpolung reactivity of strained C–C σ-bonds without transition-metal catalysis
Umpolung reactivity of strained C–C σ-bonds without transition-metal catalysis
Umpolung reactions typically focus on carbonyls or imine derivatives. Here, the authors report the umpolung reaction of C–C σ-bonds in bicyclo[1.1.0]butanes (BCBs) with electrophilic alkenes, yielding various cyclobutenes or conjugated dienes.
- Dachang Bai
- , Xiuli Guo
- & Junbiao Chang
-
Article
| Open Access Trialkoxysilane-Induced Iridium-Catalyzed para-Selective C–H Bond Borylation of Arenes
Trialkoxysilane-Induced Iridium-Catalyzed para-Selective C–H Bond Borylation of Arenes
Controlling regioselectivity is one of the most challenging aspects for the construction of aryl boron compounds, and there are few practical and general strategies for such transformations. Here, the authors report an iridium-catalyzed trialkoxysilane protecting group-assisted regioselective C–H borylation with various aromatic compounds.
- Guodong Ju
- , Zhibin Huang
- & Yingsheng Zhao
-
Article
| Open Access Stereoselective assembly of C-oligosaccharides via modular difunctionalization of glycals
Stereoselective assembly of C-oligosaccharides via modular difunctionalization of glycals
C-oligosaccharides are found in natural products and drug molecules, but their synthesis is challenging. Here, the authors report a strategy for the stereoselective and efficient synthesis of Coligosaccharides via palladium-catalyzed nondirected C1–H glycosylation/C2-alkenylation, cyanation, and alkynylation of 2-iodoglycals with glycosyl chloride donors.
- Ya-Nan Ding
- , Mei-Ze Xu
- & Yong-Min Liang
-
Article
| Open Access Electroreduction of unactivated alkenes using water as hydrogen source
Electroreduction of unactivated alkenes using water as hydrogen source
The reduction of unactivated alkyl alkenes is a difficult challenge in organic chemistry. Here, the authors present a silicon-mediated electroreduction of alkyl alkenes, using water as a hydrogen source, enabled by [Fe]-H.
- Yanwei Wang
- , Qian Wang
- & Youai Qiu
-
Article
| Open Access Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides
Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides
The direct reductive coupling of alkyl alcohol and aryl halide enables efficient access to valuable compounds, but the asymmetric pattern remains unknown. Here the authors describe the enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohol and aryl bromide in the presence of an NHC activating agent.
- Li-Li Zhang
- , Yu-Zhong Gao
- & Ze-Peng Yang
-
Article
| Open Access Enantioselective total synthesis of (‒)-lucidumone enabled by tandem prins cyclization/cycloetherification sequence
Enantioselective total synthesis of (‒)-lucidumone enabled by tandem prins cyclization/cycloetherification sequence
The Ganoderma meroterpenoids are a growing class of natural products with a wide range of biological activities. Here, the authors report the enantioselective total synthesis of the Ganoderma meroterpenoid (‒)-lucidumone, featuring a copper-catalyzed enantioselective silicon-tethered intramolecular Diels-Alder cycloaddition to assemble the bicyclo[2.2.2]octane framework and a domino deprotection/Prins reaction/cycloetherification/oxidation sequence to generate concurrenly the tetrahydrofuran and the fused indanone skeleton.
- Xian-Zhang Liao
- , Ran Wang
- & Guang Li
-
Article
| Open Access Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
While the homo N-N coupling of two NH moieties to form the hydrazide N-N bond is well developed, the crossdehydrogenative hetero N-N coupling remains unevolved. Here the authors present an efficient, PhI(OAc)2-mediated intermolecular N-N cross-coupling of primary benzamides with primary and secondary amines.
- Subban Kathiravan
- , Prakriti Dhillon
- & Ian A. Nicholls
-
Article
| Open Access Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Carbon isotope labelling of bioactive molecules is essential for accessing the pharmacokinetic and pharmacodynamic properties of new drug entities. Here, the authors propose an electrochemical isotope-labelling protocol which enables the use of near-stoichiometric 14CO2, facilitating late-stage and single-step carbon-14 labelling of pharmaceuticals and representative precursors.
