News & Views |
Featured
-
-
Article |
 Defective prelamin A processing promotes unconventional necroptosis driven by nuclear RIPK1
Defective prelamin A processing promotes unconventional necroptosis driven by nuclear RIPK1
Yang, Zhang et al. identify a non-canonical form of necroptosis driven by nuclear RIPK1-mediated nuclear membrane rupture as a result of ZMPSTE24 deficiency and defective prelamin A processing commonly observed in progeroid disorders.
- Yuanxin Yang
- , Jian Zhang
- & Daichao Xu
-
News & Views |
 Hypoxia-stabilized RIPK1 promotes cell death
Hypoxia-stabilized RIPK1 promotes cell death
The PHD–pVHL pathway is essential for oxygen-dependent prolyl hydroxylation of HIFA. A recent study identifies RIPK1 as a hydroxylation target in this pathway during hypoxia-induced cell death and presents a 2.8 Å resolution crystal structure of the pVHL–elongin B/C complex bound to hydroxylated RIPK1.
- Wei Ruan
- , Holger K. Eltzschig
- & Xiaoyi Yuan
-
Article |
 Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation
Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation
Zhang, Xu, Liu, Wang et al. identify an inhibitory mechanism for RIPK1 kinase through EGLN1/pVHL-mediated proline hydroxylation, which is disrupted upon prolonged hypoxia that activates RIPK1 activity to promote cell death and inflammation.
- Tao Zhang
- , Daichao Xu
- & Wenyi Wei
-
Article
| Open Access OASL phase condensation induces amyloid-like fibrillation of RIPK3 to promote virus-induced necroptosis
OASL phase condensation induces amyloid-like fibrillation of RIPK3 to promote virus-induced necroptosis
Lee et al. uncover a previously uncharacterized role of OASL in virus-induced necroptosis. OASL chaperones the assembly of RIPK3 and ZBP1 via liquid-liquid phase separation, which induces RIPK3 and necroptosis activation, thereby modulating inflammation and host defence against viral infection.
- Shin-Ae Lee
- , Lin-Chun Chang
- & Jae U. Jung
-
News & Views |
 Navigating ferroptosis via an NADPH sensor
Navigating ferroptosis via an NADPH sensor
NADPH levels serve as a biomarker of sensitivity to ferroptosis, but the regulators that detect cellular NADPH levels and modulate downstream ferroptosis responses are unknown. A study now identifies MARCHF6 in the ubiquitin system as an NADPH sensor that suppresses ferroptosis.
- Chao Mao
- & Boyi Gan
-
Article |
 The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis
The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis
Nguyen et al. show that the E3 ubiquitin ligase MARCHF6 acts as an NADPH sensor to suppress ferroptosis. Mechanistically, NADPH binds to MARCHF6 and activates its E3 ligase activity, enhancing the degradation of pro-ferroptosis proteins.
- Kha The Nguyen
- , Sang-Hyeon Mun
- & Cheol-Sang Hwang
-
News & Views |
 RIPK1 and RIPK3 form mosaic necrosomes
RIPK1 and RIPK3 form mosaic necrosomes
Necrosomes formed by RIPK1–RIPK3 mediate necroptosis. Super-resolution microscopy identifies the architectural features of necrosomes and provides mechanistic insights into the signalling from RIPK1 to RIPK3 when RIPK1 is activated to mediate necroptosis, and from RIPK3 to RIPK1 when RIPK3 is inhibited to mediate apoptosis.
- Weiwei Qi
- & Junying Yuan
-
Article |
 Mosaic composition of RIP1–RIP3 signalling hub and its role in regulating cell death
Mosaic composition of RIP1–RIP3 signalling hub and its role in regulating cell death
Chen et al. examine the cellular necrosome using STORM and uncover the rod-shaped structure formed by mosaics of RIP1 and RIP3 oligomers.
- Xin Chen
- , Rongfeng Zhu
- & Jiahuai Han
-
News & Views |
 Prostanoids put a brake on necroptosis in IBD
Prostanoids put a brake on necroptosis in IBD
A form of programmed cell death, necroptosis, in intestinal epithelial cells initiates mucosal inflammation. A study now finds that prostanoid EP4 receptor signalling interferes with RIPK1–RIPK3-dependent MLKL activation, thereby inhibiting necroptosis and accelerating resolution of inflammation.
- Nicole C. Kaneider
- & Arthur Kaser
-
Article |
 E-type prostanoid receptor 4 drives resolution of intestinal inflammation by blocking epithelial necroptosis
E-type prostanoid receptor 4 drives resolution of intestinal inflammation by blocking epithelial necroptosis
Patankar et al. identify E-type prostanoid receptor 4 as a negative regulator of tumour necrosis factor signalling and mixed-lineage kinase domain-like pseudokinase activation, thereby suppressing necroptosis of intestinal epithelial cells and promoting resolution of intestinal inflammation.
- Jay V. Patankar
- , Tanja M. Müller
- & Christoph Becker
-
News & Views |
 Parkin inhibits necroptosis to prevent cancer
Parkin inhibits necroptosis to prevent cancer
Loss-of-function mutations in the ubiquitin ligase Parkin are a cause of Parkinson’s disease. Parkin also has tumour-suppressor activity, although how Parkin prevents cancer is unclear. Unexpectedly, Parkin is found to suppress cancer by inhibiting an inflammatory type of cell death called necroptosis.
- Kai Cao
- & Stephen W. G. Tait
-
Article |
 The AMPK–Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome
The AMPK–Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome
AMPK and Parkin keep the necrosome in check. Lee et al. show that AMPK activates Parkin and prevents RIPK1−RIPK3 complex formation by promoting RIPK3 ubiquitination, thereby negatively regulating necroptosis, inflammation and tumour initiation.
