Abstract
Rising temperatures are impacting the range and prevalence of mosquito-borne diseases. A promising biocontrol technology replaces wild mosquitoes with those carrying the virus-blocking Wolbachia bacterium. Because the most widely used strain, wMel, is adversely affected by heat stress, we examined how global warming may influence wMel-based replacement. We simulated interventions in two locations with successful field trials using Coupled Model Intercomparison Project Phase 5 climate projections and historical temperature records, integrating empirical data on wMel’s thermal sensitivity into a model of Aedes aegypti population dynamics to evaluate introgression and persistence over one year. We show that in Cairns, Australia, climatic futures necessitate operational adaptations for heatwaves exceeding two weeks. In Nha Trang, Vietnam, projected heatwaves of three weeks and longer eliminate wMel under the most stringent assumptions of that symbiont’s thermal limits. We conclude that this technology is generally robust to near-term (2030s) climate change. Accelerated warming may challenge this in the 2050s and beyond.
Similar content being viewed by others
Main
Temperature influences both mosquito and pathogen traits, affecting the geographical range and prevalence of mosquito-borne diseases like malaria, dengue and Zika virus1,2,3,4,5. Anthropogenic warming is expected to have heterogenous impacts, with increases in seasonal suitability for the malarial Anopheles mosquito in some regions and extensions of the environmental conditions favourable to arbovirus vectors, such as the Aedes mosquito, in others6.
Disease risk under future climates underscores a need for improved vector control tools, particularly as insecticide efficacy wanes due to evolved resistance7,8,9. There has been a surge of innovation in control technologies over the past decade, including several successful field trials of a self-sustaining biocontrol approach called Wolbachia-based replacement10,11,12. This technique, which transfects wild mosquitoes with a maternally inherited endosymbiotic bacterium that is naturally occurring in many arthropods, can be highly effective at blocking the ability to transmit human pathogens13. Multiple strains of the Wolbachia bacterium, each bearing unique biological properties, have been transferred into various Aedes species14. Public health intervention trials of Aedes aegypti transfected with the wMel strain11 have been undertaken in Latin America, Asia and Oceania15,16,17,18. Most Wolbachia replacement efforts trialled for control of Aedes-borne viruses have used the wMel strain naturally occurring in Drosophila melanogaster19.
In transfected A. aegypti mosquitoes, wMel reliably causes stable maternal transmission and blocks replication of pathogenic viruses while incurring a relatively low fitness cost compared to alternative Wolbachia strains20. Under optimal laboratory conditions, wMel demonstrates complete cytoplasmic incompatibility (CI), which renders crosses between infected males and uninfected females unviable, favouring the reproduction of wMel carriers and driving its own spread through a vector population. However, recent studies have shown that CI—a property vital to wMel utility—is weakened by the cyclical heat stress that can characterize field conditions21. High temperatures also decrease the hatch rate of wMel-infected eggs and reduce wMel density in adults, impairing maternal transmission and causing the infection to fall out of a laboratory population once a threshold of 35.0 °C is reached22. Another strain, wAlbB, exhibits less susceptibility to high rearing temperatures and has been successfully established in Malaysian populations of A. aegypti23; however, its predominant use has been for the suppression (population lowering) of that vector24,25,26.
Using projections of future increases in the frequency, severity and duration of heatwaves27, together with laboratory evidence of the negative impact that oscillating temperature regimes and thermal shocks have on wMel infection21,22, we tested our hypothesis that the hotter, more prevalent heat extremes and higher average temperatures brought by climate change could impede wMel-based replacement interventions. We assess the effects of alternative climate scenarios on this technology in Cairns, Queensland, Australia—the location of the first successful field trial10—using entomological end points, including the frequency of wMel infection and the magnitude of wild-type (WT) suppression, to examine introgression and fixation in a simulated A. aegypti population over a year-long horizon. We investigate the limits of our findings through analyses of wMel interventions in the region of Nha Trang City, Vietnam, where the technology has also been trialled and where higher temperatures and longer heatwaves are expected. Our computational experiments study the compounding interaction of warming trends, wMel’s intrinsic biological thresholds and extrinsic fitness costs, and uncertainties about the functional form of temperature impacts on wMel to understand the resilience of the technology to climate change via the dynamics of its introduction and short-term persistence.
Results
wMel-based population replacement is robust to projected regional warming trends out to mid-century. Outcomes were quantified using the replacement efficacy score (RES), a metric modelled after the suppression efficacy score (SES)28, and evaluated on a 0–100 scale relative to ideal efficacy (sustained fixation). The RES summarizes the success with which the standing population is substituted by wMel carriers. It serves as a basis of comparison to assess the replacement achieved by alternative scenarios (Methods). Across the scenarios, RES scores remained above 79 (Fig. 1 and section 5 in the Supplementary Information).
Increases in average temperature impact the RES of Wolbachia-based interventions. Each circle marker represents an RES for the release simulated in an individual year, with colours corresponding to the temperature regime (2011 in black, all other historical in yellow, 2030 in orange, 2050 in red). The trend line fits are noted in each panel legend. a–c, Results from the sensitivity analysis for unique thermal thresholds (35 °C (a) 33 °C (b), 31.5 °C (c)) and daily temperature inputs to the wMel dynamic model. d, Results when seven-day moving averages are used as temperature inputs; all three thermal thresholds returned equivalent output under this assumption.
