Featured
-
-
In Your Element |
 Redefining peptide therapeutics with semaglutide
Redefining peptide therapeutics with semaglutide
Thomas Kruse and Søren Østergaard reflect on the development of the GLP-1 analogue, semaglutide, which is reshaping peptide therapeutics in type 2 diabetes, weight management, and beyond.
- Thomas Kruse
- & Søren Østergaard
-
Article |
 Molecular jackhammers eradicate cancer cells by vibronic-driven action
Molecular jackhammers eradicate cancer cells by vibronic-driven action
In contrast to photothermal therapy requiring high powers over extended times and photodynamic therapy being abrogated by inhibitors of reactive oxygen species, actuation of vibronic modes in single molecules—molecular jackhammers—can now induce efficient cancer cell death. Here, the mechanical disassembly of cell membranes is characterized as the underlying mechanism by which this vibronic-driven action promotes necrotic cell death.
- Ciceron Ayala-Orozco
- , Diego Galvez-Aranda
- & James M. Tour
-
Research Briefing |
 In vitro selection of covalent aptamers with sulfur(VI) fluoride exchange chemistry
In vitro selection of covalent aptamers with sulfur(VI) fluoride exchange chemistry
To develop covalent inhibitors with high potency and low off-target effects, combinatorial approaches that search for candidates from large libraries are desired. Here, sulfur(VI) fluoride exchange (SuFEx) in vitro selection is established for the evolution of covalent aptamers from trillions of SuFEx-modified oligonucleotides. Through this technique, covalent aptamers with optimally balanced selectivity and reactivity are identified.
-
Article |
 Discovering covalent inhibitors of protein–protein interactions from trillions of sulfur(VI) fluoride exchange-modified oligonucleotides
Discovering covalent inhibitors of protein–protein interactions from trillions of sulfur(VI) fluoride exchange-modified oligonucleotides
Covalent inhibitors offer high potency but their potential is hindered by off-target reactivity. Now, an in vitro selection method has been developed to enable the discovery of covalent inhibitors from trillions of oligonucleotides endowed with the sulfur(VI) fluoride exchange chemistry. This strategy generates covalent inhibitors of protein–protein interactions with optimally balanced selectivity and reactivity.
- Zichen Qin
- , Kaining Zhang
- & Yu Xiang
-
News & Views |
 Anticancer platinum-based photo-oxidants in a new light
Anticancer platinum-based photo-oxidants in a new light
Pharmacologically inactive prodrugs that can be activated by near-infrared light are attractive candidates for clinical applications. Now, platinum-based photo-oxidants have been shown to eradicate tumours in mice with a new mode of action.
- Gloria Vigueras
- & Gilles Gasser
-
Article
| Open Access 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
The ortho-substituted phenyl ring is a basic structural element in chemistry. Now, 2-oxabicyclo[2.1.1]hexanes have been developed as saturated bioisosteres of the ortho-substituted phenyl ring with improved physicochemical properties. Replacement of the phenyl ring with 2-oxabicyclo[2.1.1]hexanes in marketed agrochemicals fluxapyroxad and boscalid improved water solubility, reduced lipophilicity and retained bioactivity.
- Aleksandr Denisenko
- , Pavel Garbuz
- & Pavel K. Mykhailiuk
-
Article |
 Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Tigilanol tiglate is a therapeutic lead for the treatment of a broad range of cancers. Now, it has been shown that tigilanol tiglate can be synthesized in a time and step economical fashion from phorbol—its naturally abundant biosynthetic precursor. This synthesis provides rapid access to analogues with unprecedented protein kinase C binding activity.
- Paul A. Wender
- , Zachary O. Gentry
- & Edward Njoo
-
Article |
 Total synthesis of structurally diverse pleuromutilin antibiotics
Total synthesis of structurally diverse pleuromutilin antibiotics
General synthetic methods to access pleuromutilin antibiotics are limited due to their complex carbocyclic skeleton. Now, a synthetic platform has been developed to access structurally diverse pleuromutilins with variations at the quaternary C12 position and hydrindanone cores. Seventeen structurally distinct derivatives were prepared and evaluated against a panel of Gram-positive and -negative bacteria.
- Olivia Goethe
- , Mikaela DiBello
- & Seth B. Herzon
-
Article
| Open Access Controlled masking and targeted release of redox-cycling ortho-quinones via a C–C bond-cleaving 1,6-elimination
Controlled masking and targeted release of redox-cycling ortho-quinones via a C–C bond-cleaving 1,6-elimination
A strategy for protecting redox-active ortho-quinones, which show promise as anticancer agents but suffer from redox-cycling behaviour and systemic toxicity, has been developed. The ortho-quinones are derivatized to redox-inactive para-aminobenzyl ketols. Upon amine deprotection, an acid-promoted, self-immolative C–C bond-cleaving 1,6-elimination releases the redox-active hydroquinone. The strategy also enables conjugation to a carrier for targeted delivery of ortho-quinone species.