- Seung Baek Lee
- , Jung Jin Kim
- & Zhenkun Lou
-
Article |
 A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain
A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain
Necroptosis drives arthritis. Polykratis et al. show that the deubiquitinating enzyme A20 inhibits inflammasome-dependent arthritis development by regulating macrophage necroptosis and this function depends on its ZnF7 ubiquitin binding domain.
- Apostolos Polykratis
- , Arne Martens
- & Manolis Pasparakis
-
News & Views |
 TBK1 and IKKε restrain cell death
TBK1 and IKKε restrain cell death
RIPK1 plays a key role in several inflammatory and cell death signalling pathways. Understanding its regulation is pivotal for identifying diseases that might therapeutically benefit from RIPK1 inhibition. Recent studies now show that TBK1 and IKKε constitute a cell death checkpoint that restrains RIPK1 activation.
- Klaus Heger
- & Vishva M. Dixit
-
Article |
 TBK1 and IKKε prevent TNF-induced cell death by RIPK1 phosphorylation
TBK1 and IKKε prevent TNF-induced cell death by RIPK1 phosphorylation
Lafont et al. uncover a checkpoint mediated by TBK1 and IKKε, which phosphorylate RIPK1 in the TNFR1-SC. TBK1 and IKKε recruitment depends on M1 ubiquitylation and NEMO to restrict TNF-induced cell death.
- Elodie Lafont
- , Peter Draber
- & Henning Walczak
-
Article |
 RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis
RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis
RIP3 regulates mitochondrial metabolism. Yang et al. show that RIP3 activates the pyruvate dehydrogenase complex to enhance aerobic respiration and increase mitochondrial ROS during necroptosis, and MLKL is required for RIP3 translocation to mitochondria.
- Zhentao Yang
- , Yan Wang
- & Jiahuai Han
-
News & Views |
 MK2 balances inflammation and cell death
MK2 balances inflammation and cell death
The cytokine tumour necrosis factor (TNF) and the toll-like receptors (TLRs) coordinate immune responses by activating inflammatory transcriptional programs, but these signals can also trigger cell death. Recent studies identify the MAP kinase substrate MK2 as a key player in determining whether cells live or die in response to TNF and TLR signalling.
- Andrew Oberst
-
Article |
 MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death
MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death
Dondelinger et al. and Menon et al. show that MAPKAP kinase-2 (MK2) phosphorylates RIPK1 to regulate TNF-mediated cell death as well as RIPK1 signalling in inflammation and bacterial infection.
- Yves Dondelinger
- , Tom Delanghe
- & Mathieu J. M. Bertrand
-
Article |
 p38MAPK/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection
p38MAPK/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection
Dondelinger et al. and Menon et al. show that MAPKAP kinase-2 (MK2) phosphorylates RIPK1 to regulate TNF-mediated cell death as well as RIPK1 signalling in inflammation and bacterial infection.
- Manoj B. Menon
- , Julia Gropengießer
- & Klaus Ruckdeschel
-
News & Views |
 A caspase-independent way to kill cancer cells
A caspase-independent way to kill cancer cells
Cancer treatments often focus on killing tumour cells through apoptosis, which is thought to typically require mitochondrial outer membrane permeabilization (MOMP) and subsequent caspase activation. A study now shows that MOMP can trigger TNF-dependent, but caspase-independent cell death, suggesting a different approach to improve cancer therapy.
- Brent E. Fitzwalter
- & Andrew Thorburn
-
Article |
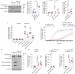 Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency
Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency
Tait and colleagues show that caspase-independent cell death induced by mitochondrial permeabilization stimulates NF-κB activity through downregulation of inhibitor of apoptosis, and enhances anti-tumour effects.
- Evangelos Giampazolias
- , Barbara Zunino
- & Stephen W. G. Tait
-
Article |
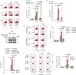 CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3
CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3
Receptor-interacting protein kinase 3 (RIPK3) is a key regulator of necroptosis. Seo et al. show that the E3 ligase CHIP mediates ubiquitylation and lysosomal degradation of RIPK3, thus regulating both necrosome formation and necroptosis.
- Jinho Seo
- , Eun-Woo Lee
- & Jaewhan Song
-
Article |
 Ppm1b negatively regulates necroptosis through dephosphorylating Rip3
Ppm1b negatively regulates necroptosis through dephosphorylating Rip3
Han and colleagues reveal that the phosphatase Ppm1b dephosphorylates the kinase Rip3 to negatively regulate necroptosis in vitro and in vivo.
- Wanze Chen
- , Jianfeng Wu
- & Jiahuai Han
-
Review Article |
 Organelle-specific initiation of cell death
Organelle-specific initiation of cell death
- Lorenzo Galluzzi
- , José Manuel Bravo-San Pedro
- & Guido Kroemer
-
-
Article |
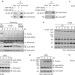 Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis
Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis
Liu and colleagues find that MLKL translocates to the plasma membrane to induce TNF-induced necroptosis, possibly through an effect on calcium influx and the action of the cation channel TRPM7.
- Zhenyu Cai
- , Siriporn Jitkaew
- & Zheng-Gang Liu
-
-
News & Views |
 Necrotic cell death: harnessing the Dark side of the Force in mammary gland involution
Necrotic cell death: harnessing the Dark side of the Force in mammary gland involution
In response to major cellular insults, a massive increase in lysosomal membrane permeability (LMP) leads to necrosis. Data now reveal that this potent lysosomal-mediated necrotic cell-death machinery can also be harnessed for complex physiological processes, such as post-lactation mammary gland involution.
- Cliff J. Luke
- & Gary A. Silverman

 RIPK1 and necroptosis role in premature ageing
RIPK1 and necroptosis role in premature ageing