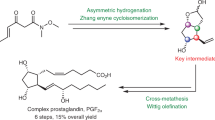
Abstract
Prostaglandins have garnered significant attention from synthetic chemists due to their exceptional biological activities. In this report, we present a concise chemoenzymatic synthesis method for several representative prostaglandins, achieved in 5 to 7 steps. Notably, the common intermediate bromohydrin, a radical equivalent of Corey lactone, is chemoenzymatically synthesized in only two steps, which allows us to complete the synthesis of prostaglandin F2α in five steps on a 10-gram scale. The chiral cyclopentane core is introduced with high enantioselectivity, while the lipid chains are sequentially incorporated through a cost-effective process involving bromohydrin formation, nickel-catalyzed cross-couplings, and Wittig reactions. This cost-efficient synthesis route for prostaglandins holds the potential to make prostaglandin-related drugs more affordable and facilitate easier access to their analogues.
Similar content being viewed by others
Introduction
Natural prostaglandins (PGs) are a family of lipid compounds generated from arachidonic acid by cyclooxygenase (COX)1. Due to the unique biological properties and the medicinal value of prostaglandins (PGs), they have gained extensive attention from medicinal chemists. However, natural prostaglandin molecules often have poor chemical stability, rapid in vivo metabolism, and are frequently associated with corresponding side effects, posing challenges for direct medical use2. In recent years, medicinal chemists have developed a series of structurally diverse prostaglandin derivatives and analogs based on this molecular framework to attempt to address these issues (Fig. 1). More than 20 prostaglandin-class drugs have been successfully approved for the treatment of various diseases, with global annual sales amounting to billions of dollars, indicating broad demand and a huge market. Therefore, developing concise and efficient routes for synthesizing prostaglandins and related drugs is of great significance in meeting market demands, reducing medication costs, and facilitating new drug development.
In the decades following the landmark synthesis of prostaglandin F2α by the Corey laboratory3,4,5,6,7,8,9,10, synthetic chemists have developed a number of efficient synthetic approaches using Corey lactone as a key intermediate for the synthesis of various prostaglandins and their analogues11,12,13,14,15,16,17,18,19,20,21,22,23,24. Chen research group has previously accomplished the shortest synthesis route for Corey lactone23,24, achieving the asymmetric synthesis of Corey lactone in just four steps starting from commercially available starting materials. A collection of highly effective synthetic methodologies, developed by more than 20 research teams from prestigious laboratories, including those led by Stork, Woodward, Nicolaou, Danishefsky, Carreira, Noyori, Aggarwal, and Zhang have been established for crafting novel synthesis pathways for prostaglandins and their derivatives25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57. These approaches notably bypass the use of the traditional Corey lactone intermediates. There are two strategies that are representative in terms of efficiency: the Zhang lab employed several elegant noble metal catalytic reactions, such as iridium, rhodium, palladium, ruthenium, to accomplish the scalable synthesis of prostaglandins47. On the other hand, the Aggarwal lab utilized an efficient organocatalytic chemistry and completed the synthesis of prostaglandins in only seven steps33.In these synthetic reports, the radical cleavage strategy by the Baran group22 and the Baeyer–Villiger reaction-based strategy by the Chen group23,24 are very inspirational to the retrosynthetic analysis of this work. Due to Chen lab’s use of a dichloro precursor as their substrate, their synthesis process totaled eight steps to reach prostaglandin F2α. Meanwhile, Baran lab opted for the more expensive Corey lactone, which requires at least four steps to synthesize from a cost-efficient starting material. In the past decade, chemists have developed many excellent conditions for radical reactions for C–C bond formation, making radical disconnection an important complement to its polar and pericyclic counterparts in retrosynthetic logic58. In recent years, chemoenzymatic strategies have also been employed by organic chemists for the synthesis of complex molecules59. Renata lab has pioneered the merger of chemoenzymatic and radical-based retrosynthetic logic, and simplified the synthesis of meroterpenoid natural products60. Thereby, we believe that there is room for efficiency improvement in the synthesis of prostaglandins through a design that combines both free radical approach and enzymatic methods.
