Abstract
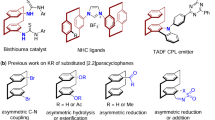
Planar chiral [2.2]paracyclophanes consist of two functionalized benzene rings connected by two ethylene bridges. These organic compounds have a wide range of applications in asymmetric synthesis, as both ligands and catalysts, and in materials science, as polymers, energy materials and dyes. However, these molecules can only be accessed by enantiomer separation via (a) time-consuming chiral separations and (b) kinetic resolution approaches, often with a limited substrate scope, yielding both enantiomers. Here, we report a simple, efficient, metal-free protocol for organocatalytic desymmetrization of prochiral diformyl[2.2]paracyclophanes. Our detailed experimental mechanistic study highlights differences in the origin of enantiocontrol of pseudo-para and pseudo-gem diformyl derivatives in NHC catalyzed desymmetrizations based on whether a key Breslow intermediate is irreversibly or reversibly formed in this process. This gram-scale reaction enables a wide range of follow-up derivatizations of carbonyl groups, producing various enantiomerically pure planar chiral [2.2]paracyclophane derivatives, thereby underscoring the potential of this method.
Similar content being viewed by others
Introduction
Asymmetric organocatalysis uses small organic molecules as chiral catalysts to mimic biocatalytic processes, thereby expanding the chemical space1,2,3,4. Organocatalytic approaches are valuable tools for preparing enantiomerically pure compounds given the operational simplicity of their reactions, which frequently include water and air tolerance. In addition, commonly used organocatalysts are available in both enantiomeric forms, and often derived from natural sources, such as amino acids and alkaloids5. Yet, despite the diversity of organocatalysts, organocatalytic approaches have been focused on the preparation of chiral molecules containing central and axial chirality. Consequently, asymmetric organocatalysis applications remain overlooked, especially in the production of planar chiral molecules, such as [2.2]paracyclophane derivatives6.
In [2.2]paracyclophanes, two benzene rings are covalently bound by two ethylene bridges at arene para positions. This molecular architecture suppresses the rotation of the benzene rings, providing [2.2]paracyclophanes with high configuration stability (up to 200 °C)7 and planar chirality upon arene derivatization8. In fact, the first planar chiral derivative of these compounds was isolated by crystallization of brucine salts of 4-carboxy[2.2]paracyclophane9 only 6 years after Brown and Farthing had pioneered the preparation of [2.2]paracyclophane10. Since then, considerable research efforts have focused on the unique 3D structure of chiral [2.2]paracyclophanes for their unusual electronical11,12 and photophysical properties13,14,15,16,17,18,19. Case in point, highly rigid planar chiral [2.2]paracyclophanes (Fig. 1) have become a valuable toolbox for developing ligands20,21,22,23,24 and organocatalysts25. Beyond synthetic chemistry, these scaffolds have also been applied in small-organic circularly polarized luminescence (CPL, Fig. 1D)26,27,28 and other phosphorescent emitters29.
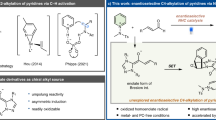
Notwithstanding these applications, enantiopure [2.2]paracyclophanes lack general and efficient synthetic pathways, a major constraint that continues to stall progress in this research field. Currently available synthetic approaches rely on enantiomer separations or various resolutions, including chemical resolution through diastereomerization and kinetic resolution30. Kinetic resolution, in particular, involves metal31,32,33,34,35 and enzyme-catalyzed processes36,37,38 and organocatalytic methods39,40,41,42 although the last approaches remain incipient. Regardless of the approach, though, kinetic resolution entails an inherent limitation, that is, the maximum enantiopure product yield is only 50%. For a high-yielding and practical synthesis of chiral [2.2]paracyclophanes, desymmetrization or dynamic kinetic resolution can be used, but only one study has reported such an approach thus far, more specifically the desymmetrization of centrosymmetric diformyl[2.2]paracyclophanes by ruthenium-catalyzed asymmetric transfer hydrogenation43. Moreover, this method still has some limitations, not least of which a limited reaction scope. Therefore, facilitating synthethic access to enantiopure [2.2]paracyclophanes requires developing high-yielding methods with a wide substrate scope.