- Lavinia Dunsmore
- , Claudio D. Navo
- & Gonçalo J. L. Bernardes
-
News & Views |
 A hydroxide lock for metallo-β-lactamases
A hydroxide lock for metallo-β-lactamases
It is extremely difficult to design a broad-spectrum inhibitor for metallo-β-lactamases (MBLs) due to the diversity in the active site. Now, indole-2-carboxylates have been developed as broad-spectrum inhibitors for MBLs. These inhibitors take advantage of key elements of both MBL substrates and products and work by locking a hydroxide.
- Hongyan Li
- & Hongzhe Sun
-
Article |
 Imitation of β-lactam binding enables broad-spectrum metallo-β-lactamase inhibitors
Imitation of β-lactam binding enables broad-spectrum metallo-β-lactamase inhibitors
The efficacy of carbapenem antibiotics can be compromised by metallo-β-lactamases, but a high-throughput screen followed by optimization has now enabled the discovery of indole-2-carboxylates (InCs) as potent broad-spectrum metallo-β-lactamase inhibitors. The results highlight the potential of InC–carbapenem combinations for clinical use as well as mechanism-guided approaches to combatting globally disseminated antibiotic resistant mechanisms.
- Jürgen Brem
- , Tharindi Panduwawala
- & Christopher J. Schofield
-
Article |
 Switching on prodrugs using radiotherapy
Switching on prodrugs using radiotherapy
Prodrugs offer one route to treat cancer, but they require activation once they have been delivered to the tumour. Now, a simultaneous chemo-radiotherapy strategy has been demonstrated in mice that uses gamma or X-ray irradiation to locally activate an anticancer prodrug.
- Jin Geng
- , Yichuan Zhang
- & Mark Bradley
-
News & Views |
 Expanding the effectiveness of screening
Expanding the effectiveness of screening
DNA-encoded libraries are a powerful tool to identify hit compounds for drug discovery. Now, two papers have reported new advances in this technology. One paper reports a method to screen for binders inside a living cell, and the other investigates the effects of stereo- and regiochemistry on ligand discovery.
- Minsoo Song
- & Gil Tae Hwang
-
News & Views |
 Protein targeting with SAF(er) electrophiles
Protein targeting with SAF(er) electrophiles
Electrophilic groups that undergo sulfur-exchange chemistry with protein nucleophiles can serve as the functional basis of chemical proteomic probes. A new addition to this class, sulfuramidimidoyl fluoride (SAF), which can be included in an array of covalent small molecule probes, exhibits a unique reactivity profile with proteins.
- Thomas E. Speltz
- & Raymond E. Moellering
-
Comment |
 Harder, better, faster
Harder, better, faster
The gap between fundamental academic research and the applied industrial research that is necessary to ensure real-world applications can be bridged by engaging in well-defined collaborative academia–industry projects and fostering better communication between the scientists involved in them.
- Danielle Schultz
- & Louis-Charles Campeau
-
Correspondence |
Crowdsourcing drug discovery for pandemics
- John Chodera
- , Alpha A. Lee
- & Frank von Delft
-
Article |
 Targeted photoredox catalysis in cancer cells
Targeted photoredox catalysis in cancer cells
Current photodynamic therapy photosensitizers require oxygen; however, tumours are often hypoxic. Now, an organoiridium complex with an unusually high redox potential, which is effective in normoxia and hypoxia, has been developed. The organoiridium complex kills cancer cells by an immunogenic apoptotic mechanism involving efficient photocatalytic oxidation of NADH to NAD radicals, and reduction of cytochrome c.
- Huaiyi Huang
- , Samya Banerjee
- & Peter J. Sadler
-
News & Views |
 A positive positive to negative
A positive positive to negative
The structure of an antibiotic that is effective against Gram-positive bacteria, but not against Gram-negative bacteria, has now been modified to improve its effectiveness against Gram-negative bacteria. The approach could help broaden the spectrum of activity of other antibiotics.
- Jed F. Fisher
- & Shahriar Mobashery
-
Article |
 Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3
Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3
New natural-product-inspired molecules are often limited by their only partial coverage of biologically relevant chemical space. Combining fragments of natural products has now been shown to yield pseudo natural products, which — while still being inspired by natural products — populate previously unexplored areas of chemical space and have novel biological activities.
- George Karageorgis
- , Elena S. Reckzeh
- & Herbert Waldmann
-
Perspective |
 Glucose-responsive insulin by molecular and physical design
Glucose-responsive insulin by molecular and physical design
Glucose-responsive insulin is a therapeutic that modulates its potency, concentration or dosing relative to a patient’s dynamic glucose concentration. This Perspective summarizes some of the recent accomplishments in this field as well as discussing new computational algorithms that may aid in the development of such therapeutics.