- Gabriel M. F. Batista
- , Ruth Ebenbauer
- & Troels Skrydstrup
-
Article
| Open Access A concise and scalable chemoenzymatic synthesis of prostaglandins
A concise and scalable chemoenzymatic synthesis of prostaglandins
Prostaglandins are of interest to synthetic chemists due to their biological activities. Here, the authors present a concise chemoenzymatic synthesis method for several representative prostaglandins, achieved in 5 to 7 steps, via the common intermediate bromohydrin, a radical equivalent of Corey lactone.
- Yunpeng Yin
- , Jinxin Wang
- & Jian Li
-
Article
| Open Access Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
Asymmetric synthesis of P-stereogenic phosphindane oxides via kinetic resolution and their biological activity
P-stereogenic heterocycles are privileged chiral ligands and bioactive compounds, but the catalytic asymmetric synthesis of P-stereogenic phosphindane derivatives is challenging. Here, the authors report a catalytic kinetic resolution of phosphindole oxides via rhodium-catalyzed diastereo- and enantioselective conjugate addition to access enantiopure Pstereogenic phosphindane and phosphindole derivatives.
- Long Yin
- , Jiajia Li
- & Dong Guo
-
Article
| Open Access Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction
Chemically modified nucleobases and oligonucleotides are essential in several fields but introducing functional groups into nucleobases requires laborious chemical synthesis. Here, the authors report site-selective alkylation at the C8-position of guanines in guanosine, GMP, GDP, and GTP, as well as late-stage alkylation of RNA/DNA oligonucleotides through photomediated Minisci reaction.
- Ruoqian Xie
- , Wanlu Li
- & Gang Chen
-
Article
| Open Access Targeted sampling of natural product space to identify bioactive natural product-like polyketide macrolides
Targeted sampling of natural product space to identify bioactive natural product-like polyketide macrolides
Polyketide macrolides are of interest for drug discovery but their inherent structural and stereochemical complexity hinders the exploration of related regions of chemical space more broadly. Here, the authors designed in silico and synthesized a library of tetrahydrofuran-containing polyketide macrolides, and screened them against a panel of biological assays, identifying biologically active library members.
- Darryl M. Wilson
- , Daniel J. Driedger
- & Robert A. Britton
-
Article
| Open Access Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Photo-induced intramolecular dearomative [5 + 4] cycloaddition of arenes for the construction of highly strained medium-sized-rings
Medium-sized-ring compounds are challenging synthetic targets in organic chemistry. Here the authors report an intramolecular dearomative [5 + 4] cycloaddition of naphthalene-derived vinylcyclopropanes to construct these molecules under visible-light irradiation and a proper triplet photosensitizer.
- Min Zhu
- , Yuan-Jun Gao
- & Shu-Li You
-
Article
| Open Access 2H-Thiopyran-2-thione sulfine, a compound for converting H2S to HSOH/H2S2 and increasing intracellular sulfane sulfur levels
2H-Thiopyran-2-thione sulfine, a compound for converting H2S to HSOH/H2S2 and increasing intracellular sulfane sulfur levels
Reactive sulfane sulfur species such as persulfides and H2S2 are important redox regulators and linked to H2S signaling, but their study is hindered by a lack of suitable donors to produce them. Here, the authors report 2H-thiopyran-2-thione sulfine (TTS), a compound which can specifically convert H2S to HSOH, and then to H2S2 in the presence of excess H2S.
- Qi Cui
- , Meg Shieh
- & Ming Xian
-
Article
| Open Access Synthesis of meta-carbonyl phenols and anilines
Synthesis of meta-carbonyl phenols and anilines
Phenols and anilines are of extreme importance for medicinal chemistry and material science but the selective preparation of meta-substituted phenols and anilines remains challenging. Here the authors report an efficient copper-catalyzed dehydrogenation strategy to exclusively synthesize meta-carbonyl phenols and anilines from carbonyl substituted cyclohexanes.
