Article
|
Open Access
Featured
-
-
Article |
 A pentameric TRPV3 channel with a dilated pore
A pentameric TRPV3 channel with a dilated pore
High-speed atomic force microscopy single-molecule imaging and cryo-EM analysis discover and reveal the structure of a TRPV3 pentamer, providing evidence for a non-canonical pentameric TRP-channel assembly, laying the foundation for new directions in TRP channel research.
- Shifra Lansky
- , John Michael Betancourt
- & Simon Scheuring
-
Article |
 Structural basis of odorant recognition by a human odorant receptor
Structural basis of odorant recognition by a human odorant receptor
Through the use of cryo-electron microscopy and molecular dynamics stimulations, mechanistic insight into the binding of an odorant to the human odorant receptor OR51E2 is provided.
- Christian B. Billesbølle
- , Claire A. de March
- & Aashish Manglik
-
Article |
 Time-resolved structural analysis of an RNA-cleaving DNA catalyst
Time-resolved structural analysis of an RNA-cleaving DNA catalyst
Using high-resolution NMR characterization, the kinetics and dynamics of the catalytic function of a DNAzyme are shown.
- Jan Borggräfe
- , Julian Victor
- & Manuel Etzkorn
-
Article |
 Systems-level effects of allosteric perturbations to a model molecular switch
Systems-level effects of allosteric perturbations to a model molecular switch
Interface mutations in the GTPase switch protein Gsp1 (the yeast homologue of human RAN) allosterically affect the kinetics of the switch cycle, revealing a systems-level mechanism of multi-specificity.
- Tina Perica
- , Christopher J. P. Mathy
- & Tanja Kortemme
-
Article |
 Cryo-EM structures of full-length Tetrahymena ribozyme at 3.1 Å resolution
Cryo-EM structures of full-length Tetrahymena ribozyme at 3.1 Å resolution
Cryo-electron microscopy has been used to determine the structure of the Tetrahymena ribozyme (a catalytic RNA) at sufficiently high resolution to model side chains and metal ions.
- Zhaoming Su
- , Kaiming Zhang
- & Wah Chiu
-
Article |
 HP1 reshapes nucleosome core to promote phase separation of heterochromatin
HP1 reshapes nucleosome core to promote phase separation of heterochromatin
The S. pombe HP1 protein Swi6 couples chromatin compaction to phase separation by dynamically exposing buried histone residues within nucleosomes.
- S. Sanulli
- , M. J. Trnka
- & G. J. Narlikar
-
Article |
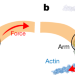 Force-induced conformational changes in PIEZO1
Force-induced conformational changes in PIEZO1
Cryo-electron microscopy and high-speed atomic force microscopy reveal that PIEZO1 can reversibly deform its shape towards a planar structure, which may explain how the PIEZO1 channel is gated in response to mechanical stimulation.
- Yi-Chih Lin
- , Yusong R. Guo
- & Simon Scheuring
-
Letter |
 Structural principles of distinct assemblies of the human α4β2 nicotinic receptor
Structural principles of distinct assemblies of the human α4β2 nicotinic receptor
Cryo-electron microscopy structures of two stoichiometries of heteromeric acetylcholine receptors in complex with nicotine reveal principles of subunit assembly and the structural basis of the distinctive biophysical and pharmacological properties of the different stoichiometries.
- Richard M. Walsh Jr
- , Soung-Hun Roh
- & Ryan E. Hibbs
-
Article |
 Force interacts with macromolecular structure in activation of TGF-β
Force interacts with macromolecular structure in activation of TGF-β
Integrin αVβ6 binds the transforming growth factor-β1 precursor (pro-TGF-β1) in an orientation that is biologically relevant for force-dependent release of TGF-β from its latent form.
- Xianchi Dong
- , Bo Zhao
- & Timothy A. Springer
-
Letter |
 Basis of catalytic assembly of the mitotic checkpoint complex
Basis of catalytic assembly of the mitotic checkpoint complex
The near-complete in vitro reconstitution of the mitotic spindle assembly checkpoint reveals how the assembly of its effector, the mitotic checkpoint complex, is catalysed.
