Abstract
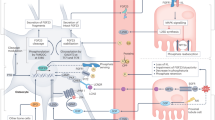
High concentrations of extracellular phosphate are toxic to cells. Impaired urinary phosphate excretion increases serum phosphate level and induces a premature-ageing phenotype. Urinary phosphate levels are increased by dietary phosphate overload and might induce tubular injury and interstitial fibrosis. Extracellular phosphate exerts its cytotoxic effects by forming insoluble nanoparticles with calcium and fetuin-A; these nanoparticles are referred to in this Review as calciprotein particles. Calciprotein particles are highly bioactive ligands that can induce various cellular responses, including the osteogenic transformation of vascular smooth muscle cells and cell death of vascular endothelial cells and renal tubular epithelial cells. Calciprotein particles are detected in the serum of animal models of kidney disease and in patients with chronic kidney disease (CKD) and might be associated with a (mal)adaptation of the endocrine axes mediated by fibroblast growth factors and Klothos that regulate phosphate homeostasis and ageing. These observations raise the possibility that calciprotein particles contribute to the pathogenesis of CKD. This theory, if verified, is expected to provide novel diagnostic markers and therapeutic targets in CKD.
Key Points
-
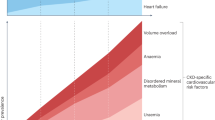
Calciprotein particles are cytotoxic nanoparticles composed of inorganic calcium–phosphate crystals and mineral binding proteins, such as fetuin-A
-
Calciprotein particles circulate in the blood of patients with chronic kidney disease (CKD) and may contribute to vascular calcification, chronic inflammation and a premature-ageing phenotype
-
Raised phosphate intake and/or decreased nephron number increase the rate of phosphate excretion per nephron, potentially causing calciprotein particle formation in the tubular lumen and tubular injury
-
Serum fibroblast growth factor 23 (FGF-23) level correlates with phosphate excretion per nephron and, when combined with 24 h urinary phosphate excretion, may be useful for estimating changes in residual nephron number
-
A new paradigm for phosphate restriction proposed in this Review argues for phosphate binders to be used in patients with CKD and an abnormally high FGF-23 level, regardless of serum phosphate level
-
The therapeutic goal is to reduce serum FGF-23 level but not serum phosphate level, with the primary aim of preventing histological kidney damage and progression of CKD without increasing vascular calcification and cardiovascular events
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wolfe-Simon, F. et al. A bacterium that can grow by using arsenic instead of phosphorus. Science 332, 1163–1166 (2011).
Hiza, H., Bente, L. & Fungwe, T. Nutrient content of the U.S. food supply, 2005. U.S. Department of Agriculture, Center for Nutritional Policy and Promotion [online], (2008).
Uribarri, J. Phosphorus additives in food and their effect in dialysis patients. Clin. J. Am. Soc. Nephrol. 4, 1290–1292 (2009).
Schiavi, S. C. & Kumar, R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int. 65, 1–14 (2004).
Mitchell, H. H., Hamilton, T. S., Steggerda, F. R. & Bean, H. W. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 96, 625–637 (1945).
Kuro-o, M. Phosphate and Klotho. Kidney Int. Suppl. 79, S20–S23 (2011).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Azuma, M. et al. Promoter methylation confers kidney-specific expression of the Klotho gene. FASEB J. 26, 4264–4274 (2012).
Nagai, T. et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 17, 50–52 (2003).
Kamemori, M. et al. Expression of Klotho protein in the inner ear. Hear. Res. 171, 103–110 (2002).
Toyama, R. et al. Impaired regulation of gonadotropins leads to the atrophy of the female reproductive system in klotho-deficient mice. Endocrinology 147, 120–129 (2006).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Suga, T. et al. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 22, 26–33 (2000).
Kawaguchi, H. et al. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J. Clin. Invest. 104, 229–237 (1999).
Tsujikawa, H., Kurotaki, Y., Fujimori, T., Fukuda, K. & Nabeshima, Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Farrow, E. G., Davis, S. I., Summers, L. J. & White, K. E. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 20, 955–960 (2009).
Olauson, H. et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 23, 1641–1651 (2012).
Goetz, R. et al. Molecular insights into the Klotho-dependent, endocrine mode of action of FGF19 subfamily members. Mol. Cell Biol. 27, 3417–3428 (2007).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005).
Choi, M. et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 12, 1253–1255 (2006).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005).
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007).
Potthoff, M. J. et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl Acad. Sci. USA 106, 10853–10858 (2009).
Badman, M. K. et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell. Metab. 5, 426–437 (2007).
Potthoff, M. J., Kliewer, S. A. & Mangelsdorf, D. J. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 26, 312–324 (2012).
Ito, S. et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech. Dev. 98, 115–119 (2000).
Ogawa, Y. et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007).
Kurosu, H. et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007).
Masuyama, R. et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Invest. 116, 3150–3159 (2006).
Kuro-o, M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol. Metab. 19, 239–245 (2008).
