Research Highlights |
Featured
-
-
News & Views |
 Knockout for malaria
Knockout for malaria
Discovering and validating new targets is urgently required to tackle the rise in resistance to antimalarial drugs. Now, inhibition of the enzyme N-myristoyltransferase has been shown to prevent the formation of a critical subcellular organelle in the parasite that causes malaria, leading to death of the parasite.
- Joanna Krysiak
- & Stephan A. Sieber
-
Article |
 Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach
Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach
Chemical validation of new drug targets is urgently required to help develop new antimalarial therapies. Here, chemical proteomic tools and selective enzyme inhibitors are combined to study protein lipidation in human malaria parasites, leading to in vitro and in vivo validation of the enzyme N-myristoyltransferase as a drug target.
- Megan H. Wright
- , Barbara Clough
- & Edward W. Tate
-
News & Views |
 A stitch in time
A stitch in time
Lengthy molecular dynamics simulations of complex systems at the atomic scale usually require supercomputers. Now, by stitching together many shorter independent simulations run 'in the cloud', this requirement has been circumvented, allowing two milliseconds of the dynamics of a G-protein-coupled receptor to be simulated.
- Xavier Deupi
-
Article |
 Cloud-based simulations on Google Exacycle reveal ligand modulation of GPCR activation pathways
Cloud-based simulations on Google Exacycle reveal ligand modulation of GPCR activation pathways
Two milliseconds of molecular dynamics simulations of a major drug-target G-protein-coupled receptor (GPCR) has been carried out using Google's Exacycle cloud computing platform. Markov state models were used to aggregate independent simulations into a statistical model that provides an atomistic description of GPCR ligand-modulated activation pathways.
- Kai J. Kohlhoff
- , Diwakar Shukla
- & Vijay S. Pande
-
Article |
 Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate
Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate
Natural products are enduring leads for exploring cell biology, yet structure–activity relationship studies and 'arming' of these small molecules for subsequent cellular probe synthesis remains a challenge. Here, a strategy for derivatization of natural products by C–H amination, aziridination and unusual N-aminations is described. Selective derivatization of eupalmarin acetate led to identification of this natural product's target.
- Jing Li
- , Justin S. Cisar
- & Daniel Romo
-
Article |
 Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90
Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90
The heat shock protein Hsp90 is a potential target for cancer and neurodegeneration drugs. Here, the introduction of a substituent into the 19-position of the naturally occurring inhibitor geldanamycin by chemical synthesis is shown to ameliorate toxicity, and also cause a favourable conformational switch that is required for protein binding.
- Russell R. A. Kitson
- , Chuan-Hsin Chang
- & Christopher J. Moody
-
-
News & Views |
 Diversifying complexity
Diversifying complexity
A synthetic strategy that uses a series of simple reactions to distort the core architecture of complex natural products could provide libraries of stereochemically rich compounds that will help in the search for new biological probes and drugs.
- Indrajeet Sharma
- & Derek S. Tan
-
Review Article |
 Inhibition of α-helix-mediated protein–protein interactions using designed molecules
Inhibition of α-helix-mediated protein–protein interactions using designed molecules
α-Helix-mediated protein–protein interactions (PPIs) play a key role in the development of numerous infection and disease states. Modulating such interactions offers considerable therapeutic potential, however, identifying suitable inhibitors has proved challenging. This Review highlights recent and generic approaches for designing inhibitors of helix-mediated PPIs.
- Valeria Azzarito
- , Kérya Long
- & Andrew J. Wilson
-
Article |
 Snapshot of the equilibrium dynamics of a drug bound to HIV-1 reverse transcriptase
Snapshot of the equilibrium dynamics of a drug bound to HIV-1 reverse transcriptase
Conformational changes are known to occur during binding of the anti-AIDS drug rilpivirine to HIV-1 reverse transcriptase, an essential enzyme for the replication of HIV. Vibrational spectroscopy, X-ray crystallography and simulations now show that water molecules play an essential role in this binding process, which may help it retain potency despite mutations within the binding pocket.
- Daniel G. Kuroda
- , Joseph D. Bauman
- & Robin M. Hochstrasser
-
Article |
 A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
An approach for the construction of complex and diverse compound libraries is described, whereby natural products are altered through a series of ring system distortion reactions. The compounds produced have markedly different physiochemical properties from those in standard screening collections and thus could offer advantages in the search for lead molecules that can be developed into drug candidates.
- Robert W. Huigens III
- , Karen C. Morrison
- & Paul J. Hergenrother
-
News & Views |
 Nature's pieces
Nature's pieces
Natural products contain a range of chemical structures optimized for biological interactions. Fragmenting these compounds could help to combine this diversity with the broad coverage of chemical space offered by fragment-based drug discovery, and help to improve the efficiency with which screening hits can become successful drugs.
