Abstract
The bone-derived hormone fibroblast growth factor 23 (FGF23) functions in concert with parathyroid hormone (PTH) and the active vitamin D metabolite, 1,25(OH)2 vitamin D (1,25D), to control phosphate and calcium homeostasis. A rise in circulating levels of phosphate and 1,25D leads to FGF23 production in bone. Circulating FGF23 acts on the kidney by binding to FGF receptors and the co-receptor α-Klotho to promote phosphaturia and reduce circulating 1,25D levels. Various other biomolecules that are produced by the kidney, including lipocalin-2, glycerol 3-phosphate, 1-acyl lysophosphatidic acid and erythropoietin, are involved in the regulation of mineral metabolism via effects on FGF23 synthesis in bone. Understanding of the molecular mechanisms that control FGF23 synthesis in the bone and its bioactivity in the kidney has led to the identification of potential targets for novel interventions. Emerging approaches to target aberrant phosphate metabolism include small molecule inhibitors that directly bind FGF23 and prevent its interactions with FGF receptors and α-Klotho, FGF23 peptide fragments that act as competitive inhibitors of intact FGF23 and small molecule inhibitors of kidney sodium-phosphate cotransporters.
Key points
-
The osteocyte-derived hormone fibroblast growth factor 23 (FGF23) controls renal handling of phosphate and active 1,25(OH)2 vitamin D(1,25D).
-
Rare heritable and common acquired disturbances in FGF23 homeostasis, including chronic kidney disease, are associated with altered mineral balance.
-
The regulation of FGF23 production in osteocytes occurs via transcriptional and post-translational mechanisms.
-
In the kidney, binding of FGF23 to its co-receptor α-Klotho and fibroblast growth factor receptors results in a reduction in blood phosphate and inhibits the production of 1,25D.
-
The kidney-derived factors glycerol-3-phophate/1-acyl lysophosphatidic acid, lipocalin-2 and erythropoietin stimulate bone FGF23 synthesis.
-
Identification of the mechanisms of FGF23 functions and the effects of FGF23 on downstream targets in the kidney could lead to the development of novel therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Consortium, A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Isakova, T. et al. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 6, 2688–2695 (2011).
Perwad, F. et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146, 5358–5364 (2005).
Hu, M. C. et al. Recombinant alpha-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int. 91, 1104–1114 (2017).
Noonan, M. L. et al. Erythropoietin and a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHDi) lowers FGF23 in a model of chronic kidney disease (CKD). Physiol. Rep. 8, e14434 (2020).
Noonan, M. L. et al. The HIF-PHI BAY 85-3934 (Molidustat) improves anemia and is associated with reduced levels of circulating FGF23 in a CKD mouse model. J. Bone Min. Res. 36, 1117–1130 (2021).
David, V. et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 89, 135–146 (2016).
Clinkenbeard, E. L. et al. Conditional deletion of MurineFgf23: interruption of the normal skeletal responses to phosphate challenge and rescue of genetic hypophosphatemia. J. Bone Miner. Res. 31, 1247–1257 (2016).
Clinkenbeard, E. L. et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 102, e427–e430 (2017).
Goetz, R. & Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14, 166–180 (2013).
Itoh, N., Nakayama, Y. & Konishi, M. Roles of FGFs as paracrine or endocrine signals in liver development, health, and disease. Front. Cell Dev. Biol. 4, 30 (2016).
Lindberg, K. et al. The kidney is the principal organ mediating Klotho effects. J. Am. Soc. Nephrol. 25, 2169–2175 (2014).
Beck-Nielsen, S. S. et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J. Rare Dis. 14, 58 (2019).
de Las Rivas, M. et al. Molecular basis for fibroblast growth factor 23 O-glycosylation by GalNAc-T3. Nat. Chem. Biol. 16, 351–360 (2020).
Tagliabracci, V. S. et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. USA 111, 5520–5525 (2014).
Farrow, E. G., Imel, E. A. & White, K. E. Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and αKlotho). Best. Pract. Res. Clin. Rheumatol. 25, 735–747 (2011).
White, K. E. et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J. Clin. Endocrinol. Metab. 86, 497–500 (2001).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Gattineni, J. & Baum, M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr. Nephrol. 25, 591–601 (2010).