Kuro-o, M. (Ed.) Endocrine FGFs and Klothos (Landes Bioscience and Springer Science, New York, 2012).
Stubbs, J. R. et al. Role of hyperphosphatemia and 1,25-Dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 18, 2116–2124 (2007).
Hesse, M., Frohlich, L. F., Zeitz, U., Lanske, B. & Erben, R. G. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 26, 75–84 (2007).
Razzaque, M. S., Sitara, D., Taguchi, T., St-Arnaud, R. & Lanske, B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 20, 720–722 (2006).
Ohnishi, M., Nakatani, T., Lanske, B. & Razzaque, M. S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 75, 1166–1172 (2009).
Ohnishi, M. & Razzaque, M. S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 24, 3562–3571 (2010).
Beck, L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. USA 95, 5372–5377 (1998).
Ohnishi, M., Nakatani, T., Lanske, B. & Razzaque, M. S. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin-D levels. Circ. Cardiovasc. Genet. 2, 583–590 (2009).
Hruska, K. A., Mathew, S., Lund, R., Qiu, P. & Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 74, 148–157 (2008).
Stenvinkel, P. & Larsson, T. E. Chronic kidney disease: a clinical model of premature aging. Am. J. Kidney Dis. http://dx.doi.org/10.1053/j.ajkd.2012.11.051.
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Koh, N. et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 280, 1015–1020 (2001).
Galitzer, H., Ben-Dov, I. Z., Silver, J. & Naveh-Many, T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 77, 211–218 (2010).
Komaba, H. et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 77, 232–238 (2010).
Krajisnik, T. et al. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 78, 1024–1032 (2010).
Kumata, C. et al. Involvement of α-klotho and fibroblast growth factor receptor in the development of secondary hyperparathyroidism. Am. J. Nephrol. 31, 230–238 (2010).
Kestenbaum, B. et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol. 16, 520–528 (2005).
Ganesh, S. K., Stack, A. G., Levin, N. W., Hulbert-Shearon, T. & Port, F. K. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 12, 2131–2138 (2001).
Martin, K. J. & Gonzalez, E. A. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin. J. Am. Soc. Nephrol. 6, 440–446 (2011).
Martin, G. M. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects 14, 5–39 (1978).
Lombard, D. B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 39, 99–105 (2007).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Flurkey, K., Papaconstantinou, J., Miller, R. A. & Harrison, D. E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA 98, 6736–6741 (2001).
Ramirez, R. et al. Stress-induced premature senescence in mononuclear cells from patients on long-term hemodialysis. Am. J. Kidney Dis. 45, 353–359 (2005).
Smogorzewski, M. J. & Massry, S. G. Liver metabolism in CRF. Am. J. Kidney Dis. 41 (Suppl. 1), S127–S132 (2003).
Inagaki, T. et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8, 77–83 (2008).
Lin, Z. et al. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS ONE 6, e18398 (2011).
Han, S. H. et al. Serum fibroblast growth factor-21 concentration is associated with residual renal function and insulin resistance in end-stage renal disease patients receiving long-term peritoneal dialysis. Metabolism 59, 1656–1662 (2010).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Di Marco, G. S. et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am. J. Physiol. Renal Physiol. 294, F1381–F1387 (2008).
Shuto, E. et al. Dietary phosphorus acutely impairs endothelial function. J. Am. Soc. Nephrol. 20, 1504–1512 (2009).
Steitz, S. A. et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 89, 1147–1154 (2001).
Jono, S. et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 87, E10–E17 (2000).
Reynolds, J. L. et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 15, 2857–2867 (2004).
Sage, A. P., Lu, J., Tintut, Y. & Demer, L. L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 79, 414–422 (2011).
Villa-Bellosta, R. & Sorribas, V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler. Thromb. Vasc. Biol. 29, 761–766 (2009).
Ewence, A. E. et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 103, e28–e34 (2008).
Li, X., Yang, H. Y. & Giachelli, C. M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 98, 905–912 (2006).
Nancollas, G. H., LoRe, M., Perez, L., Richardson, C. & Zawacki, S. J. Mineral phases of calcium phosphate. Anat. Rec. 224, 234–241 (1989).
Pak, C. Y. C., Eanes, E. D. & Ruskin, B. Spontaneous precipitation of brushite in urine: evidence that brushite Is the nidus of renal stones originating as calcium phosphate. Proc. Natl Acad. Sci. USA 68, 1456–1460 (1971).
Heiss, A., Pipich, V., Jahnen-Dechent, W. & Schwahn, D. Fetuin-A is a mineral carrier protein: small angle neutron scattering provides new insight on fetuin-a controlled calcification inhibition. Biophys. J. 99, 3986–3995 (2010).
Rochette, C. N. et al. A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-A and calcium phosphate: a time-resolved small-angle X-ray study. Chembiochem. 10, 735–740 (2009).
Heiss, A., Jahnen-Dechent, W., Endo, H. & Schwahn, D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2, 16–20 (2007).