Applicable to a broad scope of meso-symmetric substrates, metal-free organocatalytic desymmetrization44,45,46,47 induced by chiral N-heterocyclic carbenes (NHCs) yields enantiomerically pure compounds48,49,50,51. Furthermore, NHC organocatalysis features versatile reactivity modes under mild reaction conditions, broad functional-group tolerance, and bench-stable NHC precursors derived from natural sources (such as amino acids). For example, oxidative NHC catalysis was applied to the atroposelective desymmetrization of aromatic dialdehyde, producing axially chiral monoesters52,53. However, NHC-catalyzed desymmetrization to planar chiral [2.2]paracyclophanes has never been attempted before. Nevertheless, a recent study has shown that NHC facilitates access to planar chiral ferrocenes via enantioselective desymmetrization54. Accordingly, we aimed at developing a method for preparing enantiomerically pure [2.2]paracyclophane derivates using an oxidative NHC-catalyzed process.
In this study, we report a highly efficient and versatile protocol for organocatalytic desymmetrization esterification of prochiral diformyl[2.2]paracyclophanes through NHC catalysis under mild conditions. For this purpose, we used amino acid-derived precursors to induce enantiocontrol via central-to-planar chirality transfer. After optimizing the reaction conditions, we analysed the reaction scope and conducted mechanistic studies to understand differences in the origin of enantiocontrol of organocatalytic desymmetrization.
Results
Optimization of reaction conditions
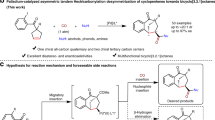
From the outset of our study, we chose the pseudo-para derivative (1a) as a model substrate considering the accessibility of prochiral diformyl[2.2]paracyclophanes. Simply mixing achiral paracyclophane 1a with an excess of methanol and in the presence of an l-valine-derived NHC-precursor (pre-C1), an oxidant (Kharash reagent, 3,3’5,5’-tetra-tert-butyldiphenoquinone, DQ), and a base (cesium carbonate) produced planar chiral monoester 3a in 51% isolated yield with enantioselectivity 92:8 er, along with an easily separable diesterification by-product (Table 1, entry 1). Based on the results from this proof-of-concept experiment, we aimed at optimizing the efficiency and stereochemical outcomes by varying the reaction conditions. For this purpose, we tested different amino acid-derived and other NHC precursors, oxidants, bases, and solvents.
The isolated yield of 3a significantly increased in the model reaction (entry 2) mediated by an l-phenylalanine-derived NHC precursor (pre-C2). Other precursors, such as morpholine-based pre-C3, failed to improve the efficiency of this reaction. In addition to these amino acid-derived NHC precursors, we also tested various other NHC precursors (for further information on the optimization survey, please refer to the Supplementary Tables 1–9). As a result, the model reaction became less tolerant to bases and solvents. For instance, with triethylamine as a base or chloroform as a solvent, the model reaction displayed lower yield and enantiocontrol (entries 4, 5). The same outcome was found when replacing DQ by the single-electron oxidant TEMPO (entry 6). Conversely, electroredox oxidation using iodide as a promoter55 produced the expected product 3a in 47% yield, albeit slightly decreasing the enantiocontrol (entry 7). Nevertheless, this experiment validated electrochemical oxidation as a potentially more suitable approach than other systems involving additional oxidants.
After further optimizing the reaction conditions, we found that increasing the amount of base (2.0 equiv., entry 8) slightly improved the stereocontrol of the model reaction. Under optimized reaction conditions, we tested the desymmetrization approach using ethanol instead of methanol, but the enantiocontrol decreased significantly (entry 9). This decrease led us to reexamine the catalyst for esterification using ethanol. Surprisingly, the reaction mediated by pre-C1 produced nearly an enantiopure product with a good yield (entry 10). Moreover, this reaction proved equally effective with methanol, providing the desired product 3a in excellent yield and enantiocontrol (entry 11).
