Featured
-
-
Letter |
 Two enzymes bound to one transfer RNA assume alternative conformations for consecutive reactions
Two enzymes bound to one transfer RNA assume alternative conformations for consecutive reactions
In most bacteria and all archaea, glutamyl-tRNA synthetase (GluRS) glutamylates both tRNAGlu and tRNAGln; Glu-tRNAGln is then converted to Gln-tRNAGln by an amidotransferase. Here the structure is reported of a bacterial complex containing tRNAGln, GluRS and the amidotransferase GatCAB. The structure provides an explanation for how the enzymes work consecutively: only one can assume a productive state at any time. There also seems to be an intermediary state in which neither enzyme is productive.
- Takuhiro Ito
- & Shigeyuki Yokoyama
-
Letter |
 Structure of a fucose transporter in an outward-open conformation
Structure of a fucose transporter in an outward-open conformation
In Escherichia coli, the uptake of L-fucose, an important source of carbon for microorganisms, is mediated by a proton symporter from the major facilitator superfamily (MFS). These authors report the first X-ray crystal structure of the outward-open conformation of an MFS proton transporter, FucP. Building on previous work, they develop a working model for how the substrate is recognized by the transporter and how the protein mediates L-fucose/proton symport.
- Shangyu Dang
- , Linfeng Sun
- & Nieng Yan
-
Letter |
 Conical intersection dynamics of the primary photoisomerization event in vision
Conical intersection dynamics of the primary photoisomerization event in vision
Chemical reactions are usually described in terms of the movement of nuclei between the potential energy surfaces of ground and excited electronic states. Crossings known as conical intersections permit efficient transitions between the surfaces. It is shown here that ultrafast optical spectroscopy, with sub-20-fs time resolution and spectral coverage from the visible to the near-infrared, can map the isomerization of rhodopsin with sufficient resolution to shown that a conical intersection is important in this crucial event in vision.
- Dario Polli
- , Piero Altoè
- & Giulio Cerullo
-
Letter |
 Structure of a cation-bound multidrug and toxic compound extrusion transporter
Structure of a cation-bound multidrug and toxic compound extrusion transporter
Transporter proteins from the MATE (multidrug and toxic compound extrusion) family are involved in metabolite transport in plants, and in multiple-drug resistance in bacteria and mammals. Here, the X-ray crystal structure of a MATE transporter from Vibrio cholerae is reported. The structure is in an outward-facing conformation, and reveals a cation-binding site near to residues previously deemed essential for transport.
- Xiao He
- , Paul Szewczyk
- & Geoffrey Chang
-
-
Article |
 Fundamental limits on the suppression of molecular fluctuations
Fundamental limits on the suppression of molecular fluctuations
Biological systems avoid molecular noise using feedback loops controlling RNA or protein synthesis, but these reactions rely on the stochastic birth and death of molecules. These authors use control and information theory to show that making a genetic network twice as accurate takes 16 times more signalling steps. Nature must therefore call on brute-force solutions to maintain accuracy, and hence does so only when noise suppression is absolutely vital.
- Ioannis Lestas
- , Glenn Vinnicombe
- & Johan Paulsson
-
Letter |
 The structure of (CENP-A–H4)2 reveals physical features that mark centromeres
The structure of (CENP-A–H4)2 reveals physical features that mark centromeres
The centromeres of chromosomes are specified epigenetically, and the histone H3 variant CENP-A is assembled into the chromatin of all active centromeres. Here, the crystal structure of CENP-A in a tetrameric complex with histone H4 reveals the physical features of centromeric chromatin. CENP-A seems to mark the centromere by altering nucleosome structure from within its folded histone core.
- Nikolina Sekulic
- , Emily A. Bassett
- & Ben E. Black
-
Letter |
 Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
The repair enzyme (6–4) photolyase uses light energy to cleave the ultraviolet-induced bond between pyrimidine dimers. These authors use ultrafast spectroscopy to examine the detailed electron and proton movements during the catalytic photocycle. Histidine 364 is identified as the crucial residue involved in the rate-limiting step.
