Abstract
Defects in the prelamin A processing enzyme caused by loss-of-function mutations in the ZMPSTE24 gene are responsible for a spectrum of progeroid disorders characterized by the accumulation of farnesylated prelamin A. Here we report that defective prelamin A processing triggers nuclear RIPK1-dependent signalling that leads to necroptosis and inflammation. We show that accumulated prelamin A recruits RIPK1 to the nucleus to facilitate its activation upon tumour necrosis factor stimulation in ZMPSTE24-deficient cells. Kinase-activated RIPK1 then promotes RIPK3-mediated MLKL activation in the nucleus, leading to nuclear envelope disruption and necroptosis. This signalling relies on prelamin A farnesylation, which anchors prelamin A to nuclear envelope to serve as a nucleation platform for necroptosis. Genetic inactivation of necroptosis ameliorates the progeroid phenotypes in Zmpste24−/− mice. Our findings identify an unconventional nuclear necroptosis pathway resulting from ZMPSTE24 deficiency with pathogenic consequences in progeroid disorder and suggest RIPK1 as a feasible target for prelamin A-associated progeroid disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Mass spectrometry data have been deposited in ProteomeXchange with the primary accession code PXD049033. All data are available in the article and its Supplementary Information as well as from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Barrowman, J. & Michaelis, S. ZMPSTE24, an integral membrane zinc metalloprotease with a connection to progeroid disorders. Biol. Chem. 390, 761–773 (2009).
Spear, E. D. et al. ZMPSTE24 missense mutations that cause progeroid diseases decrease prelamin A cleavage activity and/or protein stability. Dis. Model Mech. 11, dmm033670 (2018).
Corrigan, D. P. et al. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem. J. 387, 129–138 (2005).
Dittmer, T. A. & Misteli, T. The lamin protein family. Genome Biol. 12, 222 (2011).
Gruenbaum, Y. & Foisner, R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 84, 131–164 (2015).
Mattout, A., Dechat, T., Adam, S. A., Goldman, R. D. & Gruenbaum, Y. Nuclear lamins, diseases and aging. Curr. Opin. Cell Biol. 18, 335–341 (2006).
Young, S. G., Fong, L. G., Michaelis, S. & Prelamin, A. Zmpste24, misshapen cell nuclei, and progeria–new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J. Lipid Res. 46, 2531–2558 (2005).
Davies, B. S., Fong, L. G., Yang, S. H., Coffinier, C. & Young, S. G. The posttranslational processing of prelamin A and disease. Annu Rev. Genomics Hum. Genet. 10, 153–174 (2009).
Davies, B. S. et al. Investigating the purpose of prelamin A processing. Nucleus 2, 4–9 (2011).
Quigley, A. et al. The structural basis of ZMPSTE24-dependent laminopathies. Science 339, 1604–1607 (2013).
Barrowman, J., Hamblet, C., Kane, M. S. & Michaelis, S. Requirements for efficient proteolytic cleavage of prelamin A by ZMPSTE24. PLoS ONE 7, e32120 (2012).
Babatz, T. D. et al. Site specificity determinants for prelamin A cleavage by the zinc metalloprotease ZMPSTE24. J. Biol. Chem. 296, 100165 (2021).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423, 293–298 (2003).
De Sandre-Giovannoli, A. et al. Lamin a truncation in Hutchinson–Gilford progeria. Science 300, 2055 (2003).
Moulson, C. L. et al. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J. Invest. Dermatol. 125, 913–919 (2005).
Agarwal, A. K., Fryns, J. P., Auchus, R. J. & Garg, A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum. Mol. Genet. 12, 1995–2001 (2003).
Shackleton, S. et al. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J. Med. Genet. 42, e36 (2005).
Denecke, J. et al. A homozygous ZMPSTE24 null mutation in combination with a heterozygous mutation in the LMNA gene causes Hutchinson–Gilford progeria syndrome (HGPS): insights into the pathophysiology of HGPS. Hum. Mutat. 27, 524–531 (2006).
Navarro, C. L. et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum. Mol. Genet. 14, 1503–1513 (2005).
Pendas, A. M. et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, 94–99 (2002).
Bergo, M. O. et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl Acad. Sci. USA 99, 13049–13054 (2002).
Fong, L. G. et al. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc. Natl Acad. Sci. USA 101, 18111–18116 (2004).
Varela, I. et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437, 564–568 (2005).
Fong, L. G. et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Investig. 116, 743–752 (2006).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 (2017).
Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Xu, D., Zou, C. & Yuan, J. Genetic regulation of RIPK1 and necroptosis. Annu. Rev. Genet. 55, 235–263 (2021).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Annibaldi, A. & Meier, P. Checkpoints in TNF-induced cell death: implications in inflammation and cancer. Trends Mol. Med. 24, 49–65 (2018).
Osorio, F. G. et al. Nuclear lamina defects cause ATM-dependent NF-kappaB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 26, 2311–2324 (2012).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Wang, H. et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 (2014).
Cai, Z. et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 (2014).
Li, W. et al. Nuclear RIPK1 promotes chromatin remodeling to mediate inflammatory response. Cell Res. 32, 621–637 (2022).
Nguyen, T. M. T., Kim, J., Doan, T. T., Lee, M. W. & Lee, M. APEX proximity labeling as a versatile tool for biological research. Biochemistry 59, 260–269 (2020).
Mifflin, L., Ofengeim, D. & Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 19, 553–571 (2020).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008).
Ofengeim, D. et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 10, 1836–1849 (2015).
Berger, S. B. et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014).
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Kaiser, W. J. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
He, S., Liang, Y., Shao, F. & Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl Acad. Sci. USA 108, 20054–20059 (2011).
Amin, P. et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFalpha-mediated apoptosis. Proc. Natl Acad. Sci. USA 115, E5944–E5953 (2018).
Wang, L., Du, F. & Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
Dondelinger, Y. et al. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell 60, 63–76 (2015).
Lin, F. & Worman, H. J. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, 16321–16326 (1993).
Mandal, P. et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481–495 (2014).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Meng, H. et al. Death–domain dimerization-mediated activation of RIPK1 controls necroptosis and RIPK1-dependent apoptosis. Proc. Natl Acad. Sci. USA 115, E2001–E2009 (2018).
Xu, D. et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell 174, 1477–1491 e1419 (2018).
Dickens, L. S., Powley, I. R., Hughes, M. A. & MacFarlane, M. The ‘complexities’ of life and death: death receptor signalling platforms. Exp. Cell Res. 318, 1269–1277 (2012).
Yoon, S., Bogdanov, K., Kovalenko, A. & Wallach, D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell Death Differ. 23, 253–260 (2016).
Jang, K. H., Jang, T., Son, E., Choi, S. & Kim, E. Kinase-independent role of nuclear RIPK1 in regulating parthanatos through physical interaction with PARP1 upon oxidative stress. Biochim. Biophys. Acta Mol. Cell Res. 1865, 132–141 (2018).
Weber, K., Roelandt, R., Bruggeman, I., Estornes, Y. & Vandenabeele, P. Nuclear RIPK3 and MLKL contribute to cytosolic necrosome formation and necroptosis. Commun. Biol. 1, 6 (2018).
Kleinig, H. Nuclear membranes from mammalian liver. II. Lipid composition. J. Cell Biol. 46, 396–402 (1970).
Dondelinger, Y. et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 (2014).
Davies, B. S. et al. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum. Mol. Genet. 19, 2682–2694 (2010).
Fong, L. G. et al. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311, 1621–1623 (2006).
McClintock, D., Gordon, L. B. & Djabali, K. Hutchinson–Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc. Natl Acad. Sci. USA 103, 2154–2159 (2006).
Murtada, S. I. et al. Lonafarnib improves cardiovascular function and survival in a mouse model of Hutchinson–Gilford progeria syndrome. Elife 12, e82728 (2023).
Barcena, C. et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242 (2019).
Preston, S. P. et al. Epigenetic silencing of RIPK3 in hepatocytes prevents MLKL-mediated necroptosis from contributing to liver pathologies. Gastroenterology 163, 1643–1657 e1614 (2022).
Morgun, E. I., Pozdniakova, E. D. & Vorotelyak, E. A. Expression of protein kinases RIPK-1 and RIPK-3 in mouse and human hair follicle. Dokl. Biochem. Biophys. 494, 252–255 (2020).
Zheng, M. et al. Hair growth promotion by necrostatin-1s. Sci. Rep. 10, 17622 (2020).
Dannappel, M. et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513, 90–94 (2014).
Li, W. & Yuan, J. Targeting RIPK1 kinase for modulating inflammation in human diseases. Front. Immunol. 14, 1159743 (2023).
Ho, Y. H. et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 25, 407–418 e406 (2019).
Wang, F. et al. Generation of a Hutchinson–Gilford progeria syndrome monkey model by base editing. Protein Cell 11, 809–824 (2020).