We examined the consequences of CI and maternal inheritance—both thermally sensitive biological mechanisms—failing completely when daily average temperature reached the 35.0 °C threshold, which has been empirically shown to drop the wMel titre to zero in exposed A. aegypti eggs. We also conducted a sensitivity analysis where CI and maternal inheritance fail at lower temperatures (33.0 °C and 31.5 °C). For interventions conducted under Cairns average daily temperatures in both historical (1990–2019) and projected future (2024–2039, 2044–2059) years, RES scores and their correlation with temperature were nearly identical between the 35.0 °C and 33.0 °C threshold scenarios (Fig. 1a,b). The lowest threshold, 31.5 °C, produced outcomes that were substantially more sensitive to temperature than those generated by the 35.0 °C and 33.0 °C alternatives (Fig. 1c).
Because the data used to develop wMel temperature-responsive dynamics were from laboratory studies conducted at a resolution of one week, we performed a sensitivity analysis to investigate the effect of altering the functional form of temperature inputs. In one set of simulations, temperature inputs to the mechanistic model assumed that the same impact observed after one week of exposure would also occur after one day. In a second set of simulations, a seven-day moving average constituted the temperature inputs. The simulations driven by moving averages resulted in equivalent output under all threshold values (35.0 °C, 33.0 °C, 31.5 °C) for CI and maternal inheritance (Fig. 1c,d). These runs also generated a larger difference between the 2030 and 2050 RES scores and trends, compared to results driven by daily temperature inputs. A third sensitivity analysis examined how the wMel-induced fitness cost affected outcomes. The influence was minimal; therefore, all main text results assume a 10% cost on wMel carriers relative to WT A. aegypti (section 5 of the SupplementaryInformation for 0% and 20%).
The linear relationship of wMel replacement to increasing temperature diminishes in future, warmer decades. RES scores were calculated for interventions under average daily temperatures across both historical and future years, with the latter encompassing both the Representative Concentration Pathway (RCP) 4.5 and 8.5 scenarios (Fig. 1). Under historical climates, assuming daily inputs to the wMel model and using the thermal threshold of 35 °C, temperature as a linear covariate accounts for approximately 25% (R2 = 0.2540, P < 0.0045) of the variation in the model fit (y = 73.44 − 0.31x); each degree of temperature increase lowers the RES score by 0.31.
However, the relationship between temperature and RES is non-linear: holding the thermal threshold and daily input assumptions constant during model runs representative of 2024–2039, the effect of annual average temperature on the RES—assuming the highest threshold of 35 °C—is no longer significantly negative (R2 = 0.0157, P < 0.4948). This is also the case in the years 2044–2059 (R2 = 0.0389, P < 0.2788). Given that temperature is the only input variable permitted to change in the model over time, the non-linearity of its association with RES is evidenced by this shift in significance under future scenarios. The changing linear relationships between temperature and RES in future decades reflect the non-linear dynamics in this model system.
Altering the functional form of temperature inputs such that the wMel model is driven by a seven-day moving average yields the same reduction in temperature sensitivity. There is again a negative relationship between temperature and RES score during the historical period (y = 94.75 − 0.51x); however, annual temperature only accounts for 17.2% (R2 = 0.17219, P < 0.02259) of the variation in that linear fit. In the linear model fit for future decades, the correlation is absent (2030s: R2 = 0.00048, P < 0.90467, 2050s: R2 = 0.3110, P < 0.08316). The primary distinction from the model with daily temperature inputs is that the absolute magnitude of the slope in the linear model fit is invariably larger. This is sensible, given that the moving average approach extends the effect of a single hot day for an additional six days.
Because the RES is a single value that summarizes success over the entire period of interest, understanding the reason for this metric’s shifting relationship with temperature requires a closer look at the population dynamics (Fig. 2). Under the 2050 scenarios, the number of wMel-infected females drops towards the end of the calendar year (summer in the Southern Hemisphere). Thresholds for temperature-sensitive wMel mechanisms (CI and maternal inheritance) are exceeded, leading to the reduction of the infected population. However, higher average temperatures during the coolest part of the year also improve wMel replacement during that period. These mid-year dynamics are strong enough to mask the decrease in infected females that occurs in later months when using a summary metric.
wMel-based population replacement also remains robust to existing predictions of future heatwaves in Cairns for both the 2030 and 2050 climate regimes. For a description of the methodology used to construct the heatwave timeseries, see Methods and section 2 of the Supplementary Information. As with the average temperature change experiments, we conducted sensitivity analyses over the range of biological thermal thresholds, functional form of temperature inputs and fitness costs.
Simulations using daily temperature inputs lower wMel frequency across all thermal thresholds (35.0 °C, 33.0 °C, 31.5 °C) for select years (Fig. 3). This holds for all assumptions of fitness cost. In the most severe cases, the proportion of the population carrying wMel falls to 62% when assuming a 10% fitness cost. This is well above what is considered a critical threshold (20–30%) for achieving wMel fixation (100% infection frequency)29,30. When dynamics were driven by seven-day moving average temperature inputs, wMel frequency rose faster than with daily inputs; in no instance did frequency fall after reaching fixation.
