Key Points
-
Vector-borne diseases are on the rise globally. Although there have been some successes with vaccines and drug treatment, most of the current measures for limiting these diseases focus on vector control.
-
Habitat modification, insecticides, bed nets, biopesticides and sterile-male releases have all been used in the past, with some efficacy. There are also several emerging technologies that rely on genetic modification of the vector or bacterial symbionts for biological control of the vector.
-
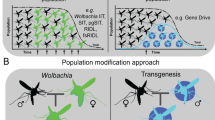
Genetic modification approaches include release of insects carrying a dominant lethal (RIDL), homing endonuclease genes (HEGs) and RNAi, with RIDL being the most progressed of these three approaches and currently in the stage of open-field releases. In this approach, mosquito populations are reduced by releasing males carrying a transgene that renders their female offspring flightless.
-
The insect endosymbiont Wolbachia is being developed for control via three potential pathways: releasing male mosquitoes that are mating-incompatible with wild-type female mosquitoes to reduce or eliminate populations, reducing the lifespan of the vector to reduce the number of insects able to transmit the disease, and reducing the ability of a range of pathogens to infect the insect. This latter approach is currently on trial in open-field release studies.
-
The roll out of all these methods will require substantial engagement with all stakeholders to ensure community and government support.
-
Each of the methods harbours risks around long-term stability and the evolution of resistance. These potential drawbacks will only truly be tested by monitoring the efficacy of the approaches following their implementation. As with other interventions, combinations of these approaches with more traditional control practices, such as the use of insecticides, vaccines and drug therapy, might offer the best solution for long-term disease control.
Abstract
Vector-borne disease is one of the greatest contributors to human mortality and morbidity throughout the tropics. Mosquito-transmitted diseases such as malaria, dengue, yellow fever and filariasis are the main contributors to this burden. Although insecticides have historically been used to try to control vector populations, over the past 15 years, substantial progress has been made in developing alternative vector control strategies ranging from biocontrol methods through to genetic modification of wild insect populations. Here, we review recent advances concerning these strategies and consider the potential impediments to their deployment, including the challenges of obtaining regulatory approval and community acceptance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
WHO. The World Health Report 2004: Changing History (WHO, 2004).
Gubler, D. J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 10, 100–103 (2002). A report that describes the breadth of the dengue problem.
Suaya, J. A. et al. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am. J. Trop. Med. Hyg. 80, 846–855 (2009).
Agnandji, S. T. et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 (2011).
Vaughan, A. M. & Kappe, S. H. Malaria vaccine development: persistent challenges. Curr. Opin. Immunol. 24, 324–331 (2012).
Whitty, C. J., Chandler, C., Ansah, E., Leslie, T. & Staedke, S. G. Deployment of ACT antimalarials for treatment of malaria: challenges and opportunities. Malar. J. 7, S7 (2008).
Mutabingwa, T. K. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 95, 305–315 (2005).
malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLoS Med. 8, 15–23 (2011).
Botting, C. & Kuhn, R. J. Novel approaches to flavivirus drug discovery. Expert Opin. Drug Discov. 7, 417–428 (2012).
Theiler, M. & Smith, H. H. The use of yellow fever virus modified by in vitro cultivation for human imunization. J. Exp. Med. 65, 787–800 (1937).
Halstead, S. B. & Thomas, S. J. Japanese encephalitis: new options for active immunization. Clin. Infect. Dis. 50, 1155–1164 (2010).
Sabchareon, A. et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380, 1559–1567 (2012). A recent report revealing the limited efficacy of the leading dengue vaccine currently under development.
Thomas, S. J. & Endy, T. P. Critical issues in dengue vaccine development. Curr. Opin. Infect. Dis. 24, 442–450 (2011).
Prichard, R. K. et al. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl. Trop. Dis. 6, e1549 (2012).
Ramirez, J. L., Garver, L. S. & Dimopoulos, G. Challenges and approaches for mosquito targeted malaria control. Curr. Mol. Med. 9, 116–130 (2009).