- Alex C. Faesen
- , Maria Thanasoula
- & Andrea Musacchio
-
Letter |
 Structural basis of co-translational quality control by ArfA and RF2 bound to ribosome
Structural basis of co-translational quality control by ArfA and RF2 bound to ribosome
The structure of the bacterial ribosome in complex with the ArfA and the release factor RF2 shows how ArfA recruits RF2 to terminate translation of messenger RNAs that lack a stop codon in the ribosome.
- Fuxing Zeng
- , Yanbo Chen
- & Hong Jin
-
Article |
 Visualizing transient Watson–Crick-like mispairs in DNA and RNA duplexes
Visualizing transient Watson–Crick-like mispairs in DNA and RNA duplexes
dG•dT and rG•rU ‘wobble’ mispairs in DNA and RNA transiently form base pairs with Watson–Crick geometry via tautomerization and ionization with probabilities that correlate with misincorporation probabilities during replication and translation.
- Isaac J. Kimsey
- , Katja Petzold
- & Hashim M. Al-Hashimi
-
Letter |
 Ubiquitin chain conformation regulates recognition and activity of interacting proteins
Ubiquitin chain conformation regulates recognition and activity of interacting proteins
Single-molecule FRET assays used to probe the conformational dynamics of ubiquitin chains reveal that conformational selection is an important mechanism of ubiquitin chain recognition.
- Yu Ye
- , Georg Blaser
- & David Komander
-
News & Views |
 A toolbox for protein design
A toolbox for protein design
Some of the principles underlying how amino-acid sequences determine the three-dimensional structures of proteins have been defined. This has enabled a successful approach to designing protein folds from scratch. See Article p.222
- Birte Höcker
-
Article |
 Visualizing transient low-populated structures of RNA
Visualizing transient low-populated structures of RNA
This study develops an NMR-based approach that can capture previously inaccessible, highly transient, low-populated ‘excited states’ in RNA; the localized rearrangements in base-pairing giving rise to these states are found to affect function by changing the exposure of residues required for a specific biological process.
- Elizabeth A. Dethoff
- , Katja Petzold
- & Hashim M. Al-Hashimi
-
Review Article |
 Functional complexity and regulation through RNA dynamics
Functional complexity and regulation through RNA dynamics
- Elizabeth A. Dethoff
- , Jeetender Chugh
- & Hashim M. Al-Hashimi
-
Article |
 X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
The X-ray crystal structure of LeuT, the bacterial homologue of the neurotransmitter sodium symporter family, is reported in the outward-open and inward-open states.
- Harini Krishnamurthy
- & Eric Gouaux
-
Article |
 Antiparallel EmrE exports drugs by exchanging between asymmetric structures
Antiparallel EmrE exports drugs by exchanging between asymmetric structures
NMR and single molecule FRET experiments show that antiparallel EmrE dimers interconvert between two identical but oppositely oriented conformations that are each open only to one side of the membrane.
- Emma A. Morrison
- , Gregory T. DeKoster
- & Katherine A. Henzler-Wildman
-
Letter |
 Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel
Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel
The crystal structure of the calcium-bound gating ring of a calcium- and voltage-activated potassium channel shows in detail how the effect of calcium binding on the gating ring produces the conformational change from closed to open.