Reaction scope
After optimizing the reaction conditions, we began exploring the scope of the desymmetrization reaction of pseudo-para derivative 1a (Fig. 2). When conducted with ent-pre-C1 derived from unnatural D-valine, the desymmetrization reaction produced the expected opposite enantiomeric product (ent-3a) in high yield, albeit with slightly diminished enantiopurity. Then, we assessed the effect of the steric hindrance of the selected aliphatic alcohols on the reaction rate and stereochemical outcome (Fig. 2A). Unsurprisingly, the reaction rate was significantly slower when using sterically hindered alcohols. Conversely, longer aliphatic alcohols, such as lauryl alcohol, produced the corresponding ester 3d in high yield (87%) and enantiopurity (94:6 er). Substituted aliphatic alcohols with halogen, methoxy, or internal and terminal alkenyl or alkynyl groups showed similar efficiency.
Subsequently, we explored the scope of this method using various aromatic alcohols (Fig. 2B). The results showed that this method was intolerant to phenols, including substituted phenols, but tolerated well benzyl alcohol and 2-phenylethanol. In addition, the expected products (3m and 3n) were formed in high yields and enantiopurities when using 2-(ferrocenyl)ethanol or tryptophol. Such functional group tolerance encouraged us to apply the desymmetrization reaction of 1a to the late-stage modification of structurally diverse alcohols derived from natural or bioactive molecules (Fig. 2C), including indomethacin, proline, biotin, and chenodeoxycholic acid, or bioactive alcohols (sulfurol, citronellol, protected glucose derivative). These desymmetrization reactions resulted in good-to-high yields of esters, with high levels of enantiopurity of the final product. For instance, the steroidal product 3r and 3s were obtained in high yields (67 and 66%) as single diastereomers (both 20:1 dr). In the reaction to steroidal product 3s, the starting material contained three unprotected hydroxy groups. In this case, differences in the reaction rates of desymmetrization of secondary alcohols resulted in regioselectivity. Moreover, thiols also worked as esterification agents in this desymmetrization reaction (Fig. 2D), but their efficiency, in terms of yield and optical purities of thioesters 3w and 3x, was lower than that of the aforementioned esters.
To assess our method (Fig. 3), we introduced another prochiral [2.2]paracyclophane, namely pseudo-gem-diformylparacyclophane (1b). We began by optimizing the reaction conditions (for more details, please refer to Supplementary Table 10). After lowering the reaction temperature, we noted that the expected product 5a was formed in excellent yield and enantiomeric purity (91%, 99.5/0.5 er) without the diester byproduct. In turn, by using the opposite enantiomeric form (ent-pre-C1), we gained access to the opposite enantiomer (ent-5a), obtaining the expected product in excellent yield and stereochemical outcomes. With sterically hindered alcohols, the reaction rate decreased, as expected, albeit without significantly affecting the enantiocontrol. Moreover, introducing different alcohols improved the yield and stereocontrol of the desymmetrization process.
Mechanistic studies
To elucidate the reaction mechanism and origin of stereocontrol, we conducted control experiments with both substrates 1 (Figs. 4 and 5). First, treating 1a (pseudo-para) with deuterated methanol-d4 (Fig. 4A) under optimized conditions provided 3a-d3 with deuterated aldehyde (~40%, validated by 2H NMR), indicating the reversible formation of the Breslow intermediate. Subsequently, we studied the parallel kinetic isotope effect (Fig. 4B) using 1a and 1a-d2 in a desymmetrization reaction with methanol under optimized reaction conditions for 1 h. The results showed a KIE (kinetic isotope effect) value of 2.8, implying that proton transfer in the formation of the Breslow intermediate is the rate-limiting step. To investigate the origin of enantiocontrol, we conducted a series of control experiments (Fig. 4C). The model reaction with a lowered amount of oxidant (55 mol%) produced 3a in 88:12 er with traces of the diesterification product, suggesting that desymmetrization is an enantiodivergent process and that kinetic resolution could be an additional enantiocontrol mechanism. To confirm this hypothesis, we conducted a kinetic resolution reaction of rac-3a under optimized reaction conditions with a lowered amount of oxidant (55 mol%), thereby forming enantioenriched product 3a and confirming the existence of an additional source of enantiocontrol. Based on our findings, we propose that 1a enantioselective desymmetrization (kR/kS = 7.6/1) is followed by kinetic resolution (s = 4.1), resulting in a high level of enantiocontrol (for details, please refer to pages 28-42 of the Supplementary Information file), in line with the slightly decreased enantiocontrol in the preparation of ent-3a.