Effect of future heatwaves on the frequency of wMel infection in an adult female A. aegypti population. Each line corresponds to one simulated intervention year; each colour corresponds to a different temperature regime (2030 in orange, 2050 in red). Each panel reflects infection frequency given unique thermal thresholds (35 °C (a) 33 °C (b), 31.5 °C (c)) and daily temperature inputs to the wMel dynamic model. d, Infection frequency when seven-day moving averages are used as temperature inputs; all three thermal thresholds returned equivalent output under this assumption.
The dynamics of wMel-infected and WT female mosquitoes under future regimes put these results into population-level perspective across assumptions of biological threshold and temperature input (Fig. 4). Dynamics occurring under heatwave years were overlaid on dynamics driven by the baseline temperatures used to construct those heatwave inputs, highlighting the delta generated by thermal shocks. The decline in wMel infection frequency and corresponding increase in vector-competent female mosquitoes indicates the potential public health impact of future temperature variability. Using daily temperature inputs, the population of wMel carriers was more than halved by December for selected years in both the 33 °C and 31.5 °C scenarios. A decline was observed for those same years in the 35 °C scenario. In all cases, this decrease corresponded to a rising proportion of vector-competent females.
Replacement (solid lines) and suppression (dotted lines) dynamics for future heatwave years (darker tones) and the average temperature baselines (lighter tones) from which they are calculated under both future regimes (2030s & 2050s). In each panel, the upper set of trajectories show the wMel-infected population and the lower set of trajectories show the WT population. a–c, Dynamics given unique thermal thresholds (35 °C (a) 33 °C (b), 31.5 °C (c)) and daily temperature inputs to the wMel dynamic model. d, Dynamics when seven-day moving averages are used as temperature inputs; all three thermal thresholds returned equivalent output under this assumption.
Current empirical data suggests that wMel falls out of A. aegypti at temperatures equal to or exceeding 35 °C (ref. 22). In the Cairns climate projections used, this threshold is reached or surpassed as a daily average in 2029 and 2049. While these examples of severe heat have negative effects on simulated wMel interventions (Figs. 3 and 4a–d), they were not sufficient to cause a complete removal of the pathogen-blocking bacterium from the mosquito population. In recent years, temperature in Cairns had greater variability and hotter extremes than the years used as baselines for climate projections31 in this location (1986–2005).
Given the robustness of wMel to the Cairns climate scenarios and the potential for warmer or more variable futures in that location and elsewhere, we conducted two additional analyses. First, we undertook a theoretical exploration of what might be required to remove wMel from a population by incorporating progressively hotter days in existing Coupled Model Intercomparison Project Phase 5 (CMIP5) heatwave years (Methods).
We illustrate the effect on the WT population when additional hot days are included in the existing time series of projected future temperatures (Fig. 5a,b). The surge in vector-competent mosquitoes is larger in the 2050 scenario than that of the 2030s due to the high thermal optima of the A. aegypti species. Three fewer ‘extra’ days of heat are required in 2050 for the WT population to rebound. However, even under the most extreme assumption of consecutive hot days (19 and 16 in the 2030s and 2050s, respectively), the wMel-infected population persists at levels sufficient to permit a full rebound (Fig. 5c,d).
The impact of additional hot days on population dynamics in future heatwave years: suppression (solid lines) and replacement (dotted lines) for the 2030s (yellow tones) and 2050s (orange tones). In all panels, darker colors correspond to an increasing number of hot days extending the CMIP5-projected heatwaves. a, In the 2030s, less wildtype (WT) suppression is achieved as heatwaves are extended. b, Similarly, the 2050s see less WT suppression under longer heatwaves. c, wMel replacement is reduced under prolonged heatwaves in the 2030s. d, wMel replacement is also lessened given lengthier heatwaves in the 2050s.
In a second investigation of wMel’s thermal limits, we studied data for replacement interventions in the Nha Trang City region, Vietnam. It has hotter historical average temperatures and the potential, according to the CMIP5 projections, for average warming and heatwaves that exceed temperatures and durations predicted for Cairns. Our simulations (Fig. 6) built on the observed environmental conditions and release schedule employed by Hien et al.16 in Vinh Luong Ward, where inputs (mean temperature, duration, frequency and amplitude of heatwaves, and intrinsic biological thresholds of wMel) reflect the most extreme assumptions given the data (Methods).
a, Replacement (wMel-infected population, solid lines) and suppression (WT population, dotted lines) dynamics during both heatwave years (dark tones) and their baselines (light tones), assuming a 2050 temperature regime. b, Effect of future heatwaves on the frequency of wMel infection in an adult female A. aegypti population under the 2030 (orange) and 2050 (red) regimes.
The results show that wMel introgression is generally robust to the tested scenarios as fixation fails only under the most extreme joint assumptions of heatwave duration and intrinsic thermal limit (Fig. 6) (RCP 8.5 2050 climate regimes together with a 31.5 °C or 33.0 °C biological threshold for wMel, more stringent than the empirically demonstrated 35.0 °C point of fallout). This underscores the impact of climate change-induced heatwaves on wMel interventions compared to increases in mean daily temperature: the replacement dynamics driven by the 2050 mean temperatures maintain a high frequency of infection through the end of the period (Fig. 6a, light red solid lines). However, the 2050 heatwaves cause wMel-infected numbers to fall (dark red solid lines). The effect of heatwave duration on sustained fixation is also highlighted: during the 2030s, the longest heatwaves last an average of 9.7 days; in the 2050s, they last 24 days (Fig. 6b).