- Brian K. Shoichet
-
Article |
 Natural-product-derived fragments for fragment-based ligand discovery
Natural-product-derived fragments for fragment-based ligand discovery
Natural products populate areas of chemical space not occupied by average synthetic molecules. Here, an analysis of more than 180,000 natural product structures results in a library of 2,000 natural-product-derived fragments, which resemble the properties of the natural products themselves and give access to novel inhibitor chemotypes.
- Björn Over
- , Stefan Wetzel
- & Herbert Waldmann
-
News & Views |
 Unleash the forces within
Unleash the forces within
Liposomes are a leading drug-delivery platform in cancer chemotherapy. Now they can be used to destroy cancer cells through a method that converts chemical energy to mechanical force. These localized disruptions can cause cell death while minimizing the collateral damage to neighbouring cells.
- Weiwei Gao
- & Liangfang Zhang
-
-
News & Views |
 Forcing an enemy into the open
Forcing an enemy into the open
A series of highly active, simplified analogues of the natural product bryostatin have been prepared. They offer an improved approach for the activation of latent HIV that could, in combination with current state-of-the-art antiretroviral therapy, offer hope for eventual eradication of the infection.
- Christian Melander
- & David M. Margolis
-
Article |
 Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro
Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro
Simplified bryostatin analogues are shown to potently induce latent HIV expression in vitro. These analogues display comparable or better potency when compared with bryostatin. Moreover, they are up to 1,000-fold more potent in inducing latent HIV expression than prostratin, the current lead preclinical candidate.
- Brian A. DeChristopher
- , Brian A. Loy
- & Paul A. Wender
-
News & Views |
 A light touch to a deadly problem
A light touch to a deadly problem
Flow chemistry has grown in stature as a technique with the potential to deliver synthetic complexity with assembly-line-like efficiency. Application of flow technology to the front-line antimalarial drug artemisinin promises to revolutionalize treatment.
- Kevin Booker-Milburn
-
Blogroll |
Blogroll: Arnie and artemisinin
-
Article |
 Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals
Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals
Acyclic cucurbituril-type molecular containers have been found to increase the solubility of insoluble pharmaceutical agents in water by up to 2,750-fold. In vitro and in vivo toxicology studies suggest that the containers are well tolerated, and paclitaxel solubilized in this manner efficiently kills HeLa and SK-OV-3 cancer cells.
- Da Ma
- , Gaya Hettiarachchi
- & Lyle Isaacs
-
-
News & Views |
 Flipping fluoride's reactivity
Flipping fluoride's reactivity
A sophisticated palladium(IV)-based species allows nucleophilic fluoride to react as an electrophilic fluorination reagent. This long-awaited reactivity will be especially useful in the preparation of radiochemically labelled molecules for positron emission tomography studies.
- Véronique Gouverneur
-
News & Views |
 Naturally inspired oligomers
Naturally inspired oligomers
The design of a small-molecule library for drug discovery attempts to combine the favourable diversity of natural product structures with the modularity of peptide synthesis.
- Jeffrey Aubé
-
Article |
 Quantifying the chemical beauty of drugs
Quantifying the chemical beauty of drugs
Drug-likeness is a key consideration when selecting compounds during the early stages of drug discovery, but its evaluation in absolute terms does not adequately reflect the spectrum of compound quality. Here, an intuitive and transparent quantitative measure of drug-likeness is proposed that attempts to capture the abstract notion of aesthetics in medicinal chemistry.
- G. Richard Bickerton
- , Gaia V. Paolini
- & Andrew L. Hopkins
-
Article |
 Binary fluorous tagging enables the synthesis and separation of a 16-stereoisomer library of macrosphelides
Binary fluorous tagging enables the synthesis and separation of a 16-stereoisomer library of macrosphelides
A 16-member diastereoisomer library known to contain macrosphelides A and E is synthesized as a mixture with the aid of a new encoding strategy for fluorous mixture synthesis. A simple process of sequential demixing and tag removal provides each of the isomers in individual, pure form. Analysis of the other library members ultimately leads to a structural reassignment for macrosphelide D.
- Dennis P. Curran
- , Mantosh K. Sinha
- & Dae-Hyun Cho
-
Article |
 Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors
Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors
Light-sensitive ligands can be used to regulate neurobiological receptors with high spatiotemporal precision. Here, the optochemical control of neuronal nicotinic acetylcholine receptors, using both photoswitchable tethered agonists and antagonists, is described. These rationally designed hybrid photoreceptors will facilitate the investigation of the physiological and pathological functions of nicotinic receptors in the brain.