We also performed another series of control experiments involving the desymmetrization of pseudo-gem derivative 1b (Fig. 5). We noticed striking differences from the desymmetrization of 1a. For example, we did not detect deuterium incorporation in the control reaction conducted with methanol-d4 (Fig. 5A), indicating that the formation of the Breslow intermediate is an irreversible process. In the desymmetrization of 1b, the KIE was significantly lower (~0.5). Accordingly, the initial carbene nucleophilic attack of 1b is most likely the rate-limiting step (Fig. 5B). The origin of enantiocontrol was clear (Fig. 5C) because we observed nearly enantiopure product formation (99.8:0.2 er) in a control reaction of 1b with a lowered amount of oxidant (55 mol%) under optimized conditions. Additionally, the kinetic resolution of rac-5a was ineffective (s = 0.1), indicating that enantioselective desymmetrization (kR/kS > 400/1) is crucial for enantiocontrol in this process (for details, please refer to pages 28-42 of the Supplementary Information file). Based on these findings, pseudo-gem[2.2]paracyclophanes, not limited to dialdehydes, stand out as candidates for further elaboration in desymmetrization processes.
Synthetic utilization of the chiral product
To showcase the practicality of this method, we performed a gram-scale desymmetrization of 1b under optimized conditions (Fig. 6A). This gram-scale reaction provided us with access to a highly enantioenriched product 5a in a high yield of 88% with 99.5/0.5 er. The follow-up reactions of the planar chiral product 5a highlighted the usefulness and modulation of the aldehydic group (Fig. 6B). Moreover, the thioesterification reaction of 5a promoted by oxidative NHC catalysis produced thioester 7 in excellent yield and retaining optical purity. Similarly, the corresponding olefin 8 was isolated in nearly quantitative yield as a product of the Wittig reaction. Through reductive methods, such as reductive amination or aldehyde reduction, we prepared secondary amine 9 and benzylic alcohol 10 in good-to-high yields, without significant changes in stereochemical outcomes. In addition, 11, a crucial enantioenriched intermediate for preparing a valuable photocatalyst56, was isolated by Pinnick oxidation in high yield and retaining optical purity.
Based on these results, we synthesized novel bifunctional catalysts by transforming both carbonyl groups (Fig. 6C). To prepare these novel catalysts, we began by conducting Bayer-Villiger oxidation followed by reduction and oxidation steps57. These steps yielded product 12 without significantly affecting the enantiomeric excess of the product. Subsequently, Pinnick oxidation of aldehyde yielded carboxylic acid 13, which has been proposed as a bifunctional organocatalyst. We tested its catalytic activity by conducting select reactions, such as aminalization58 and the Henry reaction59. In both examples, the desired products were formed in high isolated yields, without significant enantiocontrol. Furthermore, planar chiral derivatives 12 and 13 are potential key intermediates for preparing [2.2]paracyclophane-based ligands60,61,62.
In summary63, our metal-free methodology for NHC-catalyzed enantioselective desymmetrization of diformyl[2.2]paracyclophanes provides efficient access to highly enantioenriched planar chiral compounds. This operationally simple and effective strategy has a wide reaction scope with various alcohols involving natural and bioactive compounds. Moreover, the feasibility of the gram-scale desymmetrization reaction and the potential for diverse follow-up transformations underscore the value of this method. And as shown in our comprehensive experimental mechanistic studies, differences in the origin of enantiocontrol of pseudo-para and pseudo-gem diformyl derivatives in NHC-catalysed desymmetrizations identified pseudo-gem diformyl[2.2]paracyclophanes as valuable synthons for future elaborations. Accordingly, ongoing research into the synthesis of planar chiral molecules organocatalytic reactions and their applications in organocatalysis or novel ligand synthesis will continue in our laboratories.