In our retrosynthetic analysis of prostaglandins (Fig. 2a), the cis double bonds in prostaglandins would be installed using classic Wittig reaction, a polar disconnection. Next, we conceived a nickel-catalyzed reductive coupling, a radical approach, for the installation of the trans double bond side chains. This disconnection entailed that bromohydrin 8 would be used as a radical version of Corey lactone as a common intermediate for prostaglandins. In turn, 8 could be obtained by simply treating chiral lactone 9 with NBS and water. For the synthesis of chiral lactone 9, two synthetic strategies were devised. The first one involved an in vivo enzymatic Baeyer–Villiger oxidation in an enantiomer divergent fashion. Although the strategy enables the one-step preparation of lactone 9, the in vivo biotransformation reaction is more challenging to achieve in traditional chemistry laboratories or medicinal chemistry laboratories. Therefore, we developed a supplementary strategy that utilizes commercially available lipase to prepare chiral compound 11, followed by a Johnson–Claisen rearrangement reaction to obtain the lactone 9. Based on our retrosynthetic analysis, the synthesis for Corey lactone equivalent 8 can be achieved in just two steps. Meanwhile, by utilizing free radical chemistry, the entirety of the trans-olefin side chains can be installed in one step, which would result in an overall reduction in total step count (Fig. 2b). Here, we report a concise and scalable chemoenzymatic synthesis of prostaglandins by merging chemoenzymatic and radical-based retrosynthetic logic.
Results
Two methods for the preparation of chiral lactone 9
To synthesize the required chiral lactone compounds (Fig. 3), a Johnson–Claisen rearrangement strategy was first developed. The achiral diol 12 can be obtained commercially at a price of $13 per gram. The asymmetric synthesis of 11 has been achieved through lipase-mediated desymmetrization61, resulting in mono-acetate product 11 with an enantiomeric excess of 95%. The mono-acetate product 11 can be transformed into the Johnson–Claisen rearrangement product 13 by using triethyl orthoacetate as the solvent and catalyzed by o-nitrophenol. The resulting product, without the need for separation, was directly treated with K2CO3 and MeOH to obtain lactone 9 in one pot. Compared to similar strategies in the literature, our modification reduced the number of steps by half62. Although this strategy can yield 13.2 g of lactone 9 in one run and performed in any chemistry laboratory, the expensive starting materials, and the need for prolonged high-temperature heating in the Johnson–Claisen rearrangement step make it difficult to further scale-up. Therefore, an enzymatic oxidative resolution was explored method using a more affordable racemic cyclobutanone 10, available at a price of $2.3 per gram. Several enzymatic Baeyer–Villiger reactions63,64,65 of cyclobutanone 10 have been reported, including a scale-up of this reaction to 2.5 g of lactone products per kilogram of cell broth66. In our preliminary study, the incorporation of an NADPH regeneration system increased the concentration of the products in the cell broth to 9.3 g/L. We opted to construct an E. coli strain that co-expresses glucose dehydrogenase with CHMO. In this system, CHMOrhodo1 achieved a product concentration of 32 mM. In subsequent optimizations, it was discovered that the capacity of glucose dehydrogenase to regenerate NADPH was significantly lower than the efficiency of CHMOrhodo1 in our reaction system. Upon switching to the phosphite dehydrogenase Opt-13 developed by the Zhao group67, complete conversion of cyclobutanone to the product was achieved at concentrations of 40 mM and 83 mM, respectively. Control experiments demonstrated that as reaction concentrations continued to rise, the reaction activity began to decrease sharply due to the influence of high concentration of sodium phosphite. Therefore, while scaling up, the reaction can be run at 83 mM with full conversion, allowing the preparation of over 100 g of lactone product 9. The ee values of the lactone compounds 9 and 14 obtained in the various NADPH regeneration systems and reactions of different scales mentioned above remained essentially same: 95% ee for lactone 9 and 97% ee for lactone 14.