- Naveed A. Bakh
- , Abel B. Cortinas
- & Michael S. Strano
-
Review Article |
 The versatility of boron in biological target engagement
The versatility of boron in biological target engagement
Recent years have witnessed a surge of interest in targeted covalent inhibition of disease-associated proteins. Among the electrophiles used to interact with nucleophilic residues in protein structures, boron is unique for its chameleonic ability to display a range of coordination modes upon interaction with protein targets.
- Diego B. Diaz
- & Andrei K. Yudin
-
Article |
 A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations
A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations
A squaramide-based anion transporter has now been shown to cause changes in the lysosomal pH leading to impairment of lysosomal enzyme activity and disruption of autophagic processes. The study provides the first experimental evidence that synthetic ion transporters can both disrupt autophagy and induce apoptosis.
- Nathalie Busschaert
- , Seong-Hyun Park
- & Injae Shin
-
Article |
 Oxadiazole grafts in peptide macrocycles
Oxadiazole grafts in peptide macrocycles
Controlling macrocycle conformation represents a powerful tool for the construction of new bioactive molecules. Now, peptide-based macrocycles bearing a 1,3,4-oxadiazole moiety grafted into their backbone have been synthesized via a new cyclization approach. The resulting cyclic products exhibit conformationally rigid turn structures (stabilized through intramolecular hydrogen bonding) that can display passive membrane permeability.
- John R. Frost
- , Conor C. G. Scully
- & Andrei K. Yudin
-
In Your Element |
 Rhodium roles
Rhodium roles
Lars Öhrström relates the various roles played by rhodium in our daily lives, ranging from car components to drugs.
- Lars Öhrström
-
-
Article |
 Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation
Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation
A method to identify pairs of ligands that simultaneously bind to a target protein has been developed. The method uses two DNA-encoded chemical sub-libraries that self-assemble to form stable dual-display structures, and an encoding system that can be decoded by DNA sequencing and enables both ligands to be identified.
- Moreno Wichert
- , Nikolaus Krall
- & Jörg Scheuermann
-
Article |
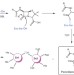 Rhodanine hydrolysis leads to potent thioenolate mediated metallo-β-lactamase inhibition
Rhodanine hydrolysis leads to potent thioenolate mediated metallo-β-lactamase inhibition
The use of β-lactam antibiotics is severely threatened by metallo-β-lactamases (MBLs), which contribute to the development of resistance. Now, crystallographic and solution studies reveal that recently reported MBL inhibition with a rhodanine can be attributed to fragmentation and complex formation with the resulting thioenolate.
- Jürgen Brem
- , Sander S. van Berkel
- & Christopher J. Schofield
-
Editorial |
Inspiration comes naturally
A collection of articles in this issue focuses on attempts to mimic aspects of natural-product biosynthesis for the identification of new drugs.
-
Interview |
 Public libraries
Public libraries
Jeffrey Bode from ETH Zürich talks with Nature Chemistry about his group's work on synthetic fermentation, and how he hopes it could bring the power of chemical synthesis into the hands of citizen scientists.
- Stephen Davey
-
Article |
 Synthetic fermentation of bioactive non-ribosomal peptides without organisms, enzymes or reagents
Synthetic fermentation of bioactive non-ribosomal peptides without organisms, enzymes or reagents
The production of biologically active compounds by microbial fermentation has proved highly successful in drug discovery. Now, a method that mimics this process has been used to prepare unnatural peptides from small building blocks without the need for additional reagents, and in a fashion that is immediately compatible with biological screening.
- Yi-Lin Huang
- & Jeffrey W. Bode
-
Article |
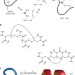 Dithiol amino acids can structurally shape and enhance the ligand-binding properties of polypeptides
Dithiol amino acids can structurally shape and enhance the ligand-binding properties of polypeptides
Disulfide bonds formed between two cysteine residues are important in the folding and stability of proteins. Now, unnatural amino acids with side-chains that contain two thiol groups are described. Incorporation of these dithiol amino acids into a serine protease inhibitor and a nicotinic acetyl choline receptor antagonist is shown to increase their inhibitory activity.
- Shiyu Chen
- , Ranganath Gopalakrishnan
- & Christian Heinis
-
News & Views |
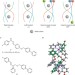 Making head–head–tails of it
Making head–head–tails of it
Enantiomerically pure head-to-head-to-tail triple-stranded helicates synthesized using a subcomponent self-assembly approach possess high anticancer activities against cancer cell lines without significant damage to DNA and with low toxicity to bacteria.
- Markus Albrecht
-
-
News & Views |
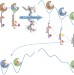 Docking points
Docking points
The flexibility and structural dynamics of proteins pose a big challenge for those trying to discover new bioactive compounds. Now, by using guiding crystallographic data, a method that uses the energetic balance between protein conformers to weight docking scores is shown to aid the hunt for new ligands.