Methods
Representative procedure
The vial (4 ml) was charged with 1 (26.4 mg, 0.1 mmol), pre-C1 (7.8 mg, 0.02 mmol), DQ (49.0 mg, 0.12 mmol), and Cs2CO3 (65.2 mg, 0.2 mmol), followed by DCM (1.0 ml), and the corresponding alcohol (0.5 mmol) at the corresponding temperature. At this temperature, the reaction mixture was stirred for the indicated time. Once the reaction was completed by thin-layer chromatography (TLC), the solvent was evaporated. The crude product was purified by column chromatography (eluting by hexane/EtOAc mixtures).
Data availability
The authors declare that the data supporting the findings of this study are available within the article and the Supplementary infomation file. The primary NMR FID files generated in this study have been deposited in the figshare repository (https://doi.org/10.6084/m9.figshare.24851235)64. Other detailed data are available from the corresponding authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2302458, 2302459, and 2309999. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
List, B. & Yang, J. W. The organic approach to asymmetric catalysis. Science 313, 1584–1586 (2006).
Xiang, S.-H. & Tan, B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 11, 3786 (2020).
Lassaletta, J. M. Spotting trends in organocatalysis for the next decade. Nat. Commun. 11, 3787 (2020).
Han, B. et al. Asymmetric organocatalysis: an enabling technology for medicinal chemistry. Chem. Soc. Rev. 50, 1522–1586 (2021).
López, R. & Palomo, C. Planar chirality: a mine for catalysis and structure discovery. Angew. Chem. Int. Ed. 61, e202113504 (2022).
Gleiter, R. & Hopf, H. Modern Cyclophane Chemistry 1st edn, Vol. 586 (Wiley, 2004), p. 435.
David, O. R. P. Syntheses and applications of disubstituted [2.2]paracyclophanes. Tetrahedron 68, 8977–8993 (2012).
Cram, D. J. & Allinger, N. L. Macro Rings. XII. stereochemical consequences of steric compression in the smallest paracyclophane. J. Am. Chem. Soc. 77, 6289–6294 (1955).
Brown, C. & Farthing, A. Preparation and structure of Di-p-xylylene. Nature 164, 915–916 (1949).
Majerz, I. & Dziembowska, T. Aromaticity and through-space interaction between aromatic rings in [2.2]paracyclophanes. J. Phys. Chem. A 120, 8138–8147 (2016).
Miyazaki, T. et al. Synthesis and electronic and photophysical properties of [2.2]- and [3.3]paracyclophane-based donor-donor′-acceptor triads. J. Org. Chem. 79, 11440–11453 (2014).
Felder, S. et al. Compact CPL emitters based on a [2.2]paracyclophane scaffold: recent developments and future perspectives. J. Mat. Chem. C. 11, 2053–2062 (2023).
Roy, I., David, A. H. G., Das, P. J., Pe, D. J. & Stoddart, J. F. Fluorescent cyclophanes and their applications. Chem. Soc. Rev. 51, 5557–5605 (2022).
Teng, J.-M., Zhang, D.-W. & Chen, C.-F. Recent progress in circularly polarized luminescence of [2.2]pracyclophane derivatives. ChemPhotoChem 6, e202100228 (2022).
Hassan, Z., Spuling, E., Knoll, D. M. & Bräse, S. Regioselective functionalization of [2.2]paracyclophanes: recent synthetic progress and perspectives. Angew. Chem. Int. Ed. 59, 2156–2170 (2020).
Hassan, Z., Spuling, E., Knoll, D. M., Lahann, J. & Bräse, S. Planar chiral [2.2]paracyclophanes: from synthetic curiosity to applications in asymmetric synthesis and materials. Chem. Soc. Rev. 47, 6947–6963 (2018).
Paradies, J. [2.2]Paracyclophane derivatives: synthesis and application in catalysis. Synthesis 23, 3749–3766 (2011).
Hopf, H. [2.2]Paracyclophanes in polymer chemistry and materials science. Angew. Chem. Int. Ed. 47, 9808–9812 (2008).