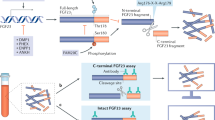
Goetz, R. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl Acad. Sci. USA 107, 407–412 (2010).
Lim, K. et al. α-Klotho expression in human tissues. J. Clin. Endocrinol. Metab. 100, E1308–E1318 (2015).
Kuro, O. M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15, 27–44 (2019).
Kuro-o, M. et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Nakatani, T. et al. In vivo genetic evidence for Klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 23, 433–441 (2009).
Olauson, H. et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 23, 1641–1651 (2012).
Takeshita, A., Kawakami, K., Furushima, K., Miyajima, M. & Sakaguchi, K. Central role of the proximal tubular αKlotho/FGF receptor complex in FGF23-regulated phosphate and vitamin D metabolism. Sci. Rep. 8, 6917 (2018).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006).
Portale, A. A. et al. Characterization of FGF23-dependent Egr-1 cistrome in the mouse renal proximal tubule. PLoS One 10, e0142924 (2015).
Andrukhova, O. et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 33, 229–246 (2014).
Andrukhova, O. et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 6, 744–759 (2014).
Knothe Tate, M. L., Niederer, P. & Knothe, U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22, 107–117 (1998).
Saito, H. et al. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005).
Bon, N. et al. Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol. Metab. 11, 197–204 (2018).
Takashi, Y. et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc. Natl Acad. Sci. USA 116, 11418–11427 (2019).
Takashi, Y. & Fukumoto, S. Phosphate-sensing and regulatory mechanism of FGF23 production. J. Endocrinol. Invest. https://doi.org/10.1007/s40618-020-01205-9 (2020).
Takashi, Y. et al. Skeletal FGFR1 signaling is necessary for regulation of serum phosphate level by FGF23 and normal life span. Biochem. Biophys. Rep. 27, 101107 (2021).
Xiao, Z. et al. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One 9, e104154 (2014).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
Akiyama, K. I. et al. Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. Kidney Int. 97, 702–712 (2020).
Akiyama, K., Kimura, T. & Shiizaki, K. Biological and clinical effects of calciprotein particles on chronic kidney disease-mineral and bone disorder. Int. J. Endocrinol. 2018, 5282389 (2018).
Nguyen-Yamamoto, L., Karaplis, A. C., St-Arnaud, R. & Goltzman, D. Fibroblast growth factor 23 regulation by systemic and local osteoblast-synthesized 1,25-dihydroxyvitamin D. J. Am. Soc. Nephrol. 28, 586–597 (2017).
Saini, R. K. et al. 1,25-dihydroxyvitamin D(3) regulation of fibroblast growth factor-23 expression in bone cells: evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif. Tissue Int. 92, 339–353 (2013).
Ito, M. et al. Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am. J. Physiol. Endocrinol. Metab. 288, E1101–E1109 (2005).
Dorr, K. et al. Randomized trial of etelcalcetide for cardiac hypertrophy in hemodialysis. Circ. Res. 128, 1616–1625 (2021).
Qin, L., Raggatt, L. J. & Partridge, N. C. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol. Metab. 15, 60–65 (2004).
Hou, Y. C., Lu, C. L. & Lu, K. C. Mineral bone disorders in chronic kidney disease. Nephrology 23, 88–94 (2018).
Murray, T. M., Rao, L. G., Divieti, P. & Bringhurst, F. R. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr. Rev. 26, 78–113 (2005).
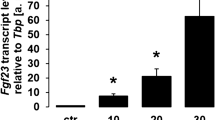
Kawata, T. et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 18, 2683–2688 (2007).
Prideaux, M. et al. Generation of two multipotent mesenchymal progenitor cell lines capable of osteogenic, mature osteocyte, adipogenic, and chondrogenic differentiation. Sci. Rep. 11, 22593 (2021).
Ito, N. et al. Sclerostin directly stimulates osteocyte synthesis of fibroblast growth factor-23. Calcif. Tissue Int. 109, 66–76 (2021).