- Ivan Tochitsky
- , Matthew R. Banghart
- & Dirk Trauner
-
Article |
 Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity
Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity
Poly(ethylene glycol) conjugates have been widely used to improve the stability of proteins for use as therapeutics, but this stability comes at the expense of binding affinity. Here, poly(carboxybetaine) — a zwitterionic polymer — is shown to provide increased stability while also enhancing binding due to its super-hydrophilic nature.
- Andrew J. Keefe
- & Shaoyi Jiang
-
Article |
 Stability of quantum dots in live cells
Stability of quantum dots in live cells
Intracellular biothiols can degrade nanoparticle monolayers, compromising the function of these potentially promising tools. Here, we describe a label-free method for quantifying the intracellular stability of quantum dot monolayers, using laser desorption/ionization mass spectrometry coupled with inductively coupled plasma mass spectrometry.
- Zheng-Jiang Zhu
- , Yi-Cheun Yeh
- & Vincent M. Rotello
-
News & Views |
 Untying a nanoscale knot
Untying a nanoscale knot
Mechanical unfolding of a single DNA G-quadruplex structure with and without a stabilizing ligand can be used to calculate the binding strength of the ligand and could help to identify drugs to target these important biological assemblies.
- Micah J. McCauley
- & Mark C. Williams
-
Commentary |
 Open science is a research accelerator
Open science is a research accelerator
An open-source approach to the problem of producing an off-patent drug in enantiopure form serves as an example of how academic and industrial researchers can join forces to make new scientific discoveries that could have a huge impact on human health.
- Michael Woelfle
- , Piero Olliaro
- & Matthew H. Todd
-
Commentary |
 Getting physical to fix pharma
Getting physical to fix pharma
Powerful technologies allow the synthesis and testing of large numbers of new compounds, but the failure rate of pharmaceutical R&D remains very high. Greater understanding of the fundamental physical chemical behaviour of molecules could be the key to greatly enhancing the success rate of drug discovery.
- Patrick R. Connelly
- , T. Minh Vuong
- & Mark A. Murcko
-
Article |
 The transcription factor FOXM1 is a cellular target of the natural product thiostrepton
The transcription factor FOXM1 is a cellular target of the natural product thiostrepton
The natural product thiostrepton is known to have anticancer properties but its mechanism of action is not known. Here, it is shown that thiostrepton binds to the protein FOXM1, preventing its interaction with several gene promoters and inhibits their expression. This illustrates the druggability of transcription factors, and provides a molecular basis for targeting FOXM1.
- Nagaratna S. Hegde
- , Deborah A. Sanders
- & Shankar Balasubramanian
-
Article |
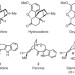 Synthesis of conolidine, a potent non-opioid analgesic for tonic and persistent pain
Synthesis of conolidine, a potent non-opioid analgesic for tonic and persistent pain
The first total synthesis of conolidine — representing the first asymmetric synthesis of a C5-nor-stemmadenine — is described. The chemical synthesis proceeds in just nine steps and gives an 18% overall yield from a commercially available pyridine — an achievement that led to the discovery that (+)-, (–)- and (±)-conolidine are potent non-opioid analgesics.
- Michael A. Tarselli
- , Kirsten M. Raehal
- & Glenn C. Micalizio
-
Research Highlights |
 Binding screens
Binding screens
Small-molecule microarrays facilitate the search for Alzheimer's disease therapeutics by screening compounds that bind to the amyloid-β peptide.
- Anne Pichon
-
Article |
 Recognition-mediated activation of therapeutic gold nanoparticles inside living cells
Recognition-mediated activation of therapeutic gold nanoparticles inside living cells
Application of supramolecular chemistry in living systems is challenging because of the inherent chemical complexity of cellular environments. Now, the use of a carefully designed host–guest system featuring diaminohexane-terminated gold nanoparticles and complementary cucurbit[7]uril macrocycles has been shown to provide triggered activation of a therapeutic system in living cells.
- Chaekyu Kim
- , Sarit S. Agasti
- & Vincent M. Rotello
-
Editorial |
A bitter pill
Cuts in pharmaceutical R&D jobs might provide short-term improvements to the bottom line, but do not bode well for the industry in the long run.
-
News & Views |
 Under control
Under control
Macrocyclic compounds can serve as hosts for smaller organic molecules, but precise control over the uptake and release of the guests remains challenging. Now, a host–guest system has been built that responds to the addition of metal ions, showing promise for drug-delivery applications.
- Werner M. Nau
-
Research Highlights |
Advancing vaccines
Replacing readily hydrolysable ester linkages with amides in a natural adjuvant has resulted in not only more stable, but significantly more active and less toxic analogues.
- Georgia Tsoukala

 Crowdsourcing drug discovery
Crowdsourcing drug discovery Two-millennia-old tablets
Two-millennia-old tablets