- Peng Yuan
- , Manuel D. Leonetti
- & Roderick MacKinnon
-
Letter |
 Electrically driven directional motion of a four-wheeled molecule on a metal surface
Electrically driven directional motion of a four-wheeled molecule on a metal surface
- Tibor Kudernac
- , Nopporn Ruangsupapichat
- & Ben L. Feringa
-
Letter |
 Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase
Structural basis for a [4Fe-3S] cluster in the oxygen-tolerant membrane-bound [NiFe]-hydrogenase
- Yasuhito Shomura
- , Ki-Seok Yoon
- & Yoshiki Higuchi
-
Letter |
 Conformational changes in the G protein Gs induced by the β2 adrenergic receptor
Conformational changes in the G protein Gs induced by the β2 adrenergic receptor
- Ka Young Chung
- , Søren G. F. Rasmussen
- & Roger K. Sunahara
-
Letter |
 Structural basis of RNA recognition and activation by innate immune receptor RIG-I
Structural basis of RNA recognition and activation by innate immune receptor RIG-I
- Fuguo Jiang
- , Anand Ramanathan
- & Joseph Marcotrigiano
-
Letter |
 Structures of the RNA-guided surveillance complex from a bacterial immune system
Structures of the RNA-guided surveillance complex from a bacterial immune system
- Blake Wiedenheft
- , Gabriel C. Lander
- & Eva Nogales
-
Letter |
 Solution structure of a minor and transiently formed state of a T4 lysozyme mutant
Solution structure of a minor and transiently formed state of a T4 lysozyme mutant
- Guillaume Bouvignies
- , Pramodh Vallurupalli
- & Lewis E. Kay
-
Letter |
 Multi-domain conformational selection underlies pre-mRNA splicing regulation by U2AF
Multi-domain conformational selection underlies pre-mRNA splicing regulation by U2AF
- Cameron D. Mackereth
- , Tobias Madl
- & Michael Sattler
-
Letter |
 Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
Structural basis for site-specific ribose methylation by box C/D RNA protein complexes
A complex of RNA and protein known as the box C/D RNP catalyses the site-specific modification of RNAs with a 2′-O-methylation group. The structure of the full complex has now been solved, including the guide RNA and either of two substrate RNAs. This structure reveals how the guide and target RNAs are aligned, and how the methyltransferase subunit, fibrillarin, facilitates placement of the target ribose into the active site.
- Jinzhong Lin
- , Shaomei Lai
- & Keqiong Ye
-
Letter |
 Structure and function of an irreversible agonist-β2 adrenoceptor complex
Structure and function of an irreversible agonist-β2 adrenoceptor complex
The X-ray crystal structure of the human β2 adrenergic receptor, a G-protein-coupled receptor (GPCR), covalently bound to a small-molecule agonist is solved. Comparison of this structure with structures of this GPCR in an inactive state and in an antibody-stabilized active state reveals how binding events at both the extracellular and intracellular surfaces stabilize the active conformation of the receptor. Molecular dynamics simulations suggest that the agonist-bound active state spontaneously relaxes to an inactive-like state in the absence of a G protein.
- Daniel M. Rosenbaum
- , Cheng Zhang
- & Brian K. Kobilka
-
Letter |
 Sensing the anomeric effect in a solvent-free environment
Sensing the anomeric effect in a solvent-free environment
The anomeric effect is a chemical phenomenon that refers to an observed stabilization of six-membered carbohydrate rings when they contain an electronegative substituent at the C1 position of the ring. This stereoelectronic effect influences the three-dimensional shapes of many biological molecules, but the underlying physical origin is unclear. Here it is shown that complexes formed between a truncated peptide motif and an isolated sugar in the gas phase are nearly identical structurally; however, the strength of the polarization of their interactions with the peptide differs greatly. It will be important to re-evaluate the influence, and biological effects, of substituents at position C2 of the six-membered carbohydrate rings.
- Emilio J. Cocinero
- , Pierre Carcabal
- & Benjamin G. Davis
-
Letter |
 Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites
During translation, tRNAs enter the ribosome and then move sequentially through three sites, known as A, P and E, as they transfer their attached amino acids onto the growing peptide chain. How the ribosome facilitates tRNA translocation between the sites remains largely unknown. Now a study uses multiparticle cryoelectron microscopy of a ribosome bound to the translation elongation factor, EF-G, to get information about tRNA movement. It identifies two new substates and sees that translocation is linked to unratcheting of the 30S ribosomal subunit.
- Andreas H. Ratje
- , Justus Loerke
- & Christian M. T. Spahn
-
Letter |
 Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography
The E1 and E2 glycoproteins of alphaviruses form heterodimers and assemble into spikes on the virus surface, which mediate receptor binding and endocytosis. When the virion encounters acidic pH in the endosome E1 and E2 dissociate and E1 triggers fusion with the endosomal membrane. Two papers now provide the first crystal structures for glycoprotein complexes incorporating E2 at acidic and neutral pH, respectively. Together they provide insight into how fusion activation is controlled in alphaviruses.