Kwan, R. et al. Hepatocyte-specific deletion of mouse lamin A/C leads to male-selective steatohepatitis. Cell Mol. Gastroenterol. Hepatol. 4, 365–383 (2017).
Nam, H. J. et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol. 81, 12260–12271 (2007).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Newton, K. et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431 (2019).
Young, S. G., Meta, M., Yang, S. H. & Fong, L. G. Prelamin A farnesylation and progeroid syndromes. J. Biol. Chem. 281, 39741–39745 (2006).
Zhang, T. et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129 e1113 (2020).
Tan, S. et al. Hepatocyte-specific TAK1 deficiency drives RIPK1 kinase-dependent inflammation to promote liver fibrosis and hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 117, 14231–14242 (2020).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Gagnon, K. T., Li, L., Janowski, B. A. & Corey, D. R. Analysis of nuclear RNA interference in human cells by subcellular fractionation and Argonaute loading. Nat. Protoc. 9, 2045–2060 (2014).
Brunt, E. M. et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53, 810–820 (2011).
Acknowledgements
We thank the staff members of the Animal Facility at the National Facility for Protein Science in Shanghai (NFPS) for providing technical support. This work was supported in part by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB39030600 to D.X. and XDB39030200 to J.Y.), the STI2030-Major Projects (2022ZD0213200 to D.X.), the National Natural Science Foundation of China (32350022 and 32070737 to D.X., and 92049303 to J.Y.), the Shanghai Science and Technology Development Funds (20JC1411600 and 20QA1411500 to D.X., and 22JC1410400 to J.Y.), the Chinese Academy of Sciences Youth Interdisciplinary Team (JCTD-2022-10 to D.X.), the Shanghai Key Laboratory of Aging Studies (19DZ2260400 to D.X. and J.Y.) and Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to D.X. and J.Y.).
Author information
Authors and Affiliations
Contributions
This project was conceived, designed and directed by D.X. Y.Y. designed and conducted the majority of the experiments. J.Z. performed the AAV-mediated gene editing in mouse liver and assisted with animal experiments. M.L. assisted with the microcomputed tomography scans. Q.S. and L.Y. assisted with animal experiments. B.S. and M.Z. conducted the mass spectrometry analysis. N.C. and C.Z. assisted with flow cytometry analysis. J.Y. provided helpful discussions. The manuscript was written by D.X. and edited by J.Y.
Corresponding author
Ethics declarations
Competing interests
Authors claim no conflict of interest.
Peer review
Peer review information
Nature Cell Biology thanks Zheng Gang Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of RIPK1 as a binding protein of Prelamin A.
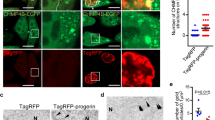
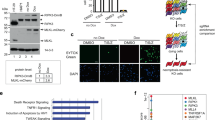
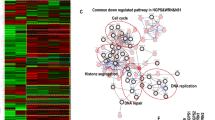
a, Biogenesis of lamin A and the failure to generate mature lamin A in the setting of ZMPSTE24 deficiency. Prelamin A normally undergoes four posttranslational processing steps (left panel). First, the cysteine of the CaaX motif is farnesylated by FTase. Second, the -aaX is released. Third, the newly exposed farnesylcysteine is methylated. Fourth, the carboxyl-terminal 15 amino acids, including the farnesylcysteine methyl ester, are clipped off by ZMPSTE24 and degraded, releasing mature lamin A. In the setting of ZMPSTE24 deficiency (right panel), the last endoproteolytic processing step does not occur, resulting in the accumulation of the farnesylated form of prelamin A. b, Overall workflow for APEX2-mediated proximity biotinylation and mass spectrometry analysis for identification of prelamin A interactors. c, The truncated RIPK1 Flag-fusion protein constructs used for domain mapping. FL, full-length; KD, kinase domain; ID, intermediate domain; DD, death domain. d, HEK293T cells were transfected with expression plasmids of Myc-tagged uncleavable prelamin A and Flag-tagged full-length RIPK1 or truncation mutants of RIPK1 as indicated for 24 h. The cell lysates were immunoprecipitated with anti-Flag-conjugated beads. The interaction of prelamin A and RIPK1 truncation mutants was revealed by immunoblotting. e, HEK293T cells were transfected with expression plasmids of Flag-tagged DD (death domain)-containing protein and Myc-tagged uncleavable prelamin A as indicated for 24 h. The cell lysates were immunoprecipitated with anti-Flag-conjugated beads. Immunoprecipitates were analyzed by immunoblotting. f, The truncated prelamin A Myc-fusion protein constructs used for domain mapping. g, HEK293T cells were transfected with expression plasmids of Flag-tagged RIPK1 and Myc-tagged uncleavable full-length prelamin A or its truncation mutants as indicated for 24 h. The cell lysates were then immunoprecipitated with anti-Flag-conjugated beads. Immunoprecipitates were analyzed by immunoblotting.