- Jiang Li
- , Zheyun Liu
- & Dongping Zhong
-
Letter |
 Structure of the LexA–DNA complex and implications for SOS box measurement
Structure of the LexA–DNA complex and implications for SOS box measurement
Normally, expression of bacterial DNA damage repair genes is repressed by the binding of LexA protein to SOS ‘boxes’ in their operators. DNA damage activates the RecA protein, which promotes autocleavage of LexA such that its repression is relieved and repair proteins are expressed. These authors solve several structures of LexA dimer bound to SOS box DNA, and find that the orientation of the DNA-binding wings can account for the strict intersite spacing.
- Adrianna P. P. Zhang
- , Ying Z. Pigli
- & Phoebe A. Rice
-
Letter |
 Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
The bacterial flagellar motor drives the rotation of flagellar filaments, propelling bacteria through viscous media. The rotation can switch from an anticlockwise to a clockwise direction, determining a smooth or tumbling motion. A protein called FliG forms a ring in the motor's rotor, and has been proposed to adopt distinct conformations that induce switching. Here, the full-length structure of FliG is presented, and conformational changes are identified that are involved in switching between clockwise and anticlockwise rotations.
- Lawrence K. Lee
- , Michael A. Ginsburg
- & Daniela Stock
-
Article |
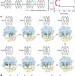 Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
During protein synthesis within the ribosome, transfer RNAs (tRNAs) move sequentially through different sites as their attached amino acids are transferred onto the growing protein chain. Large conformational movements accompany this process. Here, a staggering 1.9 million electron cryomicroscopy images of the ribosome have been processed to visualize these changes. The results reveal that the ribosome functions as a Brownian machine that couples spontaneous changes driven by thermal energy to directed movement.
- Niels Fischer
- , Andrey L. Konevega
- & Holger Stark
-
Letter |
 Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
XXXMicrotubules are nucleated in vivo by γ-tubulin complexes and comprise 13 protofilaments. How this precise geometry is controlled remains unclear. These authors report the cryo-electron microscopic structure of the universally conserved, core microtubule nucleating complex, γ-tubulin small complex. The structure provides insight into how this complex establishes thirteen-fold tubulin symmetry.
- Justin M. Kollman
- , Jessica K. Polka
- & David A. Agard
-
Letter |
 Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Large-conductance Ca2+-gated K+ (BK) channels are essential for many biological processes, such as smooth muscle contraction and neurotransmitter release. Here, the X-ray crystal structure is presented of the entire cytoplasmic region of the human BK channel in a Ca2+-free state. Moreover, a voltage-gated K+ channel pore of known structure is 'docked' onto the gating ring to generate a structural model for the full BK channel.
- Yunkun Wu
- , Yi Yang
- & Youxing Jiang
-
Letter |
 A conserved spider silk domain acts as a molecular switch that controls fibre assembly
A conserved spider silk domain acts as a molecular switch that controls fibre assembly
Spider silk proteins are remarkably soluble when stored at high concentration and yet can be converted to extremely sturdy fibres, through unknown molecular mechanisms. Here, the structure of the evolutionarily conserved carboxy-terminal domain of a silk protein is presented. The results provide evidence that the structural state of this domain is essential for controlled switching between the storage and assembly forms of silk proteins. Such molecular switches might see application in the design of versatile fibrous materials.
- Franz Hagn
- , Lukas Eisoldt
- & Horst Kessler
-
News & Views |
 Transporter in the spotlight
Transporter in the spotlight
Membrane transporter proteins switch between conformational states to move substrates across membranes. The transition between these states can now be studied using single-molecule experiments.
- Nathan K. Karpowich
- & Da-Neng Wang
-
Letter |
 Dislocation nucleation governed softening and maximum strength in nano-twinned metals
Dislocation nucleation governed softening and maximum strength in nano-twinned metals
The strength of conventional metals is determined by the interaction of dislocations with obstacles such as grain boundaries. Molecular dynamics simulations reveal that the strength of ultrafine-grained copper containing twin boundaries can be controlled by a dislocation nucleation mechanism activated below a critical twin thickness. Below this thickness the material becomes softer. The smaller the grains, the smaller the critical twin boundary spacing, and the higher the metal's maximum strength.
- Xiaoyan Li
- , Yujie Wei
- & Huajian Gao
-
Research Highlights |
 Molecular biology: Flowering time unravelled
Molecular biology: Flowering time unravelled

 The proteasome antechamber maintains substrates in an unfolded state
The proteasome antechamber maintains substrates in an unfolded state Functional roles for noise in genetic circuits
Functional roles for noise in genetic circuits