Knab, V. M. et al. Acute parathyroid hormone injection increases C-terminal but not intact fibroblast growth factor 23 levels. Endocrinology 158, 1130–1139 (2017).
Simic, P. et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J. Clin. Invest. 130, 1513–1526 (2020).
Flo, T. H. et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004).
Qiu, S., Chen, X., Pang, Y. & Zhang, Z. Lipocalin-2 protects against renal ischemia/reperfusion injury in mice through autophagy activation mediated by HIF1α and NF-κb crosstalk. Biomed. Pharmacother. 108, 244–253 (2018).
Viau, A. et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Invest. 120, 4065–4076 (2010).
Courbon, G. et al. Lipocalin 2 stimulates bone fibroblast growth factor 23 production in chronic kidney disease. Bone Res. 9, 35 (2021).
Hanudel, M. R. et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol. Dial. Transpl. 34, 2057–2065 (2019).
Daryadel, A. et al. Elevated FGF23 and disordered renal mineral handling with reduced bone mineralization in chronically erythropoietin over-expressing transgenic mice. Sci. Rep. 9, 14989 (2019).
Daryadel, A. et al. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch. 470, 1569–1582 (2018).
Wolf, M. & White, K. E. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr. Opin. Nephrol. Hypertens. 23, 411–419 (2014).
Ichikawa, S. et al. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 150, 2543–2550 (2009).
Kinoshita, Y. & Fukumoto, S. X-Linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr. Rev. 39, 274–291 (2018).
Xiao, Z. et al. Novel small molecule fibroblast growth factor 23 inhibitors increase serum phosphate and improve skeletal abnormalities in Hyp mice. Mol. Pharmacol. 101, 408–421 (2021).
Suzuki, Y. et al. FGF23 contains two distinct high-affinity binding sites enabling bivalent interactions with α-Klotho. Proc. Natl Acad. Sci. USA 117, 31800–31807 (2020).
Agrawal, A., Ni, P., Agoro, R., White, K. E. & DiMarchi, R. D. Identification of a second Klotho interaction site in the C terminus of FGF23. Cell Rep. 34, 108665 (2021).
Agrawal, A. et al. Molecular elements in FGF19 and FGF21 defining KLB/FGFR activity and specificity. Mol. Metab. 13, 45–55 (2018).
Lee, S. et al. Structures of β-Klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505 (2018).
Agoro, R. et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 32, 3752–3764 (2018).
Agoro, R. et al. C-FGF23 peptide alleviates hypoferremia during acute inflammation. Haematologica 106, 391–403 (2021).
Thomas, L. et al. PF-06869206 is a selective inhibitor of renal Pi transport: evidence from in vitro and in vivo studies. Am. J. Physiol. Renal Physiol. 319, F541–F551 (2020).
Clerin, V. et al. Selective pharmacological inhibition of the sodium-dependent phosphate cotransporter NPT2a promotes phosphate excretion. J. Clin. Invest. 130, 6510–6522 (2020).
Lamb, Y. N. Burosumab: first global approval. Drugs 78, 707–714 (2018).
Carpenter, T. O. et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 124, 1587–1597 (2014).
Acknowledgements
The authors acknowledge NIH grants R21-AR059278, R01-DK112958, and R01-HL145528 (K.E.W); The David Weaver Professorship (K.E.W); and NIDDK-K99/R00 DK129705 (R.A.).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.E.W. receives royalties for licensing FGF23 to Kyowa Hakko Kirin Co., Ltd., has previously received funding from Akebia and currently receives funding from Calico Labs. K.E.W. also owns equity interest in FGF Therapeutics. R.A. has no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agoro, R., White, K.E. Regulation of FGF23 production and phosphate metabolism by bone–kidney interactions. Nat Rev Nephrol 19, 185–193 (2023). https://doi.org/10.1038/s41581-022-00665-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00665-x
This article is cited by
-
A prospective study of the association between serum klotho and mortality among adults with rheumatoid arthritis in the USA
Arthritis Research & Therapy (2023)
-
Bone Material Properties in Bone Diseases Affecting Children
Current Osteoporosis Reports (2023)
-
Regulation of the Osteocyte Secretome with Aging and Disease
Calcified Tissue International (2023)