Raghavendra, K., Barik, T. K., Reddy, B. P., Sharma, P. & Dash, A. P. Malaria vector control: from past to future. Parasitol. Res. 108, 757–779 (2011).
WHO. Global Strategic Framework For Integrated Vector Management (WHO, 2004).
Pates, H. & Curtis, C. Mosquito behavior and vector control. Annu. Rev. Entomol. 50, 53–70 (2005).
Walker, K. A review of control methods for African malaria vectors (U.S. Agency for International Development, 2002).
Sinh Nam, V. et al. Community-based control of Aedes aegypti by using Mesocyclops in southern Vietnam. Am. J. Trop. Med. Hyg. 86, 850–859 (2012).
Kay, B. & Vu, S. N. New strategy against Aedes aegypti in Vietnam. Lancet 365, 613–617 (2005).
Yen, J. H. & Barr, A. R. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 22, 242–250 (1973).
Yen, J. H. & Barr, A. R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232, 657–658 (1971).
Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatability. Nature 216, 383–384 (1967). The demonstration that the release of Wolbachia -infected males can eradicate wild mosquito populations.
Enayati, A. & Hemingway, J. Malaria management: past, present, and future. Annu. Rev. Entomol. 55, 569–591 (2010).
Gratz, N. G. Space sprays for control of Aedes aegypti in South-East Asia and the Western Pacific. Dengue Bull. 23, 80–84 (1999).
WHO. Dengue Haemorrhagic Fever: Diagonsis, Treatment, Prevention And Control. 2nd edn (WHO, 1997).
Lindblade, K. A. et al. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. JAMA 291, 2571–2580 (2004).
Gamble, C., Ekwaru, P. J., Garner, P. & ter Kuile, F. O. Insecticide-treated nets for the prevention of malaria in pregnancy: a systematic review of randomised controlled trials. PLoS Med. 4, e107 (2007).
Binka, F. & Akweongo, P. Prevention of malaria using ITNs: potential for achieving the millennium development goals. Curr. Mol. Med. 6, 261–267 (2006).
Moiroux, N. et al. Changes in Anopheles funestus biting behavior. Following universal coverage of long-lasting insecticidal nets in benin. J. Infect. Dis. 206, 1622–1629 (2012).
Russell, T. L. et al. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10, 80 (2011).
Alphey, L. et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis.Vector Borne Zoonotic Dis. 10, 295–311 (2010).
Wise de Valdez, M. R. et al. Genetic elimination of dengue vector mosquitoes. Proc. Natl Acad. Sci. USA 108, 4772–4775 (2011). A description of the RIDL technology and the efficacy of this technology in caged mosquito populations.
Benedict, M. Q. & Robinson, A. S. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 19, 349–355 (2003).
Patterson, R. S., Weidhaas, D. E., Ford, H. R. & Lofgren, C. S. Suppression and elimination of an island population of Culex pipiens quinquefasciatus with sterile males. Science 168, 1368–1370 (1970).
Lofgren, C. S. et al. Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. 3. Field methods and population control. Am. J. Trop. Med. Hyg. 23, 288–297 (1974).
Helinski, M. E. et al. Towards a sterile insect technique field release of Anopheles arabiensis mosquitoes in Sudan: irradiation, transportation, and field cage experimentation. Malar. J. 7, 65 (2008).
El Sayed, B. B. et al. Ethical, legal and social aspects of the approach in Sudan. Malar. J. 8, S3 (2009).
Oliva, C. F. et al. The sterile insect technique for controlling populations of aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS ONE 7, e49414 (2012).
Vreysen, M. J. et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 93, 123–135 (2000).
Lindquist, D. A., Abusowa, M. & Hall, M. J. The New World screwworm fly in Libya: a review of its introduction and eradication. Med. Vet. Entomol. 6, 2–8 (1992).
Henneberry, T. J. & Naranjo, S. E. Integrated management approaches for pink bollworm in the southwestern United States. Integr. Pest Manag. Rev. 3, 31–52 (1998).