Extended Data Fig. 2 ZMPSTE24 deficiency promotes cell death in response to TNF stimulation.
a, b, Primary Zmpste24+/+ and Zmpste24-/- MEFs (a) and BMDMs (b) were stimulated with TNF (10 ng/ml) for the indicated time and the whole-cell lysates were immunoblotted as indicated. c, Representative images of SytoxGreen staining of primary Zmpste24+/+ and Zmpste24-/- MEFs treated with TNF (T, 10 ng/ml) in the presence or absence of Nec-1s (N, 10 μM) for 24 h. Quantification of SytoxGreen+ cells is shown below. Data are represented as mean ± s.d. of three independent wells of one representative experiment. Unpaired two-tailed Student’s t test. d, MEFs were transfected with the indicated siRNA targeting Zmpste24 for 48 h, and then stimulated with TNF (10 ng/ml) in the presence or absence of Nec-1s (10 μM) for 24 h. Cell death was measured by SytoxGreen positivity assay. Data are represented as mean ± s.d. of three independent wells of one representative experiment. One-way ANOVA, post hoc Dunnett’s test. Knockdown efficiency of Zmpste24 by siRNA was confirmed by RT-PCR using specific primers for Zmpste24 mRNA and is shown on the right. PCR bands were normalized using the internal control Actb.
Extended Data Fig. 3 ZMPSTE24 deficiency sensitizes cells to necroptosis.
a, Primary Zmpste24+/+ and Zmpste24-/- MEFs were pretreated with SM-164 (100 nM) and zVAD (10 μM) in the presence or absence of Nec-1s (10 μM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. Data are represented as mean ± s.d. of three independent wells of one representative experiment. b, Primary Zmpste24+/+ and Zmpste24-/- MEFs were pretreated with SM-164 (100 nM) and zVAD (10 μM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. The levels of p-RIPK1, p-RIPK3 and p-MLKL were determined by immunoblotting. c, Primary Zmpste24+/+ and Zmpste24-/- BMDMs were pretreated with zVAD (10 μM) in the presence or absence of Nec-1s (10 μM) for 0.5 h followed by LPS (50 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. Data are represented as mean ± s.d. of three independent wells of one representative experiment. d, Primary Zmpste24+/+ and Zmpste24-/- BMDMs were pretreated with zVAD (10 μM) for 0.5 h followed by LPS (50 ng/ml) stimulation for the indicated time. The levels of p-RIPK1, p-RIPK3 and p-MLKL were determined by immunoblotting. e, Representative images of SytoxGreen staining of primary BMDMs of the indicated genotypes treated with zVAD (10 μM) and LPS (50 ng/ml) for 12 h. Quantification of SytoxGreen+ cells is shown on the right. Data are represented as mean ± s.d. of three independent wells of one representative experiment. One-way ANOVA, post hoc Dunnett’s test.
Extended Data Fig. 4 ZMPSTE24 deficiency does not promote RIPK1-dependent caspase activation.
a, Primary Zmpste24+/+ and Zmpste24-/- MEFs were pretreated with 5z7 (100 nM) in the presence or absence of Nec-1s (10 μM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. b, Primary MEFs of the indicated genotypes were pretreated with 5z7 (100 nM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. c, Primary Zmpste24+/+ and Zmpste24-/- MEFs were pretreated with 5z7 (100 nM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. The levels of CC3, p-RIPK1, p-RIPK3 and p-MLKL were determined by immunoblotting. d, Primary MEFs of the indicated genotypes were pretreated with 5z7 (100 nM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. e, f, Primary Zmpste24+/+ and Zmpste24-/- MEFs were pretreated with SM-164 (100 nM) in the presence or absence of Nec-1s (10 μM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay (e). The levels of CC3, p-RIPK1, p-RIPK3 and p-MLKL were determined by immunoblotting (f). g, Primary MEFs of the indicated genotypes were pretreated with SM-164 (100 nM) for 0.5 h followed by TNF (10 ng/ml) stimulation for the indicated time. Cell death was measured as a function of time by SytoxGreen positivity assay. Data are represented as mean ± s.d. of n = 4 (a and b), 5 (d) or 3 (e and g) independent wells of one representative experiment.