The Vietnam simulations for the 2030 and 2050 climate regimes reveal an important relationship between infection frequency, heatwaves and the timing of wMel releases. Using daily temperature inputs and a thermal threshold of 31.5 °C, infection frequency was notably increased when intervention releases occurred after the longest heatwave concluded (P < 0.0006). However, the average temperature of that heatwave depressed infection frequency (P < 0.0001; full model R2 = 0.8938, corrected Akaike information criterion = 2.1597).
Discussion
The growing literature on mosquito thermal biology highlights its role in the impact of climate change on vector-borne disease. However, new intervention technologies may also be subject to the complexities of global warming. No research has yet examined the effect of climate change on Wolbachia-based biocontrol methods, nor used empirical data on the thermal sensitivity of Wolbachia to model the dynamics of this tool. Furthermore, published modelling work that explores the influence of temperature on mosquito-borne disease focuses on trends (increasing average temperatures) not variability (the magnitude, duration and frequency of heatwaves).
In this study, we demonstrate that rising average temperatures in Cairns, Australia under CMIP5-generated predictions may benefit wMel replacement during winter months; however, future summer average temperatures may surpass the symbiont’s thermal thresholds and cause transient population decline. While simulations reflecting the Nha Trang City region of Vietnam reinforce the negative impact of future mean temperatures on wMel replacement under the most stringent assumptions of the bacterium’s thermal thresholds, many non-climatological, non-biological covariates that differ across spatiotemporal settings could also affect the symbiont’s introgression and fixation16. This includes the heat-buffering effects of larger or shaded aquatic habitats that can differentially mitigate climate impacts on juvenile microorganisms across built environments, affecting the establishment and persistence of both wMel and their A. aegypti hosts16,32.
Our results exhibit the potential vulnerability of wMel-based replacement to temperature variability under climate change as simulations with hotter and more frequent thermal extremes show diminished efficacy relative to simulations that strictly consider mean temperature. CMIP5-projected heatwaves in Cairns lower wMel frequencies; however, frequencies recover under even the most severe projections of local heat shocks. Outputs for the 2030 scenarios in the Nha Trang City region bolster the finding that wMel technology may withstand near-term future periods of extreme temperature, suggesting that our conclusions can be extrapolated to additional geographical contexts.
In 2018 (26–28 November), a heatwave in Cairns where wMel infection had been established since 2011 presented a natural experiment that produced findings aligning with ours33. Recorded maximum temperatures reached 43.6 °C, and while wMel frequency fell, it never dropped below 83% in juvenile mosquitoes. It recovered to nearly 100% by April 2019. However, throughout this period, the highest average daily temperature was 33.75 °C. To explore the edge case in which wMel is severely reduced or eliminated from a population after heat shocks, empirical experimentation should assess the effect of a daily average greater than or equal to 35 °C, and evaluate the interaction of temperature spikes with mosquito developmental stages on pathogen-blocking efficacy34.
Increasingly acute and prolonged heatwaves around the world highlight the need to test the thermal limits of wMel technology beyond the theoretical examples presented in this article, where approximately two to three weeks at or above 35 °C reduced wMel frequency during the examined period. Simulation results for the 2050 heatwaves in the Nha Trang City region show wMel elimination under the lower thresholds tested (31.5 °C, 33.0 °C). While such dynamics may be relevant to a proposed toxin–antidote gene drive based on the Wolbachia alleles responsible for CI35, they also highlight a possible 30-year time horizon in which to advance the heat tolerance of current wMel-based replacement technology.
Further investigation is required to discern the minimal exposure time at or above wMel’s biological threshold before critical mechanisms such as CI and maternal inheritance are impacted, and to understand the generational duration of deleterious effects after heat spikes. The vector–pathogen relationship under heat stress also merits more study: dengue virus increases the thermal sensitivity of A. aegypti, and coinfection with Wolbachia does not furnish protection from the dengue-induced thermotolerance effects36. However, there is evidence that diurnal temperature fluctuations negatively impact the vector–pathogen relationship at mean temperatures greater than 18.0 °C, with A. aegypti having shorter lifespans and being less likely to become infected with dengue virus as the diurnal temperature range increases37.
Our modelling omits the potential heat avoidance of mosquitoes38,39 and thus the possibility that Wolbachia-infected A. aegypti may seek cooler temperatures where bacterial titre is maintained38. Such heat-induced behaviour14, as well as the connection between Wolbachia density and CI mechanisms40, require further research. Finally, our results are necessarily a function of structural decisions made when developing the ordinary differential equation (ODE) model, including the choice of functions to define temperature-sensitive vital rate parameterizations41. Therefore, analyses using alternative formulations may also be merited.
Wolbachia experts recognize that strain selection must account for local environmental realities14. Recent studies focused on deployment in extreme environments42 and the development of strains with increased heat tolerance43. Field trials across an increasing variety of geographies will furnish critical data on the effect of climate and non-climate drivers on wMel introgression and persistence. For decision-makers to weigh the choice between wMel and its alternatives as we move into an uncertain and more variable climate future, computational and bench scientists should explicitly account for predictions of regional warming—particularly heat extremes—in their experimental designs. In the short term, temperature-conscious operational planning, such as augmented deployment schedules, may be able to compensate for the already observed risk that infection frequencies drop or remain at low levels due to heat stress.