Franz, G. & Robinson, A. S. Molecular technologies to improve the effectiveness of the sterile insect technique. Genetica 139, 1–5 (2011).
Simmons, G. S. et al. Field performance of a genetically engineered strain of pink bollworm. PLoS ONE 6, e24110 (2011).
Allen, M. L., Handler, A.M., Berkebile, D. R. & Skoda, S. R. piggyBac transformation of the New World screwworm, Cochliomyia hominivorax, produces multiple distinct mutant strains. Med. Vet. Entomol. 18, 1–9 (2004).
Fu, G. et al. Female-specific flightless phenotype for mosquito control. Proc. Natl Acad. Sci. USA 107, 4550–4554 (2010).
Phuc, H. K. et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5, 11 (2007).
Bargielowski, I., Nimmo, D., Alphey, L. & Koella, J. C. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti. PLoS ONE 6, e20699 (2011).
Harris, A. F. et al. Field performance of engineered male mosquitoes. Nature Biotech. 29, 1034–1037 (2011). The finding that released mosquitoes carrying a dominant-negative lethal allele mate with wild females in an open-field release.
Lacroix, R. et al. Open field release of genetically engineered sterile male Aedes aegypti in Malaysia. PLoS ONE 7, e42771 (2012). A description of the regulatory and community engagement process for RIDL, and the fitness of mosquitoes in the field following open-field release.
Franz, A. W. et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl Acad. Sci. USA 103, 4198–4203 (2006). A discussion about RNAi constructs that reduce the ability of mosquitoes to become infected with dengue.
Franz, A. W. et al. Stability and loss of a virus resistance phenotype over time in transgenic mosquitoes harbouring an antiviral effector gene. Insect Mol. Biol. 18, 661–672 (2009).
Gu, J., Liu, M., Deng, Y., Peng, H. & Chen, X. Development of an efficient recombinant mosquito densovirus-mediated RNA interference system and its preliminary application in mosquito control. PLoS ONE 6, e21329 (2011).
Arjona, A., Wang, P., Montgomery, R. R. & Fikrig, E. Innate immune control of West Nile virus infection. Cellular Microbiol. 13, 1648–1658 (2011).
Campbell, C. L. et al. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 8, 47 (2008).
Keene, K. M. et al. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl Acad. Sci. USA 101, 17240–17245 (2004).
Catteruccia, F. & Levashina, E. A. RNAi in the malaria vector, Anopheles gambiae. Methods Mol. Biol. 555, 63–75 (2009).
Windbichler, N. et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (2011). An example of the HEG system and its ability to successfully invade caged populations.
Burt, A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci. 270, 921–928 (2003). Population modelling demonstrating the potential efficacy of HEGs.
Deredec, A., Godfray, H. C. & Burt, A. Requirements for effective malaria control with homing endonuclease genes. Proc. Natl Acad. Sci. USA 108, E874–E880 (2011).
Traver, B. E., Anderson, M. A. & Adelman, Z. N. Homing endonucleases catalyze double-stranded DNA breaks and somatic transgene excision in Aedes aegypti. Insect Mol. Biol. 18, 623–633 (2009).
Hilgenboecker, K., Hammerstein, P., Schlattmann, P., Telschow, A. & Werren, J. H. How many species are infected with Wolbachia?-A statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 (2008).
Xi, Z., Khoo, C. C. & Dobson, S. L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310, 326–328 (2005).
McMeniman, C. J. et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323, 141–144 (2009). A report showing the lifespan reduction of the dengue vector following transinfection with Wolbachia.
Walker, T. et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 (2011).
Jin, C., Ren, X. & Rasgon, J. L. The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl. Environ. Microbiol. 75, 3373–3376 (2009).
O'Connor, L. et al. Open release of male mosquitoes infected with a wolbachia biopesticide: field performance and infection containment. PLoS Negl. Trop. Dis. 6, e1797 (2012). The demonstration that Wolbachia -infected males prevent the reproduction of naturally uninfected wild females in an open-field release.
Chambers, E. W., Hapairai, L., Peel, B. A., Bossin, H. & Dobson, S. L. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl. Trop. Dis. 5, e1271 (2011).