Extended Data Fig. 5 Prelamin A-initiated necroptosis induces nuclear envelope disruption and requires farnesylation.
a, Schematic diagram of antisense oligonucleotide (ASO)-mediated knockdown of prelamin A/lamin A. b, Primary MEFs were stimulated by Flag-TNF (100 ng/ml) for indicated time. Complex I was isolated and analyzed by immunoblotting. c, Primary MEFs were transfected with siRNA targeting RIPK1 for 48 h, then treated with TNF (10 ng/ml)/CHX (1 μg/ml)/zVAD (10 μM) for 4 h. Activated RIPK1, RIPK3 and MLKL binding with prelamin A was revealed by immunoprecipitation-immunoblotting. d, Nuclear cytoplasmic fractionation and immunoblotting were carried out on MEFs of indicated genotypes. C, cytoplasm; N, nucleus. e, f, Primary MEFs were treated with TNF (10 ng/ml)/CHX (1 μg/ml)/zVAD (10 μM) for 4 h. Then, cells were stained with anti-LAP2b and DAPI. A reconstructed 3Ds image characterizing the nuclear envelope disruption and DNA leakage are shown (e). Quantification of cells with DNA leakage is shown (f). g, Primary MEFs were treated with TNF (10 ng/ml)/CHX (1 μg/ml)/zVAD (10 μM) in the presence of GSK’872 (1 μM) for 4 h. Then, cells were stained with anti-lamin B1 and DAPI. Arrows point to envelope breaches (lamin B1 staining) and nuclear DNA herniation and leakage (DAPI). Quantification of cells with DNA leakage is shown on the right. Unpaired two-tailed Student’s t test. h, i, Primary MEFs were preincubated with 5 μM FTI-277 (h) or 5 μM SCH66336 (i) for 72 h, then treated with TNF (10 ng/ml)/5z7 (100 nM) for indicated time. Cell death was measured by SytoxGreen positivity assay. j, Primary MEFs were preincubated with FTI-277 (5 μM) for 72 h, then treated with TNF (10 ng/ml)/CHX (1 μg/ml)/zVAD (10 μM) for 3 h. Nuclear cytoplasmic fractionation and immunoblotting were carried out. Data are represented as mean ± s.d. of n = 8 (f and g), 4 (h and i) independent wells of one representative experiment.
Extended Data Fig. 6 Activation of RIPK1 and necroptosis in multiple tissues of Zmpste24-/- mice.
a, b, p-S166 RIPK1 (a) and p-T231/S232 RIPK3 (b) immunostaining was carried out on skin, intestine, liver, lung, kidney, spleen, and heart sections from 3-month-old mice of the indicated genotypes. Representative images are shown. n = 6 mice for each group. Arrows point to bulge region. Quantification of p-RIPK1+ and p-RIPK3+ cells in each tissue is shown in Fig. 7a, b, respectively. c, Representative images of p-MLKL immunostaining in skin and intestine sections from 3-month-old mice of the indicated genotypes. n = 8 mice for each group.
Extended Data Fig. 7 Genetic inhibition of RIPK1 and necroptosis suppress inflammation in Zmpste24-/- mice.
a, p-S345 MLKL immunostaining and TUNEL staining were carried out on liver sections from 3-month-old mice of the indicated genotypes. Representative images out of n = 6 mice for each group were shown. b, Quantitative RT-PCR analysis of relative mRNA levels of inflammatory gene expression in the livers from 3-month-old mice of the indicated genotypes. Data are represented as mean ± s.e.m. of n = 6 (Zmpste24+/+ and Zmpste24-/-;Ripk1D138N/D138N), n = 8 (Zmpste24-/-) mice. c, CD45 immunohistochemistry was carried out on liver sections from 3-month-old mice of the indicated genotypes. Representative images are shown. Arrowheads indicate foci of macrophage infiltration. Quantification of CD45+ foci is shown below. Data are represented as mean ± s.e.m. of n = 8 mice for each group. d, The concentration of TNF in serum from 3-month-old mice of the indicated genotypes was determined by ELISA. Data are represented as mean ± s.e.m. of n = 6 (Zmpste24+/+, Zmpste24-/-;Ripk1D138N/D138N, Zmpste24-/-;Ripk3-/-, and Zmpste24-/-;Mlkl-/-), n = 9 (Zmpste24-/-) mice. e, Flow cytometry gating strategy. Representative schema describes the sequential gating to identify specific peripheral blood subsets. Red cell lysed whole blood was stained with CD45, CD11b and Ly6G. Single cell suspensions were first gated using forward scatter/side scatter (FSC vs SSC) to exclude debris, then gated using CD45 and CD11b to identify myeloid cells (CD45− and CD11b+), neutrophils were then identified as CD11b+ and Ly6G+. f, Representative flow cytometric analysis of neutrophil levels in whole blood from 3-month-old male mice of the indicated genotypes. Neutrophils were gated using CD11b and Ly6G antibodies. Quantification of neutrophil levels in mice is shown below. Data are represented as mean ± s.e.m. of n = 6 mice for each group. Each dot represents an individual mouse (b-d and f). One-way ANOVA, post hoc Dunnett’s test (b-d and f).