Bromohydrin formation and radical connection of ω-chains
The formation of bromohydrin 8 proved to be more challenging than expected. In the literature, the reaction of the same substrate with NBS-water system has been studied, showing that the reaction typically affords the undesired bromohydrin diastereomer68. Inspired by refs. 69,70,71,72, DMSO was used as a Lewis base to alter the behavior of the bromonium ion. In the presence of DMSO, a reversal in the direction of bromonium formation was observed. Upon further screening of other co-solvent system, the reaction’s overall yield and the ratio of our desired product reached their highest levels when DMSO was used as a co-solvent with chloroform (Fig. 4a). With bromide 8 in hand, the radical coupling reaction of the first sidechain was tested. Since the pioneering work of the Weix research group73,74,75, a wide range of conditions for nickel-catalyzed reductive coupling reactions have been reported76. Some examples showed direct involvement of bromohydrins in the coupling reaction77. The condition reported by the Gong group was attempted77, which fortunately afforded the coupling product in 49% yield (Fig. 4b). After analyzing the composition of the products, we found that due to the weak alkaline nature of the coupling conditions, one of the main by-products is compound 17, a product of epoxide ring closure. Catalytic amount of bidentate ligand 18 were employed as a replacement for the equivalent use of pyridine; however, the reaction produced more epoxide. To fundamentally suppress the formation of epoxides, in Entry 3, 1.1 equivalents of N-(Trimethylsilyl)imidazole was added to protect the hydroxyl group in situ. We anticipate that the one equivalent of imidazole generated in situ, as a product of the temporary protection of hydroxyl groups by N-(Trimethylsilyl)imidazole, can act as a ligand in the reaction. Entry 3 provided the coupling product in 52% yield along with 37% yield of the elimination product 9. To our delight, under this condition no epoxidation formation was found. Considering that imidazole may not serve as a good ligand, upon adding an equivalent amount of pyridine or 15% of a bidentate ligand 18 together with N-(Trimethylsilyl)imidazole, the reaction yield increased to above 83%. The substrate scope was then expanded based on the optimal conditions, allowing the installation of three different side chains of prostaglandins with high yields. Due to the cost consideration that ligand prices are significantly higher than the nickel source in nickel-catalyzed reactions, we opted for coupling conditions with an equivalent amount of pyridine for subsequent reaction scale-up. In addition, coupling reactions with aromatic and heterocyclic substrates were attempted, which also resulted in a high yield of the coupled products using ligand 19.
a Screening the optimal condition for bromohydrin 8 formation reaction. b Screening the optimal condition for reductive coupling reaction of bromohydrin 8. c Bidentate ligands used in this work. d Reaction scope of reductive coupling reaction of bromohydrin 8. NBS N-bromosuccinimide, DMSO dimethyl sulfoxide, DMA dimethylacetamide, TSIM N-(trimethylsilyl)imidazole.
Completing the syntheses of prostaglandins
After the optimization of chiral skeleton construction and key radical coupling reaction, the remaining steps were relatively straightforward. During the scale-up process, the yield of the bromohydroxylation reaction and nickel-catalyzed reductive coupling reactions remained consistent. Furthermore, through recrystallization bromohydrin 8 was obtained with a yield of 86% and an enantiomeric excess of greater than 99%. The nickel-catalyzed reductive coupling reactions exhibited a decreased yield of 73% at a 10-g scale, allowing us to obtain 12 g of product 7 in a single batch. After obtaining compound 7, some improvements were made to known methods and completed the 10-g scale synthesis of prostaglandin F2α through lactone reduction and Wittig reaction (Fig. 5a). It is worth noting that we synthesized 10.6 g of prostaglandin F2α using only 14.2 g of lactone compound 9. Using a similar strategy, we finished the synthesis of 2.13 g of fluprostenol, 1.89 g of bimatoprost, 1.82 g of cloprostenol, and 1.29 g of latanoprost (Fig. 5b, c), each of the synthesis is initiated with 1.6 g of lactone compound 9. For the synthesis of latanoprost, to avoid the use of noble metals, Raney nickel was used as a catalyst and obtained the product of double bond hydrogenation in 99% yield.
Discussion
In summary, We have achieved a scalable synthesis of prostaglandins with high enantioselectivity using cost-effective commercial starting materials. Our synthesis of prostaglandins represents one of the shortest route reported to date, among which the synthesis of prostaglandin F2α was accomplished in just five steps. Two distinct methodologies for the synthesis of chiral lactone 9 were developed: (1) a Johnson–Claisen strategy, which is readily feasible in any chemistry laboratory, and (2) an enzymatic oxidative resolution strategy that is more scalable and cost-effective. Furthermore, by incorporating a radical-based strategic bond disconnection, we can divergently synthesize various prostaglandin drugs through nickel-catalyzed reductive couplings and olefination reactions. The route has high industrial application value due to its cost-effectiveness and the fact that it does not utilize any noble metals.