Atyame, C. M. et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl Trop. Dis. 5, e1440 (2011).
Min, K. T. & Benzer, S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl Acad. Sci. USA 94, 10792–10796 (1997).
Chan, M. & Johansson, M. A. The incubation periods of dengue viruses. PLoS ONE 7, e50972 (2012).
Cook, P. E., McMeniman, C. J. & O'Neill, S. L. Modifying insect population age structure to control vector-borne disease. Adv. Exp. Med. Biol. 627, 126–140 (2008).
Molineux, L., Dietz, K. & Thomas, A. Further epidemiological evaluation of a malaria model. Bull. World Health Organ. 56, 565–571 (1978).
Rasgon, J. L. & Scott, T. W. Impact of population age structure on Wolbachia transgene driver efficacy: ecologically complex factors and release of genetically modified mosquitoes. Insect Biochem. Mol. Biol. 34, 707–713 (2004).
Moreira, L. A. et al. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl. Trop. Dis. 3, e568 (2009).
Turley, A. P., Moreira, L. A., O'Neill, S. L. & McGraw, E. A. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl. Trop. Dis. 3, e516 (2009).
McMeniman, C. J. & O'Neill, S. L. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl. Trop. Dis. 4, e748 (2010).
McMeniman, C. J., Hughes, G. L. & O'Neill, S. L. A Wolbachia symbiont in Aedes aegypti disrupts mosquito egg development to a greater extent when mosquitoes feed on nonhuman versus human blood. J. Med. Entomol. 48, 76–84 (2011).
Teixeira, L., Ferreira, A. & Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e2 (2008).
Hedges, L. M., Brownlie, J. C., O'Neill, S. L. & Johnson, K. N. Wolbachia and virus protection in insects. Science 322, 702 (2008).
Moreira, L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009). A paper reporting pathogen blocking following transinfection of the dengue vector with Wolbachia.
Bian, G., Xu, Y., Lu, P., Xie, Y. & Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6, e1000833 (2010).
Glaser, R. L. & Meola, M. A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5, e11977 (2010).
Hughes, G. L., Koga, R., Xue, P., Fukatsu, T. & Rasgon, J. L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7, e1002043 (2011).
Blagrove, M. S., Arias-Goeta, C., Failloux, A. B. & Sinkins, S. P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl Acad. Sci. USA 109, 255–260 (2012).
Lu, P., Bian, G., Pan, X. & Xi, Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop. Dis. 6, e1754 (2012).
Kambris, Z., Cook, P. E., Phuc, H. K. & Sinkins, S. P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326, 134–136 (2009).
van den Hurk, A. F. et al. Impact of Wolbachia on infection with Chikungunya and Yellow Fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1892 (2012).
Hughes, G. L., Vega-Rodriguez, J., Xue, P. & Rasgon, J. L. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl. Environ. Microbiol. 78, 1491–1495 (2012).
Hoffmann, A. A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 (2011). An article summarizing the regulatory and community engagement processes for Wolbachia , and data showing that Wolbachia infection can spread into mosquito populations in an open release.
Mousson, L. et al. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol. Ecol. 19, 1953–1964 (2010).
Rances, E., Ye, Y. H., Woolfit, M., McGraw, E. A. & O'Neill, S. L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 8, e1002548 (2012).
McNaughton, D., Clough, A., Johnson, P., Ritchie, S. A. & O'Neill, S. L. Beyond the 'back yard': lay knowledge about Aedes aegypti in northern Australia and its implications for policy and practice. Acta Trop. 116, 74–80 (2010).
Harrington, L. C. et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 72, 209–220 (2005).
Turelli, M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64, 232–241 (2010).
McGraw, E. A., Merritt, D. J., Droller, J. N. & O'Neill, S. L. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl Acad. Sci. USA 99, 2918–2923 (2002).
Carrington, L. B., Leslie, J., Weeks, A. R. & Hoffmann, A. A. The popcorn Wolbachia infection of Drosophila melanogaster: can selection alter Wolbachia longevity effects? Evolution 63, 2648–2657 (2009).