Extended Data Fig. 8 Farnesyltransferase inhibitor inhibits necroptosis and inflammation in Zmpste24-/- mice.
a, Zmpste24-/- mice were intraperitoneally injected with 40 mg/kg FTI-277 or vehicle (saline) daily beginning at 2 months of age for one month. Then, p-RIPK1, p-RIPK3, and p-MLKL immunostaining was carried out on skin and intestine sections from these mice. Representative images are shown. Quantification of p-RIPK1+, p-RIPK3+, and p-MLKL+ cells is shown on the right. Data are represented as mean ± s.e.m. of n = 6 mice for each group. Each dot represents an individual mouse. Unpaired two-tailed Student’s t test. b, TUNEL staining was carried out on the skin and intestine sections from the mice in (a). Representative images are shown. Quantification of TUNEL+ cells is shown on the right. Data are represented as mean ± s.e.m. of n = 6 mice for each group. Each dot represents an individual mouse. Unpaired two-tailed Student’s t test. c-e, Quantitative RT-PCR analysis of relative mRNA levels of inflammatory gene expression in the skins (c), intestines (d), and livers (e) from mice in (a). Age-matched Zmpste24+/+ mice were used as controls. Data are represented as mean ± s.e.m. of n = 6 mice for each group. Each dot represents an individual mouse. Unpaired two-tailed Student’s t test.
Extended Data Fig. 9 RIPK1-mediated necroptosis does not contribute to disease phenotypes caused by Lmna deficiency.
a, Prelamin A/Lamin A immunoblotting was carried out on bone, liver, skin, and intestine from 3-month-old mice of the indicated genotypes. b, Representative fluorescent microscopy images of liver from 4-mon-old male mice with the indicated genotypes two months after injections with pAAV8-U6-shRNA(Lmna)-Cbh-EGFP-WPRE (AAV8-shRNA-Lmna) or pAAV8-U6-shRNA(Scramble)-Cbh-EGFP-WPRE (AAV8-shRNA-NC). The construct expresses EGFP, which allows for verification of the site and efficacy of infection. Representative images out of n = 6 mice for each group were shown. c, Quantitative RT-PCR analysis of the Lmna mRNA expression levels in livers from (b). Data are presented as mean ± s.e.m. of n = 6 mice for each group. One-way ANOVA, post hoc Dunnett’s test. d, Serum levels of ALT from mice treated in (b). Data are presented as mean ± s.e.m. of n = 6 mice for each group. One-way ANOVA, post hoc Dunnett’s test. e, HE staining of liver sections of mice from (b). Arrows indicate steatosis. f, Nonalcoholic fatty liver disease (NAFLD) activity scores of liver sections of mice from (b). Data are presented as mean ± s.e.m. of n = 6 mice for each group. One-way ANOVA, post hoc Dunnett’s test.
Supplementary information
Supplementary Table 1
Supplementary Table 1. Proteomics analysis of prelamin A-binding proteins using APEX2-based proximity labelling. An excel file of the proteomics data showing the quantification of the abundance of each protein enriched by APEX2-based proximity labelling in APEX2-prelamin A-expressing versus APEX2-lamin A-expressing HEK293T cells. Unpaired two-tailed Student’s t-test.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots and/or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Zhang, J., lv, M. et al. Defective prelamin A processing promotes unconventional necroptosis driven by nuclear RIPK1. Nat Cell Biol 26, 567–580 (2024). https://doi.org/10.1038/s41556-024-01374-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-024-01374-2
This article is cited by
-
RIPK1 and necroptosis role in premature ageing
Nature Cell Biology (2024)