- Bao-Yin Zhao
- , Qiong Jia
- & Yong-Qiang Wang
-
Article
| Open Access N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
N-Heterocyclic carbene-catalyzed enantioselective synthesis of planar-chiral cyclophanes via dynamic kinetic resolution
Planar-chiral cyclophanes are important for drug discovery but the enantioselective synthesis of planar-chiral cyclophanes remains challenging. Here the authors describe an N-heterocyclic carbene-catalyzed asymmetric construction of planar-chiral cyclophanes.
- Jiayan Li
- , Ziyang Dong
- & Changgui Zhao
-
Article
| Open Access Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Valence-isomer selective cycloaddition reaction of cycloheptatrienes-norcaradienes
Combining data science and organic synthesis to achieve the rapid and precise creation of complex molecules while controlling multiple selectivities is an emerging trend, but few successful examples are reported. Here, the authors develop an artificial neural network regression model using bond orbital data to predict chemical reactivities.
- Shingo Harada
- , Hiroki Takenaka
- & Tetsuhiro Nemoto
-
Article
| Open Access Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis
Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis
Characterizing highly-reactive glycosyl cation intermediates and understanding their glycosylation mechanisms are essential to the stereoselective synthesis of complex carbohydrates. Here the authors report a workflow that is utilized to characterize rhamnosyl 1,3-bridged dioxanium ions derived from C-3 p-anisoyl esterified donors.
- Peter H. Moons
- , Floor ter Braak
- & Thomas J. Boltje
-
Article
| Open Access Enantioselective functionalization of unactivated C(sp3)–H bonds through copper-catalyzed diyne cyclization by kinetic resolution
Enantioselective functionalization of unactivated C(sp3)–H bonds through copper-catalyzed diyne cyclization by kinetic resolution
Site- and stereoselective C–H functionalization is challenging in the synthetic chemistry, and the catalytic enantioselective C(sp3)–H functionalization based on vinyl cations has scarcely explored. Here, the authors report an asymmetric copper-catalyzed tandem diyne cyclization/unactivated C(sp3)–H insertion reaction via a kinetic resolution.
- Yang-Bo Chen
- , Li-Gao Liu
- & Long-Wu Ye
-
Article
| Open Access Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Cobalt catalyzed practical hydroboration of terminal alkynes with time-dependent stereoselectivity
Stereodefined vinylboron compounds are important organic synthons, but the selective generation of the thermodynamically unfavorable Z-isomers remains challenging. Here, the authors develop a cobalt catalytic system, enabling the transformation of terminal alkynes with excellent Z-selectivity, even in the presence of bulky substituents.
- Jinglan Wen
- , Yahao Huang
- & Peng Hu
-
Article
| Open Access Identification of a potent palladium-aryldiphosphine catalytic system for high-performance carbonylation of alkenes
Identification of a potent palladium-aryldiphosphine catalytic system for high-performance carbonylation of alkenes
The development of stable and efficient ligands is of vital significance to enhance the catalytic performance of carbonylation reactions of alkenes. Here, the authors develop an aryldiphosphine ligand, used in palladium-catalyzed regioselective carbonylation of alkenes, exhibiting high catalytic performance and strong oxygen-resistance stability.
- Kang Zhao
- , Hongli Wang
- & Feng Shi
-
Article
| Open Access Silver-catalyzed direct conversion of epoxides into cyclopropanes using N-triftosylhydrazones
Silver-catalyzed direct conversion of epoxides into cyclopropanes using N-triftosylhydrazones
Epoxides are prominent small-ring O-heterocycles found in a variety of bioactive natural products and pharmaceuticals. Here, the authors report a silver carbene strategy to achieve O-to-C atom exchange of epoxides in a single step, affording diverse fluoroalkylcyclopropanes.
- Linxuan Li
- , Paramasivam Sivaguru
- & Xihe Bi
-
Article
| Open Access On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
On-surface synthesis relies on carefully designed molecular precursors that are thermally activated to afford desired, covalently coupled architectures. Here, the authors study the intramolecular reactions of vinyl groups in a poly-para-phenylene-based model system and provide a comprehensive description of the reaction steps taking place on the Au(111) surface under ultrahigh vacuum conditions.