Lipsitch, M. et al. Strain characteristics of Streptococcus pneumoniae carriage and invasive disease isolates during a cluster-randomized clinical trial of the 7-valent pneumococcal conjugate vaccine. J. Infect. Dis. 196, 1221–1227 (2007).
Rai, K. S., Grover, K. K. & Suguna, S. G. Genetic manipulation of Aedes aegypti: incorporation and maintenance of a genetic marker and a chromosomal translocation in natural populations. Bull. World Health Organ. 48, 49–56 (1973).
Grover, K. K. et al. Competitiveness of chemosterilised males and cytoplasmically incompatible translocated males of Culex pipiens fatigans Wiedemann (Diptera, Culicidae) in the field. Bull. Entomol. Res. 66, 469–480 (1976).
Grover, K. K. et al. Field experiments on the competitiveness of males carrying genetic control systems for Aedes aegypti. Entomol. Exp. Appl. 20, 8–18 (1976).
Curtis, C. F. et al. A field trial on control of Culex quinquefasciatus by release of males of a strain integrating cytoplasmic incompatibility and a translocation. Entomol. Exp. Appl. 31, 181–190 (1982).
Curtis, C. F. & Von Borstol, R. C. Allegations against Indian research refuted. Nature 273, 96 (1978). A description of how a negative media campaign damaged the work of a mosquito control group in India.
Walgate, R. Research in third world countries: pugwash plans controls. Nature 272, 8–9 (1978).
Tomiche, F. J. The WHO and mosquitoes. Nature 257, 175 (1975).
Wood, R. J. Mosquitoes. Nature 258, 102 (1975).
McNaughton, D. The importance of long-term social research in enabling participation and developing engagement strategies for new dengue control technologies. PLoS Negl. Trop. Dis. 6, e1785 (2012).
De Barro, P. J., Murphy, B., Jansen, C. C. & Murray, J. The proposed release of the yellow fever mosquito, Aedes aegypti containing a naturally occurring strain of Wolbachia pipientis, a question of regulatory responsibility. J. Verbrauch. Lebensm. 6, 33–40 (2011).
Murphy, B., Jansen, C. C., Murray, J. & De Barro, P. J. (CSIRO Entomology, 2010).
Popovici, J. et al. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem. Inst. Oswaldo Cruz 105, 957–964 (2010).
Hurst, T. P. et al. Impacts of Wolbachia infection on predator prey relationships: evaluating survival and horizontal transfer between wMelPop infected Aedes aegypti and its predators. J. Med. Entomol. 49, 624–630 (2012).
Roux, V. & Raoult, D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146, 385–396 (1995).
Wu, M. et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2, e69 (2004).
Cho, K. O., Kim, G. W. & Lee, O. K. Wolbachia bacteria reside in host Golgi-related vesicles whose position is regulated by polarity proteins. PLoS ONE 6, e22703 (2011).
O'Neill, S. L., Hoffmann, A. A. & Werren, J. H. (eds) Influential Passengers (Oxford Univ. Press, 1998).
Charlat, S., Hurst, G. D. & Mercot, H. Evolutionary consequences of Wolbachia infections. Trends Genet. 19, 217–223 (2003).
Turelli, M. & Hoffmann, A. A. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140, 1319–1338 (1995).
Dobson, S. L. et al. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29, 153–160 (1999).
Peng, Y., Nielsen, J. E., Cunningham, J. P. & McGraw, E. A. Wolbachia infection alters olfactory-cued locomotion in Drosophila spp. Appl. Environ. Microbiol. 74, 3943–3948 (2008).
Evans, O. et al. Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J. Exp. Biol. 212, 1436–1441 (2009).
Harcombe, W. & Hoffmann, A. A. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J. Invertebr. Pathol. 87, 45–50 (2004).
Fenn, K. & Blaxter, M. Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol. Evol. 19, 163–166 (2004).
Baldo, L. et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110 (2006).