Methods
General procedure for biocatalytic oxidation with recombinant E. coli
An overnight culture of E. coli BL21(DE3) cells harboring pET-22b(+)-based vector for expressing the appropriate CHMOrhodo1 and pRSF-Opt-13 plasmid was used to inoculate 500 mL TB media (in 2 L Erlenmeyer flask) containing 50 µg/mL kanamycin, 50 µg/mL ampicillin. The cultures were shaken at 250 rpm at 37 °C until an optical density of OD600 = 0.7–1.0 was reached. The cultures were cooled on ice for 20 min and then induced with riboflavin and IPTG to final concentrations of 1.0 µM and 0.5 mM, respectively. The cultures were shaken at 150 rpm at 20 °C for a further 20 h. Cells were harvested by centrifugation (4 °C, 15 min, 4121 g), and resuspended in 500 mL kPi buffer (50 mM, pH = 8.00) to an OD600 = 10 into a 2 L Erlenmeyer flask. To the mixture was sequentially added a pre-dissolved solution of 4.5 g ketone 10 in 25 mL DMSO, Na2NADP · 4H2O (654 mg, 0.83 mmol), Na2HPO3 · 5H2O (9.3 g, 43.0 mmol). The Erlenmeyer flask was shaken at 150 rpm at 25 °C for 20 h. The mixture was extracted with EtOAc (300 mL × 3), and the combined organic extracts were concentrated in vacuo gives 1:1 mixture of lactone 9 and 14.
Data availability
Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 2312333 (8) and CCDC 2319091 (21). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. All other characterization data and detailed experimental procedures are available in the supplementary materials.
References
Gibson, K. H. Prostaglandins, thromboxanes, PGX: biosynthetic products from arachidonic acid. Chem. Soc. Rev. 6, 489–510 (1977).
Peng, H. & Chen, F.-E. Recent advances in asymmetric total synthesis of prostaglandins. Org. Biomol. Chem. 15, 6281–6301 (2017).
Corey, E. J., Weinshenker, N. M., Schaaf, T. K. & Huber, W. Stereo-controlled synthesis of prostaglandins F2α and E2 (dl). J. Am. Chem. Soc. 91, 5675–5677 (1969).
Corey, E. J., Schaaf, T. K., Huber, W., Koelliker, U. & Weinshenker, N. M. Total synthesis of prostaglandins F2α and E2 as the naturally occurring forms. J. Am. Chem. Soc. 92, 397–398 (1970).
Corey, E. J., Noyori, R. & Schaaf, T. K. Total synthesis of prostaglandins F1α, E1, F2α, and E2 (natural forms) from a common synthetic intermediate. J. Am. Chem. Soc. 92, 2586–2587 (1970).
Corey, E. J. et al. Stereospecific total synthesis of prostaglandins E3 and F3α. J. Am. Chem. Soc. 93, 1490–1491 (1971).
Schaaf, T. K. & Corey, E. J. Total synthesis of prostaglandins F1α and E1. J. Org. Chem. 37, 2921–2922 (1972).
Corey, E. J. & Ensley, H. E. Preparation of an optically active prostaglandin intermediate via asymmetric induction. J. Am. Chem. Soc. 97, 6908–6909 (1975).
Corey, E. J., Imai, N. & Pikul, S. Catalytic enantioselective synthesis of a key intermediate for the synthesis of prostanoids. Tetrahedron Lett. 32, 7517–7520 (1991).
Corey, E. J. & Loh, T. P. First application of attractive intramolecular interactions to the design of chiral catalysts for highly enantioselective Diels-Alder reactions. J. Am. Chem. Soc. 113, 8966–8967 (1991).
Yankee, E. W., Axen, U. & Bundy, G. L. Total synthesis of 15-methylprostaglandins. J. Am. Chem. Soc. 96, 5865–5876 (1974).
Schaaf, T. K. et al. N-(Methanesulfonyl)−16-phenoxyprostaglandin carboxamides: tissue-selective, uterine stimulants. J. Med. Chem. 24, 1353–1359 (1981).
Ogawa, Y., Nunomoto, M. & Shibasaki, M. A novel synthesis of prostaglandin D2. J. Org. Chem. 51, 1625–1627 (1986).
Doyle, M. P. & Catino, A. J. A short stereoselective synthesis of (+)- and (−)−2-oxabicyclo[3.3.0]oct-6-en-3-one by intramolecular carbon–hydrogen insertion catalyzed by chiral dirhodium(II) carboxamidates. Tetrahedron. Asymmetry 14, 925–928 (2003).