- Marco Di Giovannantonio
- , Zijie Qiu
- & Roman Fasel
-
Article
| Open Access Dimethyl sulfate and diisopropyl sulfate as practical and versatile O-sulfation reagents
Dimethyl sulfate and diisopropyl sulfate as practical and versatile O-sulfation reagents
O-Sulfation is a vital post-translational modification in bioactive molecules, yet there are significant challenges for the synthesis. Here, the authors report a general and robust approach to O-sulfation by harnessing the tunable reactivity of dimethyl sulfate or diisopropyl sulfate under tetrabutylammonium bisulfate activation.
- Shuaishuai Yue
- , Guoping Ding
- & Jiakun Li
-
Article
| Open Access Dioxygen compatible electron donor-acceptor catalytic system and its enabled aerobic oxygenation
Dioxygen compatible electron donor-acceptor catalytic system and its enabled aerobic oxygenation
The photochemical properties of Electron Donor-Acceptor (EDA) complexes present exciting opportunities, but often require an inert atmosphere to maintain high efficiency. Here, the authors develop a photocatalytic system through rational design, which overcomes the oxygen-sensitive limitation of traditional EDA photocatalytic systems.
- Jialiang Wei
- , Junhong Meng
- & Ning Jiao
-
Article
| Open Access General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs
General alkyl fluoride functionalization via short-lived carbocation-organozincate ion pairs
Many alkyl fluorides are readily available, but the synthetic applications trail behind the widely accepted utility of other halides. Here, the authors report a practical Csp3-F bond functionalization method that expands the currently restricted synthetic space of unactivated C(sp3)-F bonds, but also uses benzylic, propargylic and acyl fluorides.
- D. Lucas Kane
- , Bryan C. Figula
- & Christian Wolf
-
Article
| Open Access Zirconium and hafnium catalyzed C–C single bond hydroboration
Zirconium and hafnium catalyzed C–C single bond hydroboration
Selective cleavage and subsequent functionalization of C−C single bonds present a fundamental challenge, and the use of inexpensive and Earth-abundant group IV metals for catalytic C−C single-bond cleavage is largely underdeveloped. Here, the authors report zirconium-catalyzed C−C single-bond cleavage and subsequent hydroboration reactions.
- Sida Li
- , Haijun Jiao
- & Lipeng Wu
-
Article
| Open Access Nickel-catalyzed electrophiles-controlled enantioselective reductive arylative cyclization and enantiospecific reductive alkylative cyclization of 1,6-enynes
Nickel-catalyzed electrophiles-controlled enantioselective reductive arylative cyclization and enantiospecific reductive alkylative cyclization of 1,6-enynes
Transition metal-catalyzed asymmetric cyclization of 1,6-enynes is a powerful tool for the construction of chiral nitrogen-containing heterocycles, but limiting to the use of aryl or alkenyl metal reagents. Here, the authors report Ni-catalyzed enantioselective anti-arylative cyclization and enantiospecific cis-alkylative cyclization of 1,6-enynes.
- Wenfeng Liu
- , Yunxin Xing
- & Kun Shen
-
Article
| Open Access Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
Remote-carbonyl-directed sequential Heck/isomerization/C(sp2)–H arylation of alkenes for modular synthesis of stereodefined tetrasubstituted olefins
The synthesis of highly substituted olefins has been pursued for decades. Here, the authors show a carbonyl-directed sequential Heck/isomerization/C–H arylation of alkenes for modular synthesis of stereodefined all-carbon tetrasubstituted olefins.
- Runze Luan
- , Ping Lin
- & Weiping Su
-
Article
| Open Access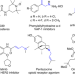 Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
Cooperative Cu/azodiformate system-catalyzed allylic C–H amination of unactivated internal alkenes directed by aminoquinoline
The oxidative intermolecular amination of C-H bonds represents a straightforward method to construct aliphatic allylic amines. However, the utilization of widely available internal alkenes remains a synthetic challenge. Here, the authors present a regioselective Cu-catalyzed oxidative allylic C-H amination of internal olefins with azodiformates.
- Le Wang
- , Cheng-Long Wang
- & Shu-Yu Zhang
-
Article
| Open Access Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Metal-free photocatalytic cross-electrophile coupling enables C1 homologation and alkylation of carboxylic acids with aldehydes
Enhancing the sp3-hybridized character of molecular structures in drug discovery is paramount. Here, the authors introduce a deoxygenative cross-electrophile coupling technique that pairs easily accessible carboxylic acid-derived redox-active esters with aldehyde sulfonyl hydrazones, employing Eosin Y as an organophotocatalyst under visible light irradiation.