- James E. Voss
- , Marie-Christine Vaney
- & Félix A. Rey
-
Letter |
 Structure of a fucose transporter in an outward-open conformation
Structure of a fucose transporter in an outward-open conformation
In Escherichia coli, the uptake of L-fucose, an important source of carbon for microorganisms, is mediated by a proton symporter from the major facilitator superfamily (MFS). These authors report the first X-ray crystal structure of the outward-open conformation of an MFS proton transporter, FucP. Building on previous work, they develop a working model for how the substrate is recognized by the transporter and how the protein mediates L-fucose/proton symport.
- Shangyu Dang
- , Linfeng Sun
- & Nieng Yan
-
Letter |
 Structure of a cation-bound multidrug and toxic compound extrusion transporter
Structure of a cation-bound multidrug and toxic compound extrusion transporter
Transporter proteins from the MATE (multidrug and toxic compound extrusion) family are involved in metabolite transport in plants, and in multiple-drug resistance in bacteria and mammals. Here, the X-ray crystal structure of a MATE transporter from Vibrio cholerae is reported. The structure is in an outward-facing conformation, and reveals a cation-binding site near to residues previously deemed essential for transport.
- Xiao He
- , Paul Szewczyk
- & Geoffrey Chang
-
Letter |
 Structure of the LexA–DNA complex and implications for SOS box measurement
Structure of the LexA–DNA complex and implications for SOS box measurement
Normally, expression of bacterial DNA damage repair genes is repressed by the binding of LexA protein to SOS ‘boxes’ in their operators. DNA damage activates the RecA protein, which promotes autocleavage of LexA such that its repression is relieved and repair proteins are expressed. These authors solve several structures of LexA dimer bound to SOS box DNA, and find that the orientation of the DNA-binding wings can account for the strict intersite spacing.
- Adrianna P. P. Zhang
- , Ying Z. Pigli
- & Phoebe A. Rice
-
Letter |
 Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching
The bacterial flagellar motor drives the rotation of flagellar filaments, propelling bacteria through viscous media. The rotation can switch from an anticlockwise to a clockwise direction, determining a smooth or tumbling motion. A protein called FliG forms a ring in the motor's rotor, and has been proposed to adopt distinct conformations that induce switching. Here, the full-length structure of FliG is presented, and conformational changes are identified that are involved in switching between clockwise and anticlockwise rotations.
- Lawrence K. Lee
- , Michael A. Ginsburg
- & Daniela Stock
-
Article |
 Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy
During protein synthesis within the ribosome, transfer RNAs (tRNAs) move sequentially through different sites as their attached amino acids are transferred onto the growing protein chain. Large conformational movements accompany this process. Here, a staggering 1.9 million electron cryomicroscopy images of the ribosome have been processed to visualize these changes. The results reveal that the ribosome functions as a Brownian machine that couples spontaneous changes driven by thermal energy to directed movement.
- Niels Fischer
- , Andrey L. Konevega
- & Holger Stark
-
Letter |
 Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry
XXXMicrotubules are nucleated in vivo by γ-tubulin complexes and comprise 13 protofilaments. How this precise geometry is controlled remains unclear. These authors report the cryo-electron microscopic structure of the universally conserved, core microtubule nucleating complex, γ-tubulin small complex. The structure provides insight into how this complex establishes thirteen-fold tubulin symmetry.
- Justin M. Kollman
- , Jessica K. Polka
- & David A. Agard
-
Letter |
 Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel
Large-conductance Ca2+-gated K+ (BK) channels are essential for many biological processes, such as smooth muscle contraction and neurotransmitter release. Here, the X-ray crystal structure is presented of the entire cytoplasmic region of the human BK channel in a Ca2+-free state. Moreover, a voltage-gated K+ channel pore of known structure is 'docked' onto the gating ring to generate a structural model for the full BK channel.
- Yunkun Wu
- , Yi Yang
- & Youxing Jiang
-
News & Views |
 Transporter in the spotlight
Transporter in the spotlight
Membrane transporter proteins switch between conformational states to move substrates across membranes. The transition between these states can now be studied using single-molecule experiments.
- Nathan K. Karpowich
- & Da-Neng Wang

 Structures of a sperm-specific solute carrier gated by voltage and cAMP
Structures of a sperm-specific solute carrier gated by voltage and cAMP