Matsumura, Y. et al. Synthesis of the highly potent prostanoid FP receptor agonist, AFP-168: a novel 15-deoxy-15,15-difluoroprostaglandin F2α derivative. Tetrahedron Lett. 45, 1527–1529 (2004).
Ohta, C. et al. An improved synthesis of the selective EP4 receptor agonist ONO-4819. J. Org. Chem. 74, 8298–8308 (2009).
Ramón, R. S. et al. Gold-catalyzed Meyer−Schuster rearrangement: application to the synthesis of prostaglandins. Organometallics 29, 3665–3668 (2010).
Chen, Y., Yan, H., Chen, H.-X., Weng, J. & Lu, G. An improved and efficient process for the preparation of (+)-cloprostenol. Chirality 27, 392–396 (2015).
Krupa, M., Chodyński, M., Ostaszewska, A., Cmoch, P. & Dams, I. A novel convergent synthesis of the potent antiglaucoma agent tafluprost. Molecules 22, 217 (2017).
Umekubo, N., Suga, Y. & Hayashi, Y. Pot and time economies in the total synthesis of corey lactone. Chem. Sci. 11, 1205–1209 (2020).
Umekubo, N. & Hayashi, Y. Asymmetric synthesis of corey lactone and latanoprost. Eur. J. Org. Chem. 39, 6221–6227 (2020).
Edwards, J. T. et al. Decarboxylative alkenylation. Nature 545, 213–218 (2017).
Zhu, K., Hu, S., Liu, M., Peng, H. & Chen, F. E. Access to a key building block for the prostaglandin family via stereocontrolled organocatalytic Baeyer–Villiger oxidation. Angew. Chem. Int. Ed. 58, 9923–9927 (2019).
Zhu, K. et al. A unified strategy to prostaglandins: chemoenzymatic total synthesis of cloprostenol, bimatoprost, PGF2α, fluprostenol, and travoprost guided by biocatalytic retrosynthesis. Chem. Sci. 12, 10362–10370 (2021).
Sih, C. J. et al. Total synthesis of prostaglandins. II. Prostaglandin E1. J. Am. Chem. Soc. 94, 3643–3644 (1972).
Woodward, R. B. et al. Novel synthesis of prostaglandin F2α. J. Am. Chem. Soc. 95, 6853–6855 (1973).
Stork, G. & Isobe, M. A general approach to prostaglandins via methylenecyclopentanones. Total synthesis of (±)-prostaglandin F2α. J. Am. Chem. Soc. 97, 4745–4746 (1975).
Stork, G. & Isobe, M. Simple total synthesis of prostaglandins from 4-cumyloxy-2-cyclopentenone. J. Am. Chem. Soc. 97, 6260–6261 (1975).
Stork, G., Sher, P. M. & Chen, H. L. Radical cyclization-trapping in the synthesis of natural products. A simple, stereocontrolled route to prostaglandin F2α. J. Am. Chem. Soc. 108, 6384–6385 (1986).
Noyori, R. & Suzuki, M. Prostaglandin syntheses by three-component coupling. New synthetic methods. Angew. Chem. Int. Ed. 23, 847–876 (1984).
Suzuki, M., Yanagisawa, A. & Noyori, R. An extremely short way to prostaglandins. J. Am. Chem. Soc. 107, 3348–3349 (1985).
Danishefsky, S. J., Cabal, M. P. & Chow, K. Novel stereospecifc silyl group transfer reactions: practical routes to the prostaglandins. J. Am. Chem. Soc. 111, 3456–3457 (1989).
Coulthard, G., Erb, W. & Aggarwal, V. K. Stereocontrolled organocatalytic synthesis of prostaglandin PGF2α in seven steps. Nature 489, 278–281 (2012).
Prévost, S. et al. Synthesis of prostaglandin analogues, latanoprost and bimatoprost, using organocatalysis via a key bicyclic enal intermediate. Org. Lett. 17, 504–507 (2015).
Baars, H., Classen, M. J. & Aggarwal, V. K. Synthesis of alfaprostol and PGF2α through 1,4-addition of an alkyne to an enal intermediate as the key step. Org. Lett. 19, 6008–6011 (2017).
Pelšs, A. et al. Reoptimization of the organocatalyzed double aldol domino process to a key enal intermediate and its application to the total synthesis of Δ12-prostaglandin J3. Chem. Eur. J. 24, 9542–9545 (2018).