- Xavier Barril
-
Article |
 Incorporation of protein flexibility and conformational energy penalties in docking screens to improve ligand discovery
Incorporation of protein flexibility and conformational energy penalties in docking screens to improve ligand discovery
The adoption of multiple conformations by proteins presents a challenge for ligand discovery using docking simulations. Now, a method for representing the conformational behaviour of a flexible protein in docking screens, which is guided by experimental crystallography data, is shown to predict protein conformation, ligand pose and aid the discovery of new ligands.
- Marcus Fischer
- , Ryan G. Coleman
- & Brian K. Shoichet
-
-
-
-
News & Views |
 Knockout for malaria
Knockout for malaria
Discovering and validating new targets is urgently required to tackle the rise in resistance to antimalarial drugs. Now, inhibition of the enzyme N-myristoyltransferase has been shown to prevent the formation of a critical subcellular organelle in the parasite that causes malaria, leading to death of the parasite.
- Joanna Krysiak
- & Stephan A. Sieber
-
Article |
 Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach
Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach
Chemical validation of new drug targets is urgently required to help develop new antimalarial therapies. Here, chemical proteomic tools and selective enzyme inhibitors are combined to study protein lipidation in human malaria parasites, leading to in vitro and in vivo validation of the enzyme N-myristoyltransferase as a drug target.
- Megan H. Wright
- , Barbara Clough
- & Edward W. Tate
-
Article |
 Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90
Synthesis of 19-substituted geldanamycins with altered conformations and their binding to heat shock protein Hsp90
The heat shock protein Hsp90 is a potential target for cancer and neurodegeneration drugs. Here, the introduction of a substituent into the 19-position of the naturally occurring inhibitor geldanamycin by chemical synthesis is shown to ameliorate toxicity, and also cause a favourable conformational switch that is required for protein binding.
- Russell R. A. Kitson
- , Chuan-Hsin Chang
- & Christopher J. Moody
-
News & Views |
 Diversifying complexity
Diversifying complexity
A synthetic strategy that uses a series of simple reactions to distort the core architecture of complex natural products could provide libraries of stereochemically rich compounds that will help in the search for new biological probes and drugs.
- Indrajeet Sharma
- & Derek S. Tan
-
Review Article |
 Inhibition of α-helix-mediated protein–protein interactions using designed molecules
Inhibition of α-helix-mediated protein–protein interactions using designed molecules
α-Helix-mediated protein–protein interactions (PPIs) play a key role in the development of numerous infection and disease states. Modulating such interactions offers considerable therapeutic potential, however, identifying suitable inhibitors has proved challenging. This Review highlights recent and generic approaches for designing inhibitors of helix-mediated PPIs.
- Valeria Azzarito
- , Kérya Long
- & Andrew J. Wilson
-
Article |
 A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
An approach for the construction of complex and diverse compound libraries is described, whereby natural products are altered through a series of ring system distortion reactions. The compounds produced have markedly different physiochemical properties from those in standard screening collections and thus could offer advantages in the search for lead molecules that can be developed into drug candidates.
- Robert W. Huigens III
- , Karen C. Morrison
- & Paul J. Hergenrother
-
News & Views |
 Nature's pieces
Nature's pieces
Natural products contain a range of chemical structures optimized for biological interactions. Fragmenting these compounds could help to combine this diversity with the broad coverage of chemical space offered by fragment-based drug discovery, and help to improve the efficiency with which screening hits can become successful drugs.
- Brian K. Shoichet
-
Article |
 Natural-product-derived fragments for fragment-based ligand discovery
Natural-product-derived fragments for fragment-based ligand discovery
Natural products populate areas of chemical space not occupied by average synthetic molecules. Here, an analysis of more than 180,000 natural product structures results in a library of 2,000 natural-product-derived fragments, which resemble the properties of the natural products themselves and give access to novel inhibitor chemotypes.
- Björn Over
- , Stefan Wetzel
- & Herbert Waldmann
-
Commentary |
 Getting physical to fix pharma
Getting physical to fix pharma
Powerful technologies allow the synthesis and testing of large numbers of new compounds, but the failure rate of pharmaceutical R&D remains very high. Greater understanding of the fundamental physical chemical behaviour of molecules could be the key to greatly enhancing the success rate of drug discovery.
- Patrick R. Connelly
- , T. Minh Vuong
- & Mark A. Murcko

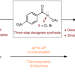 Discovery of N–X anomeric amides as electrophilic halogenation reagents
Discovery of N–X anomeric amides as electrophilic halogenation reagents Sensitive after a knockdown
Sensitive after a knockdown Crowdsourcing drug discovery
Crowdsourcing drug discovery