Liang, H., Guo, W., Li, J., Jiang, J. & Wang, J. Chiral arene ligand as stereocontroller for asymmetric C−H activation. Angew. Chem. Int. Ed. 61, e202204926 (2022).
Kumar, S. V. & Guiry, P. J. Zinc-catalyzed enantioselective [3+2] cycloaddition of azomethine ylides using planar chiral [2.2]paracyclophane-imidazoline N,O-ligands. Angew. Chem. Int. Ed. 61, e202205516 (2022).
Zippel, C., Hassan, Z., Nieger, M. & Bräse, S. Design and synthesis of a [2.2]paracyclophane-based planar chiral dirhodium catalyst and its applications in cyclopropanation reaction of vinylarenes with α-methyl-α-diazo esters. Adv. Synth. Catal. 362, 3431–3436 (2020).
Knoll, D. M., Hu, Y., Hassan, Z., Nieger, M. & Bräse, S. Planar chiral [2.2]paracyclophane-based bisoxazoline ligands and their applications in Cu-Mediated N-H insertion reaction. Molecules 24, 4122 (2019).
Bestgen, S. et al. Double-Strand DNA Breaks induced by paracyclophane gold(I) complexes. Chem. Eur. J. 23, 6315–6322 (2017).
Schneider, J. F., Fröhlich, R. & Paradies, J. Paracyclophane-derived planar-chiral hydrogen-bond receptors. Isr. J. Chem. 52, 76–91 (2012).
Chen, C.-H. & Zheng, W.-H. Planar chiral B-N heteroarenes Based on [2.2]paracyclophane as circularly polarized luminescence emitters. Org. Lett. 23, 5554–5558 (2021).
Gon, M., Morisaki, Y. & Chujo, Y. Optically active cyclic compounds based on planar chiral [2.2]paracyclophane: extension of the conjugated systems and chiroptical properties. J. Mater. Chem. C. 3, 521–529 (2015).
Morisaki, Y., Gon, M., Sasamori, T., Tokitoh, N. & Chujo, Y. Planar chiral tetrasubstituted [2.2]paracyclophane: optical resolution and functionalization. J. Am. Chem. Soc. 136, 3350–3353 (2014).
Sharma, N. et al. Turn on of sky-blue thermally activated delayed fluorescence and circularly polarized luminescence (CPL): via increased torsion by a bulky carbazolophane donor. Chem. Sci. 10, 6689–6696 (2019).
Felder, S., Wu, S., Brom, J., Micouin, L. & Benedetti, E. Enantiopure planar chiral [2.2]paracyclophanes: synthesis and applications in asymmetric organocatalysis. Chirality 33, 506–527 (2021).
Zhao, Y., Ding, Y.-X., Wu, B. & Zhou, Y.-G. Nickel-catalyzed asymmetric hydrogenation for kinetic resolution of [2.2]paracyclophane-derived. Cycl. N.-Sulfonylimines. J. Org. Chem. 86, 10788–10798 (2021).
Zhao, Y., Wang, X.-Q., Yu, Y.-J. & Zhou, Y.-G. Kinetic resolution of [2.2]paracyclophane-derived cyclic N-sulfonylimines via palladium-catalyzed addition of arylboronic acids. J. Org. Chem. 86, 1262–1272 (2021).
Zippel, C., Hassan, Z., Parsa, A. Q., Hohmann, J. & Bräse, S. Multigram-scale kinetic resolution of 4-acetyl[2.2]paracyclophane via Ru-catalyzed enantioselective hydrogenation: accessing [2.2]paracyclophanes with planar and central chirality. Adv. Synth. Catal. 363, 2861–2865 (2021).
Zhao, Y., Wang, H., Wu, B. & Zhou, Y.-G. Synthesis of paracyclophanes with planar and central chirality: Kinetic resolution of [2.2]paracyclophane aldimines via palladium-catalyzed addition of arylboronic acids. Org. Chem. Front. 6, 3956–3960 (2019).
Delcourt, M.-L. et al. Highly enantioselective asymmetric transfer hydrogenation: a practical and scalable method to efficiently access planar chiral [2.2]paracyclophanes. J. Org. Chem. 84, 5369–5382 (2019).