Jing, C. & Aggarwal, V. K. Total synthesis of thromboxane B2 via a key bicyclic enal intermediate. Org. Lett. 22, 6505–6509 (2020).
Bennett, S. H. & Aggarwal, V. K. Organocatalytic dimerization of succinaldehyde. Org. Synth. 99, 139–158 (2022).
Bennett, S. H., Coulthard, G. & Aggarwal, V. K. Prostaglandin total synthesis enabled by the organocatalytic dimerization of succinaldehyde. Chem. Rec. 20, 936–947 (2020).
Jing, C. et al. Synthesis, stability, and biological studies of fluorinated analogues of thromboxane A2. ACS Cent. Sci. 6, 995–1000 (2020).
Yi, X. et al. Asymmetric total synthesis of prostaglandin C2 TBS ether. Chem. Commun. 58, 6000–6003 (2022).
Wang, Z.-S. et al. De novo synthesis of dihydrobenzofurans and indolines and its application to a modular, asymmetric synthesis of beraprost. J. Am. Chem. Soc. 145, 14124–14132 (2023).
Hayashi, Y. & Umemiya, S. Pot economy in the synthesis of prostaglandin A1 and E1 methyl esters. Angew. Chem. Int. Ed. 52, 3450–3452 (2013).
Umemiya, S., Sakamoto, D., Kawauchi, G. & Hayashi, Y. Enantioselective total synthesis of beraprost using organocatalyst. Org. Lett. 19, 1112–1115 (2017).
Kawauchi, G., Umemiya, S., Taniguchi, T., Monde, K. & Hayashi, Y. Enantio- and diastereoselective synthesis of latanoprost using an organocatalyst. Chem. Eur. J. 24, 8409–8414 (2018).
Kawauchi, G., Suga, Y., Toda, S. & Hayashi, Y. Organocatalyst-mediated, pot-economical total synthesis of latanoprost. Chem. Sci. 14, 10081–10086 (2023).
Zhang, F. et al. Concise, scalable and enantioselective total synthesis of prostaglandins. Nat. Chem. 13, 692–697 (2021).
Egger, J., Bretscher, P., Freigang, S., Kopf, M. & Carreira, E. M. Synthesis of epoxyisoprostanes: effects in reducing secretion of pro-inflammatory cytokines IL-6 and IL-12. Angew. Chem. Int. Ed. 52, 5382–5385 (2013).
Egger, J., Bretscher, P., Freigang, S., Kopf, M. & Carreira, E. M. Discovery of a highly potent anti-inflammatory epoxyisoprostane-derived lactone. J. Am. Chem. Soc. 136, 17382–17385 (2014).
Egger, J. et al. Total synthesis of prostaglandin 15d-PGJ2 and investigation of its effect on the secretion of IL-6 and IL-12. Org. Lett. 17, 4340–4347 (2015).
Wolleb, H. et al. Synthesis and structure–activity relationship studies of anti- inflammatory epoxyisoprostane analogues. Org. Lett. 20, 3014–3016 (2018).
Nicolaou, K. C. et al. Total synthesis of Δ12-prostaglandin J3, a highly potent and selective antileukemic agent. Angew. Chem. Int. Ed. 53, 10443–10447 (2014).
Nicolaou, K. C. et al. Synthesis and biological investigation of Δ12-prostaglandin J3 (Δ12-PGJ3) analogues and related compounds. J. Am. Chem. Soc. 138, 6550–6560 (2016).
Nicolaou, K. C. et al. Total synthesis of Δ12-prostaglandin J3: evolution of synthetic strategies to a streamlined process. Chem. Eur. J. 22, 8559–8570 (2016).
Kučera, R., Goetzke, F. W. & Fletcher, S. P. An asymmetric Suzuki-Miyaura approach to prostaglandins: synthesis of tafluprost. Org. Lett. 22, 2991–2994 (2020).
Cunningham, L., Mishra, S., Matthews, L. & Fletcher, S. P. A general catalyst controlled route to prostaglandin F2α. Org. Lett. 24, 8886–8889 (2022).
Li, J., Ahmed, T. S., Xu, C., Stoltz, B. M. & Grubbs, R. H. Concise syntheses of Δ12-prostaglandin J natural products via stereoretentive metathesi. J. Am. Chem. Soc. 141, 154–158 (2019).
Smith, J. M., Harwood, S. J. & Baran, P. S. Radical retrosynthesis. Acc. Chem. Res. 51, 1807–1817 (2018).