McGraw, E. A., Merritt, D. J., Droller, J. N. & O'Neill, S. L. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. Biol. Sci. 268, 2565–2570 (2001).
Ebert, D. Virulence and local adaptation of a horizontally transmitted parasite. Science 265, 1084–1086 (1994).
Levin, B. R. & Svanborg Eden, C. Selection and evolution of virulence in bacteria: an ecumenical excursion and modest suggestion. Parasitology 100, S103–S15 (1990).
Jasinskiene, N. et al. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc. Natl Acad. Sci. USA 95, 3743–3747 (1998).
Labbe, G. M. Nimmo, D.D. & Alphey, L. piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse). PLoS Negl. Trop. Dis. 4, e788 (2010).
Jacobs-Lorena, M. Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes. J. Vector Borne Dis. 40, 73–77 (2003).
Catteruccia, F. et al. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature 405, 959–962 (2000).
Perera, O. P., Harrell, I. R. & Handler, A. M. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol. Biol. 11, 291–297 (2002).
Catteruccia, F., Benton, J. P. & Crisanti, A. An Anopheles transgenic sexing strain for vector control. Nature Biotech. 23, 1414–1417 (2005).
Allen, M. L., O'Brochta, D. A., Atkinson, P. W. & Levesque, C. S. Stable, germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 38, 701–710 (2001).
Kettle, D. S. Medical and Veterinary Entomology (CAB International, 1995).
Mullen, G. & Durden, L. (eds) Medical and Veterinary Entomology (Academic Press, 2002).
Kitron, U. & Spielman, A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Rev. Infect. Dis. 11, 391–406 (1989).
Utzinger, J., Tozan, Y. & Singer, B. H. Efficacy and cost-effectiveness of environmental management for malaria control. Trop. Med. Int. Health 6, 677–687 (2001).
Imbahale, S. S., Githeko, A., Mukabana, W. R. & Takken, W. Integrated mosquito larval source management reduces larval numbers in two highland villages in western Kenya. BMC Publ. Health 12, 362 (2012).
Nandha, B. & Krishnamoorthy, K. Impact of education campaign on community-based vector control in hastening the process of elimination of lymphatic filariasis in Tamil Nadu, South India. Health Educ. Res. 27, 585–594 (2012).
Gubler, D. J. & Clark, G. G. Community involvement in the control of Aedes aegypti. Acta Trop. 61, 169–179 (1996).
Thammapalo, S., Meksawi, S. & Chongsuvivatwong, V. Effectiveness of space spraying on the transmission of dengue/dengue hemorrhagic fever (DF/DHF) in an urban area of Southern Thailand. J. Trop. Med. 2012, 652564 (2012).
Ogoma, S. B. et al. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl. Trop. Dis. 4, e773 (2010).
Seng, C. M. et al. Community-based use of the larvivorous fish Poecilia reticulata to control the dengue vector Aedes aegypti in domestic water storage containers in rural Cambodia. J. Vector Ecol. 33, 139–144 (2008).
Kusumawathie, P. H., Wickremasinghe, A. R., Karunaweera, N. D. & Wijeyaratne, M. J. Larvivorous potential of the guppy, Poecilia reticulata, in anopheline mosquito control in riverbed pools below the Kotmale dam Sri Lanka. Asia Pac. J. Publ. Health 20, 56–63 (2008).
Chen, C. D. et al. Field effectiveness of Bacillus thuringiensis israelensis (Bti) against Aedes (Stegomyia) aegypti (Linnaeus) in ornamental ceramic containers with common aquatic plants. Trop. Biomed. 26, 100–105 (2009).
Tchicaya, E. S. et al. Effect of repeated application of microbial larvicides on malaria transmission in central Cote d'Ivoire. J. Am. Mosq. Control Assoc. 25, 382–385 (2009).
Darbro, J. M. et al. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am. J. Trop. Med. 86, 656–664 (2012).
Howard, A. F. et al. First report of the infection of insecticide-resistant malaria vector mosquitoes with an entomopathogenic fungus under field conditions. Malar. J. 10, 24 (2011).