- Stefano Bonciolini
- , Antonio Pulcinella
- & Timothy Noël
-
Article
| Open Access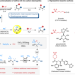 Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay
Stoichiometric amounts of metal reductant and the requirement of a directing group limit the application of transition metal-catalyzed reductive difunctionalization of alkenes with alkyl halides. Here, the authors report a reductive difunctionalization of alkenes via a radical-anion relay with Na2S2O4 as both reductant and sulfone-source.
- Xiaoya Hou
- , Hongchi Liu
- & Hanmin Huang
-
Review Article
| Open Access Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids
α,α-Disubstituted α-amino acids (α-AAs) have improved properties compared to other amino acids. Here, the authors provide an overview of the recent advancements since 2015 and discuss existing challenges for their synthesis.
- Yu Zhang
- , Jaro Vanderghinste
- & Shoubhik Das
-
Article
| Open Access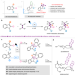 Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Conformationally rigid 3D molecules are of high interest. Here the authors develop intermolecular aza-Paternò-Büchi reactions to access ladder-shape azetidine-fused indoline pentacycles with excellent regiodivergency and stereoselectivity.
- Jianjian Huang
- , Tai-Ping Zhou
- & Fangrui Zhong
-
Article
| Open Access Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Photoexcitation of the electron-donor-acceptor (EDA) complexes are an effective approach to achieve radicals by triggering electron transfer but the catalytic version of EDA complex photoactivation remains underdeveloped. Here, the authors introduce 4-nitrophthalonitrile as an electron acceptor to facilitate π-stacking with electron-rich aromatics to form EDA complexes.
- Ting Xue
- , Cheng Ma
- & Rong Zeng
-
Article
| Open Access Cross-catenation between position-isomeric metallacages
Cross-catenation between position-isomeric metallacages
The study of cross-catenated metallacages could provide facile insights into achieving more precise control over low-symmetry/high-complexity hierarchical assembly systems but is currently lacking. Here, the authors report a cross-catenane formed between two position-isomeric Pt(II) metallacages in the solid state.
- Yiliang Wang
- , Taotao Liu
- & Jun Li
-
Article
| Open Access Water-stable boroxine structure with dynamic covalent bonds
Water-stable boroxine structure with dynamic covalent bonds
Despite the structural significance of boroxines in different classes of materials, their applicability in aqueous media is limited by their hydrolytic instability. Here, the authors discovered a water-stable boroxine structure with excellent pH stability and water-compatible dynamic covalent bonds.
- Xiaopei Li
- , Yongjie Zhang
- & Guangyan Qing
-
Article
| Open Access Carbene organic catalytic planar enantioselective macrolactonization
Carbene organic catalytic planar enantioselective macrolactonization
Macrolactones exhibit distinct conformational and configurational properties and are widely found in natural products but catalysts to govern both macrolactone formation and stereochemical control remain largely unexplored. Here, the authors disclose an N-heterocyclic carbene (NHC)-catalyzed enantioselective synthesis of chiral macrolactones varying in ring size from sixteen to twenty members that feature distinct configurationally stable planar stereogenicity.
- Xiaokang Lv
- , Fen Su
- & Yonggui Robin Chi
-
Article
| Open Access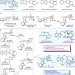 Collective total synthesis of stereoisomeric yohimbine alkaloids
Collective total synthesis of stereoisomeric yohimbine alkaloids
Nature generates the stereoisomeric yohimbine alkaloids using bioavailable monoterpene secolaganin as the building block. Here, the authors reset the stage by the development of a bioinspired coupling, in which the pentacyclic skeleton is constructed through enantioselective kinetic resolution of an achiral, easily accessible synthetic surrogate.
- Meiyi Tang
- , Haigen Lu
- & Liansuo Zu

 Pathway-divergent coupling of 1,3-enynes with acrylates through cascade cobalt catalysis
Pathway-divergent coupling of 1,3-enynes with acrylates through cascade cobalt catalysis