Li, J., Amatuni, A. & Renata, H. Recent advances in the chemoenzymatic synthesis of bioactive natural products. Curr. Opin. Chem. Biol. 55, 111–118 (2020).
Li, J., Li, F., King-Smith, E. & Renata, H. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids. Nat. Chem. 12, 173–179 (2020).
Theil, F., Schick, H., Winter, G. & Reck, G. Lipase-catalyzed transesterification of meso-cyclopentane diols. Tetrahedron 47, 7569–7582 (1991).
Laumen, K. & Schneider, M. P. A facile chemoenzymatic route to optically pure building blocks for cyclopentanoid natural products. J. Chem. Soc. Chem. Commun. 16, 1298–1299 (1986).
Beneventi, E., Niero, M., Mottoerle, R., Fraaije, M. & Bergantino, E. Discovery of Baeyer–Villiger monooxygenases from photosynthetic eukaryotes. J. Mol. Catal. B: Enzym. 98, 145–154 (2013).
Romero, E., Castellanos, J. R. G., Mattevi, A. & Fraaije, M. W. Characterization and crystal structure of a robust cyclohexanone monooxygenase. Angew. Chem. Int. Ed. 55, 15852–15855 (2016).
Fürst, M. J. L. J. et al. Polycyclic ketone monooxygenase from the thermophilic fungus thermothelomyces thermophila: a structurally distinct biocatalyst for bulky substrates. J. Am. Chem. Soc. 139, 627–630 (2017).
Baldwin, C. V. F., Wohlgemuth, R. & Woodley, J. M. The first 200-L scale asymmetric Baeyer−Villiger oxidation using a whole-cell biocatalyst. Org. Process Res. Dev. 12, 660–665 (2008).
McLachlan, M. J., Johannes, T. W. & Zhao, H. Further improvement of phosphite dehydrogenase thermostability by saturation mutagenesis. Biotechnol. Bioeng. 99, 268–274 (2008).
Tello-Aburto, R., Rios, M. Y., Swenson, D. C. & Olivo, H. F. Bromohydrin reactions of Grieco’s bicyclic lactone. Tetrahedron Lett. 49, 6853–6855 (2008).
Dalton, D. R., Dutta, V. P. & Jones, D. C. Bromohydrin formation in dimethyl sulfoxide. J. Am. Chem. Soc. 90, 5498–5501 (1968).
Song, S. et al. DMSO-catalysed late-stage chlorination of (hetero)arenes. Nat. Catal. 3, 107–115 (2020).
Song, S. et al. From simple organobromides or olefins to highly value-added bromohydrins: a versatile performance of dimethyl sulfoxide. Green. Chem. 17, 2727–2731 (2015).
Guha, S., Kazi, I., Nandy, A. & Sekar, G. Role of lewis-base-coordinated halogen(I) intermediates in organic synthesis: the journey from unstable intermediates to versatile reagents. Eur. J. Org. Chem. 37, 5497–5518 (2017).
Everson, D. A., Shrestha, R. & Weix, D. J. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 132, 920–921 (2010).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Johnson, K. A., Biswas, S. & Weix, D. J. Cross-electrophile coupling of vinyl halides with alkyl halides. Chem. Eur. J. 22, 7399–7402 (2016).
Diccianni, J. B. & Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 1, 830–844 (2019).
Gu, J. et al. Nickel-catalyzed reductive cross-coupling of vinyl bromides with unactivated alkyl halides. Synthesis 49, 1867–1873 (2017).
Acknowledgements
The authors thank Professor Hans Renata and Professor Ang Li for valuable suggestions and helpful discussions. This work was supported by the National Key R&D Program of China (grant no. 2023YFA1506700 to J.L.), The Shanghai Science and Technology Development Funds (grant no. 22QA1404100 to J.L.), and Fundamental Research Funds for the Central Universities (23×010301599 to J.L.). The authors thank the Prof. Shuangjun Lin for generous access to their lab space and instrumentations.
Author information
Authors and Affiliations
Contributions
J.L. conceived this project. Y.Y., J.W., and J.L. performed the experiment. J.L. and Y.Y. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, Y., Wang, J. & Li, J. A concise and scalable chemoenzymatic synthesis of prostaglandins. Nat Commun 15, 2523 (2024). https://doi.org/10.1038/s41467-024-46960-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46960-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.