While the results presented in this article furnish reassurance that wMel replacement is a resilient technology in the face of near-term climate change, they are predicated on temperature profiles constructed from pre-2006 baselines. Thus, they remain conceivably best-case scenarios, given the virtual certainty that hot extremes will continue to increase in both frequency and intensity44. This highlights the inextricable link between climate mitigation policy and infectious disease management. Failure to curb carbon dioxide emissions will not only increase the proportion of the world exposed to deadly mosquito-borne illnesses due to rising temperatures; it may also undercut the utility of an otherwise demonstrably effective tool for vector control.
Methods
Climate data for Cairns, Australia
We obtained historical daily temperature data for Cairns, Queensland, Australia (latitude 16° 52' 25.9594" S, longitude 145° 44' 44.88" E) from the Global Historical Climatology Network database maintained by the National Centers for Environmental Information of the United States National Oceanic and Atmospheric Administration (NCEI–NOAA). We selected Cairns as the geographical location of interest because two towns in that region, Yorkeys Knob and Gordonvale, were the sites of the first successful field trial for population replacement using wMel; therefore, the reported trial outcomes serve as experimental validation for simulations using local environmental variables10. Additional Wolbachia releases have since taken place in the area, including in central Cairns17,18,45.
Projected future data for Cairns, including both average temperatures and heatwaves under the RCP scenarios 4.5 and 8.5, were developed from dynamically downscaled CMIP5 global climate projections obtained from the Queensland Future Climate Dashboard, which is maintained by the Science Division of the Queensland Department of Environment and Sciences46,47. These data are recorded in fractions of degrees of long-term change (°C) relative to the reference period (1986–2005). Because the available daily historical temperature data begin in 1990, we used the period 1990–2005 as the historical baseline and developed daily future climate scenarios using the anomaly method for the years 2024–2059. While the years from 2060 onwards exhibit more drastic temperature rise, we excluded these from the analysis to focus on outcomes most relevant to near-term technological decisions concerning Wolbachia-based public health interventions. Full details on the methodology used to construct future daily average temperatures and heatwaves are shown in section 2 of the Supplementary Information.
Climate data for Nha Trang City, Vietnam
We sourced historical observed daily temperature records for the region of Nha Trang City (Nha Trang), Vietnam (latitude 12° 15' 12.2394" N, longitude 109° 11' 13.56" E) from the NCEI–NOAA. We chose this location given its higher average baselines and longer, more frequent and hotter future heatwaves compared to the Cairns case study. Furthermore, two villages in the Nha Trang region, Vinh Luong Ward (Vinh Luong) and Tri Nguyen Village (Tri Nguyen), are sites of recent wMel-infected A. aegypti releases. The field study associated with these trial interventions used weather data from Nha Trang to conduct subsequent analysis given the absence of such data from these two smaller nearby population centres16. Future average temperature data for Vietnam under RCP scenario 8.5 were sourced from the Climate Change Knowledge Portal48; future heatwave data were furnished by Dong et al.49. Both projected mean and heatwave scenarios were produced using historical temperature data for the baseline period (1990–2005) and the anomaly method described above. For additional details, see section 2 of the Supplementary Information.
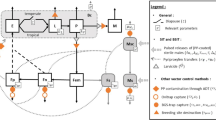
Simulation model of population dynamics
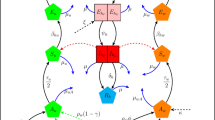
We developed a system of ODEs to simulate mosquito population dynamics. The model, described in detail in section 3 of the Supplementary Information, uses a daily time step and portrays four life stages of the mosquito lifecycle: egg, larva, pupa and adult50,51. Adults emerging from the pupal stage are evenly divided between males and females; the population is assumed to be randomly mixing and all organisms within a particular life stage are equal with respect to their birth, death and maturation rates. Larval stage mortality is modulated by logistic density dependence52. Parameterization reflects the species-specific thermal biology of A. aegypti, which was selected for this study because it has been a primary host for wMel in the context of population replacement experiments including in the Cairns and Nha Trang regions. The model was initialized at equilibrium. The equilibrium of a dynamic system generated by a system of ODEs is defined as a solution that does not change over time; it is a steady state.
A. aegypti vital rates were calculated using temperature-responsive functional forms unique to each life stage. These formulations and their parameterization were developed by Rossi et al.53 to probe the effect of climate change on the A. aegypti life cycle and its relevance to disease incidence. The assumptions used to adapt these equations to the underlying population model included using literature-derived values for oviposition (63 eggs daily per female) and excluding the consideration of carrying capacity for egg laying54. Field studies of wMel replacement interventions in the Cairns region informed the deployment schedule used for the simulations using climate inputs from that geographical location, where the frequency of model releases paralleled reality to occur every seven time steps from day 4 through day 63 (ref. 10). The interventions modelled using the Nha Trang temperature data used a schedule that emulated studies conducted in nearby Vinh Luong, with releases taking place every seven days from day 65 through day 177 (ref. 16). The Vinh Luong trials furnished the basis of our experimental design because interventions at that location occurred during one of the hottest years on record for the region (2018).
Biological parameterization of wMel temperature sensitivity
Wolbachia carriers in this work are assumed to have temperature-responsive hatch rates, maternal inheritance rates and CI levels. Empirical data generated from the cyclical heat exposure of wMel-infected A. aegypti mosquitoes was used to develop the equations that reflect thermal sensitivity22. In these laboratory experiments, eggs were subjected to weekly temperature regimes that cycled daily between the maximum and minimum of a given 10 °C range, with the coolest regime averaging 29 °C and the warmest averaging 37 °C. Data were also collected for eggs held at a consistent 26 °C for the seven-day study period.