Lenhart, A. et al. Insecticide-treated bednets to control dengue vectors: preliminary evidence from a controlled trial in Haiti. Trop. Med. Int. Health 13, 56–67 (2008).
Dutta, P. et al. The effect of insecticide-treated mosquito nets (ITMNs) on Japanese encephalitis virus seroconversion in pigs and humans. Am. J. Trop. Med. Hyg. 84, 466–472 (2011).
Phillips-Howard, P. A. et al. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 68, 23–29 (2003).
Rowland, M., Freeman, T., Downey, G., Hadi, A. & Saeed, M. DEET mosquito repellent sold through social marketing provides personal protection against malaria in an area of all-night mosquito biting and partial coverage of insecticide-treated nets: a case-control study of effectiveness. Trop. Med. Int. Health 9, 343–350 (2004).
Rapley, L. P. et al. A lethal ovitrap-based mass trapping scheme for dengue control in Australia: II. Impact on populations of the mosquito Aedes aegypti. Med. Vet. Entomol. 23, 303–316 (2009).
Okumu, F. O. et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE 5, e8951 (2010).
Barbosa, R. M., Souto, A., Eiras, A. E. & Regis, L. Laboratory and field evaluation of an oviposition trap for Culex quinquefasciatus (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 102, 523–529 (2007).
Acknowledgements
The authors are supported by grants from the Foundation for the US National Institutes of Health (through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation), the National Health and Medical Research Council of Australia and the Queensland State Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- DALY
-
(Disability-adjusted life year). The number of years lost owing to morbidity or mortality of a disease. This measure is preferable to simple mortality measures, as it better captures the disease burden for debilitating but often self-limiting diseases like dengue and malaria.
- Brackish
-
Slightly salty; pertaining to water such as that present in estuaries.
- Anthropophilic
-
Preferring humans over other animals as a blood meal source.
- Copepods
-
Small freshwater crustaceans (in the context of this Review, of the genus Mesocyclops) that prey on mosquito larvae.
- DDT
-
(Dichlorodiphenyl-trichloroethane). An organochlorine-based insecticide that has been used since the Second World War to control insects. The insecticide is banned in some countries because of its potential ill effects on human health and non-target species, but it is still used intensively in Africa in regions of high malaria transmission.
- Transgenes
-
Genes or genetic material that has been introduced into another organism using genetic engineering techniques.
- RNAi
-
The process by which animals cleave double-stranded RNAs into small fragments, the presence of which directs transcriptional silencing of the corresponding gene. RNAi also has a role in immunity, as it is responsible for cutting and degrading the RNA of invading viruses.
- Cytoplasmic incompatibility
-
The failure of embryo development in the early stages, as the result of a Wolbachia-infected male mating with an uninfected female. This leads to poor or no survival of the offspring. By contrast, when two Wolbachia-infected adults mate, the egg of the infected female 'rescues' Wolbachia-mediated changes to the sperm and allows the offspring to develop normally.
- Transinfect
-
To transfer a bacterial or viral infection from one host to another by microinjection.
- Bidirectional incompatibility
-
A phenomenon that occurs when mating males and females are infected with different Wolbachia strains. Eggs from the female may not be able to rescue the Wolbachia-induced changes in the sperm of the male. The consequence is an incompatibility in the embryo such that few or no offspring survive, despite the fact that both parents carry Wolbachia.
Rights and permissions
About this article
Cite this article
McGraw, E., O'Neill, S. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol 11, 181–193 (2013). https://doi.org/10.1038/nrmicro2968
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2968
This article is cited by
-
Pyrethroid insecticides susceptibility profiles and evaluation of L1014F kdr mutant alleles in Culex quinquefasciatus from lymphatic filariasis endemic communities
Scientific Reports (2023)
-
Surfactants alter mosquito’s flight and physical condition
Scientific Reports (2023)
-
Larvicidal and anti-termite activities of microbial biosurfactant produced by Enterobacter cloacae SJ2 isolated from marine sponge Clathria sp.
Scientific Reports (2023)