Braddock, D. C., MacGilp, I. D. & Perry, B. G. Improved synthesis of (±)−4,12-dihydroxy[2.2]paracyclophane and Its enantiomeric resolution by enzymatic methods: planar chiral (R)- and (S.) Phanol. J. Org. Chem. 67, 8679–8681 (2002).
Pamperin, D., Hopf, H., Syldatk, C. & Pietzsch, M. Synthesis of planar chiral [2.2]paracyclophanes by biotransformations: kinetic resolution of 4-formyl-[2.2]paracyclophane by asymmetric reduction. Tetrahedron Asymetry 8, 319–325 (1997).
Cipiciani, A. et al. Enzymatic kinetic resolution of (±)−4-acetoxy[2.2] paracyclophane by candida cylindracea lipase. An efficient route for the preparation of (+)-R-4-Hydroxy- and (+)-S-4-acetoxy[2.2)paracyclophane. Tetrahedron 53, 11853–11858 (1997).
Yu, S., Bao, H., Zhang, D. & Yang, X. Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination. Nat. Commun. 14, 5239 (2023).
Weinzierl, D. & Waser, M. Synthesis of [2.2]paracyclophane-based glycidic amides using chiral ammonium ylides. Helv. Chim. Acta 104, e2100073 (2021).
Weinzierl, D. & Waser, M. Chiral isothiourea-catalyzed kinetic resolution of 4-hydroxy[2.2]paracyclophane. Beilstein J. Org. Chem. 17, 800–804 (2021).
Akagawa, K., Nishi, N., Yoshikawa, I. & Kudo, K. Kinetic resolution of a planar-chiral [2.2]paracyclophane derivative by helical-peptide-catalyzed michael addition of nitromethane. Eur. J. Org. Chem. 2015, 5055–5059 (2015).
Delcourt, M.-L., Felder, S., Benedetti, E. & Micouin, L. Highly enantioselective desymmetrization of centrosymmetric pseudo-para-diformyl[2.2]paracyclophane via asymmetric transfer hydrogenation. ACS Catal. 8, 6612–6616 (2018).
Nájera, C., Foubelo, F., Sansano, J. M. & Yus, M. Enantioselective desymmetrization reactions in asymmetric catalysis. Tetrahedron 106-107, 132629 (2022).
Di Iorio, N., Crotti, S. & Bencivenni, G. Organocatalytic desymmetrization reactions for the synthesis of axially chiral compounds. Chem. Rec. 19, 2095–2104 (2019).
Borissov, A. et al. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev. 45, 5474–5540 (2016).
Xu, Y., Zhai, T.-Y., Xu, Z. & Ye, L.-W. Recent advances towards organocatalytic enantioselective desymmetrizing reactions. Trends Chem. 4, 191–205 (2022).
Zhang, Y., Cai, H., Gan, X. & Jin, Z. N-Heterocyclic carbene-catalyzed enantioselective (dynamic) kinetic resolutions and desymmetrizations. Sci. China Chem. 67, 482–511 (2024).
Song, R., Xie, Y., Jin, Z. & Chi, Y. R. Carbene-catalyzed asymmetric construction of atropisomers. Angew. Chem. Int. Ed. 60, 26026–26037 (2021).
De Risi, C., Bortolini, O., Di Carmine, G., Ragno, D. & Massi, A. Kinetic resolution, dynamic kinetic resolution and asymmetric desymmetrization by N-heterocyclic carbene catalysis. Synthesis 51, 1871–1891 (2019).
Wang, Z., Pan, D., Li, T. & Jin, Z. N-Heterocyclic carbene (NHC)-organocatalyzed kinetic resolutions, dynamic kinetic resolutions, and desymmetrizations. Chem. Asian J. 13, 2149–2163 (2018).
Wu, Y., Li, M., Sun, J., Zheng, G. & Zhang, Q. Synthesis of axially chiral aldehydes by N-heterocyclic-carbene-catalyzed desymmetrization followed by kinetic resolution. Angew. Chem. Int. Ed. 61, e202117340 (2022).