Extrapolating the results of week-long experiments to a dynamic model with daily time steps requires assumptions about the unknown functional form of heat stress accumulation in wMel. To address this uncertainty and account for the lack of required temporal resolution in the empirical data-generating process, we included a sensitivity analysis that assesses two theoretical alternatives: first, all temperature inputs to the thermally sensitive wMel equations were calibrated to consist of a seven-day moving average. This reflects an assumption that the physiological impact of heat stress is evenly distributed, with the effect of one hot day prolonged for a subsequent six days. In the second sensitivity analysis, all temperature inputs were allowed to be entered as daily values. This reflects an assumption that the impact of heat stress is primarily immediate and does not necessarily result in a cumulative effect within the organism.
To formulate the biological model of wMel, we fitted a function to the mean value of experimental replicates for each temperature regime. Equation (1) reflects the statistical best fit to egg hatch data. The hatch rate T(C) for infected eggs below 26 °C was fixed at 0.91833; above 36 °C, it was fixed at 0 in accordance with Ross et al.22. Supplementary Fig. 1 compares the equation (1) dynamics with the observed hatch rates:
The temperature-sensitive status of wMel infection and consequent vertical transmission from mother to offspring was modelled using matrix operations informed by the data underpinning Figs. 7b,c of Ross et al.22, where above 35.0 °C wMel inheritance, I, falls to 0 and previously complete CI, Y, stops functioning entirely. These conditional relationships are formalized in equations (2) and (3):
The conservative assumptions embodied by this approach, which rely on the simple presence or absence of wMel infection, are made in acknowledgement of the fact that the mappings between infection titre and the biological properties it confers are not yet well defined. The chosen method results in a probable overestimation of both wMel maternal transmission rates and CI at temperatures lower than the 35.0 °C threshold. Therefore, we include additional sensitivity analyses varying this biological threshold, imposing it instead at 31.5 °C and 33.0 °C. Future work, including empirical experimentation to improve scientific understanding of the temperature sensitivities of wMel infection and the mechanisms of CI, will enable refined mathematical estimations of these processes.
A range of fixed fitness costs was modelled to reflect heightened mortality in wMel carriers, drawing on empirically derived values in the literature. This sensitivity analysis tested costs that augmented adult mortality rates in wMel-infected mosquitoes by 0%, 10% and 20%, with reported results reflecting use of the middle value (10%). Section 5 of the Supplementary Information contains outcomes using the upper and lower bounds of the fitness range. Field studies of wMel replacement interventions in the Cairns region are consistent with model dynamics, as illustrated in Supplementary Fig. 2 comparing observed data with simulated results (SupplementaryInformation).
Metrics used to assess and compare simulation results
In addition to characterizing the results using qualitative visual comparisons, a new standard entomological metric (SEM) is proposed; it was used to evaluate the replacement capacity of wMel-based interventions under past and future climate scenarios, that is, the RES. Like the SES put forward in Vásquez et al.28, the RES is a single value that summarizes relative success over the period of interest, and can be used to compare the performance of alternative interventions to each other. The RES is assessed with respect to the frequency of Wolbachia infection in the standing vector population over this time. A conceptual explanation of the score is furnished by Supplementary Fig. 3 (see section 1 as well as section 4 of the SupplementaryInformation; the latter includes illustrative example applications).
There are a total of T time steps in the intervention period being evaluated; these are equal to that period’s length, τf − τ0, divided by the discretization, δτ. This is explained in equation (4):
The frequency of wMel is estimated by calculating the proportion of the infected female population Pg,t and integrating the cumulative change in that population over the duration of an intervention. \(A^F_{g,t}\), the area under the curve of the observed frequency trajectory, is defined in equation (5):
This area is then divided by the outcome of an idealized intervention Γg wherein fixation (100% infection of the standing population) is achieved immediately and for the duration of the period of interest; this yields the RES score described by equation (6). Like the previously defined SEMs, indexing the RES score Rg and vector population change AFg,t according to patterns of inheritance g allows the metric to be generalized beyond wMel-specific applications. When evaluating public health intervention technologies that use genetic modification, g is used to represent the frequency of the refractory genotype rather than Wolbachia infection status:
Theoretically testing the thermal limits of wMel
Using 2029 RCP 8.5 and 2049 RCP 8.5 as examples, we augmented each time series of temperature inputs to include, beginning on day 355, consecutive additional days of heat equivalent to the maximum value in that time series. For 2029, the maximum value was 35.21 °C and for 2049 it reached 36.6 °C. These temperatures are high enough to drop maternal transmission of wMel and hatch rates for Wolbachia-infected eggs to 0. CI for crosses between wild females and wMel-carrying males is also eliminated. We selected the first time step to begin appending new hot days based on the culmination of the heat surge in the 2029 example, which ended on day 354. To maintain comparability, the same time step was chosen for the 2049 simulation. The time horizon was then extended by a second year and no additional wMel-infected releases were conducted.