Heiss, A. et al. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J. Biol. Chem. 278, 13333–13341 (2003).
Schafer, C. et al. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 112, 357–366 (2003).
Khoshniat, S. et al. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone 48, 894–902 (2010).
Herrmann, M. et al. Clearance of fetuin-A--containing calciprotein particles is mediated by scavenger receptor-A. Circ. Res. 111, 575–84 (2012).
Hamano, T. et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J. Am. Soc. Nephrol. 21, 1998–2007 (2010).
Matsui, I. et al. Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int. 75, 915–928 (2009).
Smith, E. R. et al. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol. Dial. Transplant. 27, 1957–1966 (2012).
Block, G. A. et al. Effects of phosphate binders in moderate CKD. J. Am. Soc. Nephrol. 23, 1407–1415 (2012).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
Mackay, E. M. & Oliver, J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J. Exp. Med. 61, 319–334 (1935).
Ori, Y. et al. Acute phosphate nephropathy-an emerging threat. Am. J. Med. Sci. 336, 309–314 (2008).
Haut, L. L., Alfrey, A. C., Guggenheim, S., Buddington, B. & Schrier, N. Renal toxicity of phosphate in rats. Kidney Int. 17, 722–731 (1980).
Bank, N., Su, W. S. & Aynedjian, H. S. A micropuncture study of renal phosphate transport in rats with chronic renal failure and secondary hyperparathyroidism. J. Clin. Invest. 61, 884–894 (1978).
Lau, K. Phosphate excess and progressive renal failure: the precipitation-calcification hypothesis. Kidney Int. 36, 918–937 (1989).
Aihara, K., Byer, K. J. & Khan, S. R. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 64, 1283–1291 (2003).
Miller, P. D. The kidney and bisphosphonates. Bone 49, 77–81 (2011).
Francis, M. D., Russell, R. G. & Fleisch, H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science 165, 1264–1266 (1969).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Zhou, X. J. et al. The aging kidney. Kidney Int. 74, 710–720 (2008).
Ibrahim, H. N. et al. Long-term consequences of kidney donation. N. Engl. J. Med. 360, 459–469 (2009).
Tan, J. C. et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 78, 686–692 (2010).
Young, A. et al. Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am. J. Kidney Dis. 59, 761–769 (2011).
Robertson, W. G. & Morgan, D. B. The distribution of urinary calcium excretions in normal persons and stone-formers. Clin. Chim. Acta 37, 503–508 (1972).
Gallagher, J. C. et al. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J. Clin. Invest. 64, 729–736 (1979).
Cebotaru, V. et al. High citrate diet delays progression of renal insufficiency in the ClC-5 knockout mouse model of Dent's disease. Kidney Int. 68, 642–652 (2005).
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Vervloet, M. G. et al. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol. 6, 383–389 (2010).
Antoniucci, D. M., Yamashita, T. & Portale, A. A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J. Clin. Endocrinol. Metab. 91, 3144–3149 (2006).
Ito, N. et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J. Bone Miner. Metab. 25, 419–422 (2007).
Gutierrez, O. M., Wolf, M. & Taylor, E. N. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin. J. Am. Soc. Nephrol. 6, 2871–2878 (2011).
Larsson, T., Nisbeth, U., Ljunggren, O., Juppner, H. & Jonsson, K. B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 64, 2272–2279 (2003).
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Nelson, R. G. & Tuttle, K. R. The new KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and CKD. Blood Purif. 25, 112–114 (2007).
Togao, O. et al. Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology 255, 772–780 (2010).
Doi, S. et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 286, 8655–8665 (2011).
Gutierrez, O. M. & Wolf, M. Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin. Dial. 23, 401–406 (2010).
Moe, S. M. et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 257–264 (2011).
Bell, R. R., Draper, H. H., Tzeng, D. Y., Shin, H. K. & Schmidt, G. R. Physiological responses of human adults to foods containing phosphate additives. J. Nutr. 107, 42–50 (1977).
Acknowledgements
The author's work is supported in part by NIH (R01 AG019712 and DK091392).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
M. Kuro-o has received honararia from Chugai Pharmaceutical and has received research funding from Genzyme.
Rights and permissions
About this article
Cite this article
Kuro-o, M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9, 650–660 (2013). https://doi.org/10.1038/nrneph.2013.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.111
This article is cited by
-
Machine learning based biomarker discovery for chronic kidney disease–mineral and bone disorder (CKD-MBD)
BMC Medical Informatics and Decision Making (2024)
-
Assessment of a serum calcification propensity test for the prediction of all-cause mortality among hemodialysis patients
BMC Nephrology (2023)
-
Targeting aging with the healthy skeletal system: The endocrine role of bone
Reviews in Endocrine and Metabolic Disorders (2023)
-
The association of normal-range serum phosphorus with immunoglobulin A nephropathy progression: a retrospective cohort study
International Urology and Nephrology (2023)
-
Association of circulating calciprotein particle levels with skeletal muscle mass and strength in middle-aged and older adults
Hypertension Research (2022)