Zhao, W. et al. N-Heterocyclic carbene (NHC)-catalyzed desymmetrization of biaryldialdehydes to construct axially chiral aldehydes. Chin. J. Org. Chem. 42, 2504–2514 (2022).
Lv, X. et al. Access to planar chiral ferrocenes via N-heterocyclic carbene-catalyzed enantioselective desymmetrization reactions. ACS Catal. 12, 2706–2713 (2022).
Zhou, P., Li, W., Lan, J. & Zhu, T. Electroredox carbene organocatalysis with iodide as promoter. Nat. Commun. 13, 3827 (2022).
Huo, S.-C., Indurmuddam, R. R., Hong, B.-C., Lu, C.-L. & Chien, S.-Y. The hamburger-shape photocatalyst: thioxanthone-based chiral [2.2]paracyclophane for enantioselective visible-light photocatalysis of 3-methylquinoxalin-2(1H)-one and styrenes. Org. Biomol. Chem. 21, 9330–9336 (2023).
Rozenberg, V. I. et al. Enantiomerically pure (R)- and (S)−15-Hydroxy[2.2]paracyclophane-4-carbaldehyde (iso-FHPC): a novel parent compound for planar chiral ligands. Eur. J. Org. Chem. 2003, 2056–2061 (2003).
Sui, Y. et al. Highly enantioselective synthesis of cyclic aminals with a cyclopentadiene-based chiral carboxylic acid. Eur. J. Org. Chem. 2018, 215–218 (2018).
Kitagaki, S., Ueda, T. & Mukai, C. Planar chiral [2.2]paracyclophane-based bis(thiourea) catalyst: application to asymmetric henry reaction. Chem. Commun. 49, 4030–4032 (2013).
Bolm, C. & Whelligan, D. K. The synthesis of pseudo-geminal, pseudo-ortho and ortho hydroxy-oxazolinyl[2.2]paracyclophanes for use as ligands in asymmetric catalysis. Adv. Synth. Catal. 348, 2093–2100 (2006).
Bolm, C. & Whelligan, D. K. Synthesis of pseudo-geminal-, pseudo-ortho-phosphinyl-oxazolinyl-[2.2]paracyclophanes for Use as ligands in asymmetric catalysis. J. Org. Chem. 71, 4609–4618 (2006).
Wu, X.-W., Zhang, T.-Z., Yuan, K. & Hou, X.-L. Regulation of the flexibility of planar chiral [2.2]paracyclophane ligands and its significant impact on enantioselectivity in asymmetric reactions of diethylzinc with carbonyl compounds. Tetrahedron; Asymmetry 15, 2357–2365 (2004).
Dočekal, V., Koucký, F., Císařová, I. & Veselý, J. Organocatalytic desymmetrization prompts central-to-planar chirality transfer to [2.2]Paracyclophanes. ChemRxiv https://doi.org/10.26434/chemrxiv-2023-fkvmc (2023).
Dočekal, V., Koucký, F., Císařová, I. & Veselý, J. Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes. Figshare https://doi.org/10.6084/m9.figshare.24851235 (2024).
Acknowledgements
The authors gratefully acknowledge the Czech Science Foundation (24-12575 S, J.V.) for financial support. The authors also thank Dr. Štícha and Dr. Urban (both from Charles University) for the MS and IR analysis. Furthermore, the authors thank Dr. Carlos V. Melo (Charles University) for editing of the manuscript, and Dr. Tomáš Slanina (IOCB Prague) for discussing our mechanistic studies.
Author information
Authors and Affiliations
Contributions
V.D. designed project and performed the synthesis of all compounds. F. K. performed selected NMR experiments. I. C. performed X-ray analysis. J. V. conceived the study and directed the project. V.D., and J.V. wrote the manuscript. All authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shufeng Chen, Zhichao Jin and Laurent Micouin for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dočekal, V., Koucký, F., Císařová, I. et al. Organocatalytic desymmetrization provides access to planar chiral [2.2]paracyclophanes. Nat Commun 15, 3090 (2024). https://doi.org/10.1038/s41467-024-47407-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-47407-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.