Data availability
The raw data outputs featuring the population dynamics that resulted from all model runs is stored on Figshare: https://doi.org/10.6084/m9.figshare.20722270; https://doi.org/10.6084/m9.figshare.20721928; https://doi.org/10.6084/m9.figshare.20719234; https://doi.org/10.6084/m9.figshare.20716117; https://doi.org/10.6084/m9.figshare.20607765; https://doi.org/10.6084/m9.figshare.22589623; https://doi.org/10.6084/m9.figshare.22589626; https://doi.org/10.6084/m9.figshare.22589632 (refs. 55,56,57,58,59,60,61,62).
Code availability
The GeneDrive.jl software extended to implement this work is available via GitHub at https://github.com/vnvasquez/GeneDrive.jl (ref. 63). All code adaptations required to reproduce the specific experiments in this paper are available via GitHub at https://github.com/vnvasquez/WolbachiaClimatePaper (ref. 64).
Change history
14 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41558-023-01797-z
References
Ryan, S. J., Carlson, C. J., Mordecai, E. A. & Johnson, L. R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 13, e0007213 (2019).
Mordecai, E. A., Ryan, S. J., Caldwell, J. M., Shah, M. M. & LaBeaud, A. D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 4, e416–e423 (2020).
Mordecai, E. A. et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 22, 1690–1708 (2019).
Romanello, M. et al. The 2021 report of the Lancet Countdown on health and climate change: code red for a healthy future. Lancet 398, 1619–1662 (2021).
Caminade, C., McIntyre, K. M. & Jones, A. E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 1436, 157–173 (2019).
Colón-González, F. J. et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet. Health 5, e404–e414 (2021).
Hawkins, N. J., Bass, C., Dixon, A. & Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 94, 135–155 (2019).
Hemingway, J. et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet 387, 1785–1788 (2016).
Howard, W. E. Biological control of vertebrate pests. In Proc. Vertebrate Pest Conference 3 (1967).
Hoffmann, A. A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 (2011).
Utarini, A. et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 384, 2177–2186 (2021).
Gesto, J. S. M. et al. Large-scale deployment and establishment of Wolbachia into the Aedes aegypti population in Rio de Janeiro, Brazil. Front. Microbiol. 12, 711107 (2021).
Hilgenboecker, K., Hammerstein, P., Schlattmann, P., Telschow, A. & Werren, J. H. How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 (2008).
Ross, P. A. Designing effective Wolbachia release programs for mosquito and arbovirus control. Acta Trop. 222, 106045 (2021).
Pinto, S. B. et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: a quasi-experimental study. PLoS Negl. Trop. Dis. 15, e0009556 (2021).
Hien, N. T. et al. Environmental factors influence the local establishment of Wolbachia in Aedes aegypti mosquitoes in two small communities in central Vietnam. Gates Open Res. 5, 147 (2022).
O’Neill, S. L. et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2, 36 (2019).
Ryan, P. A. et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 3, 1547 (2019).
Denton, J. A., Joubert, D. A., Goundar, A. A. & Gilles, J. R. L. International shipments of Wolbachia-infected mosquito eggs: towards the scaling-up of World Mosquito Program operations. Rev. Sci. Tech. 41, 91–99 (2022).
Ross, P. A. et al. A decade of stability for wMel Wolbachia in natural Aedes aegypti populations. PLoS Pathog. 18, e1010256 (2022).
Ross, P. A., Ritchie, S. A., Axford, J. K. & Hoffmann, A. A. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl. Trop. Dis. 13, e0007357 (2019).
Ross, P. A. et al. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 13, e1006006 (2017).
Nazni, W. A. et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 29, 4241–4248 (2019).
Beebe, N. W. et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proc. Natl Acad. Sci. USA 118, e2106828118 (2021).
Crawford, J. E. et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 38, 482–492 (2020).
Project Wolbachia—Singapore Consortium & Ching, N. L. Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. Preprint at medRxiv https://doi.org/10.1101/2021.06.16.21257922 (2021).
Zhongming, Z., Linong, L., Xiaona, Y., Wangqiang, Z. & Wei, L. AR6 climate change 2021: the physical science basis. UNFCC https://unfccc.int/documents/310264?gclid=CjwKCAjw44mlBhAQEiwAqP3eVi-X8GWfpUM306iVIVrghRNoHZe18s0lA5HxX06HrKWPw5P2ACi6fBoCpCMQAvD_BwE (2021).
Vasquez, V. N., Reddy, M. R. & Marshall, J. M. Environmentally appropriate vector control is facilitated by standard metrics for simulation-based evaluation. Front. Trop. Dis. 3, 953212 (2022).
Turelli, M. & Barton, N. H. Why did the Wolbachia transinfection cross the road? drift, deterministic dynamics, and disease control. Evol. Lett. 6, 92–105 (2022).
Turelli, M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64, 232–241 (2010).
Carvalho, D. et al. How well have CMIP3, CMIP5 and CMIP6 future climate projections portrayed the recently observed warming. Sci. Rep. 12, 11983 (2022).
Franklinos, L. H., Jones, K. E., Redding, D. W. & Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 19, e302–e312 (2019).
Ross, P. A. et al. Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Negl. Trop. Dis. 14, e0007958 (2020).
Chrostek, E., Martins, N., Marialva, M. S. & Teixeira, L. Wolbachia-conferred antiviral protection is determined by developmental temperature. mBio 12, e02923-20 (2021).
Li, J. & Champer, J. Harnessing Wolbachia cytoplasmic incompatibility alleles for confined gene drive: a modeling study. PLoS Genet. 19, e1010591 (2023).
Ware-Gilmore, F. et al. Microbes increase thermal sensitivity in the mosquito Aedes aegypti, with the potential to change disease distributions. PLoS Negl. Trop. Dis. 15, e0009548 (2021).
Lambrechts, L. et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA 108, 7460–7465 (2011).
Hague, M. T. J., Caldwell, C. N. & Cooper, B. S. Pervasive effects of Wolbachia on host temperature preference. mBio 11, e01768-20 (2020).
Truitt, A. M., Kapun, M., Kaur, R. & Miller, W. J. Wolbachia modifies thermal preference in Drosophila melanogaster. Environ. Microbiol. 21, 3259–3268 (2019).
Wang, W., Cui, W. & Yang, H. Toward an accurate mechanistic understanding of Wolbachia-induced cytoplasmic incompatibility. Environ. Microbiol. 24, 4519–4532 (2022).
Nørgaard, L. S., Álvarez-Noriega, M., McGraw, E., White, C. R. & Marshall, D. J. Predicting the response of disease vectors to global change: the importance of allometric scaling. Glob. Change Biol. 28, 390–402 (2022).
Ross, P. A. et al. Developing Wolbachia-based disease interventions for an extreme environment. PLoS Pathog. 19, e1011117 (2023).
Gu, X. et al. A wMel Wolbachia variant in Aedes aegypti from field-collected Drosophila melanogaster with increased phenotypic stability under heat stress. Environ. Microbiol. 24, 2119–2135 (2022).
Pörtner, H.-O. et al. Climate Change 2022: Impacts, Adaptation and Vulnerability (IPCC, 2022).
Ritchie, S. A. Wolbachia and the near cessation of dengue outbreaks in Northern Australia despite continued dengue importations via travellers. J. Travel Med. 25, 10.1093/jtm/tay084 (2018).
Syktus, J., Trancoso, R., Ahrens, D., Toombs, N. & Wong, K. Queensland future climate dashboard. Queensland Government https://www.longpaddock.qld.gov.au/qld-future-climate (2023).
Syktus, J., Toombs, N., Wong, K. K.-H., Trancoso, R. & Ahrens, D. Queensland Future Climate Dataset: downscaled CMIP5 climate projections for RCP8.5 and RCP4.5. Terrestrial Ecosystem Research Network https://researchdata.edu.au/queensland-future-climate-rcp85-rcp45/1885395?source=suggested_datasets (2020).
Climate change knowledge portal. World Bank Group https://climateknowledgeportal.worldbank.org/ (2021).
Dong, Z. et al. Heatwaves in Southeast Asia and their changes in a warmer world. Earths Future 9, e2021EF001992 (2021).
Hancock, P. A. & Godfray, H. C. J. Application of the lumped age-class technique to studying the dynamics of malaria-mosquito-human interactions. Malar. J. 6, 98 (2007).
Gurney, W. S. C., Nisbet, R. M. & Lawton, J. H. The systematic formulation of tractable single-species population models incorporating age structure. J. Anim. Ecol. 52, 479–495 (1983).
Sibly, R. M., Barker, D., Denham, M. C., Hone, J. & Pagel, M. On the regulation of populations of mammals, birds, fish, and insects. Science 309, 607–610 (2005).
Rossi, M. M., Ólivêr, L. & Massad, E. in Ecological Modelling Applied to Entomology 81–107 (Springer, 2014).
Otero, M., Solari, H. G. & Schweigmann, N. A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bull. Math. Biol. 68, 1945–1974 (2006).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.20722270 (2022).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.20721928 (2022).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.20719234 (2022).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.20716117 (2022).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.20607765 (2022).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.22589623 (2023).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.22589626 (2023).
Vasquez, V. N. Aedes aegypti population dynamics. figshare https://doi.org/10.6084/m9.figshare.22589632 (2023).
Vasquez, V. N. GeneDrive.jl. GitHub https://github.com/vnvasquez/GeneDrive.jl (2022).
Vasquez, V. N. Experimental code for ‘wMel replacement of dengue-competent mosquitoes is robust to near-term climate change’. GitHub https://github.com/vnvasquez/WolbachiaClimatePaper (2023).
Acknowledgements
We thank L. Wang for the data characterizing future heatwaves in Vietnam and guidance concerning these projections. V.N.V. was supported by a Microsoft Research PhD Fellowship. G.R. and J.M.M. were supported by a National Institutes of Health R01 Grant (no. 1R01AI143698-01A1). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
V.N.V., L.M.K., G.R. and J.M.M. conceived and designed the study. V.N.V. collected the data, performed the analysis and wrote the first manuscript draft. V.N.V., L.M.K., G.R. and J.M.M. verified the data and analysis. V.N.V., L.M.K., G.R. and J.M.M. revised and approved the final manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Grigori Chapiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Figs. 1–8 and Tables 1–27.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vásquez, V.N., Kueppers, L.M., Rašić, G. et al. wMel replacement of dengue-competent mosquitoes is robust to near-term climate change. Nat. Clim. Chang. 13, 848–855 (2023). https://doi.org/10.1038/s41558-023-01746-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-023-01746-w
This article is cited by
-
Effects of climate change and human activities on vector-borne diseases
Nature Reviews Microbiology (2024)
-
Susceptibility of Wolbachia mosquito control to temperature shifts
Nature Climate Change (2023)