Key Points
-
Elevated levels of LDL, remnant lipoproteins, and lipoprotein(a) all cause cardiovascular disease, whereas elevated levels of triglyceride-rich lipoproteins can cause acute pancreatitis in some individuals
-
Small molecule and antibody-based drugs such as statins, ezetimibe, and PCSK9 inhibitors efficiently reduce LDL-cholesterol levels and lower the risk of cardiovascular disease
-
In addition, these drugs can modestly reduce remnant lipoproteins, triglyceride-rich lipoproteins, and/or lipoprotein(a) levels
-
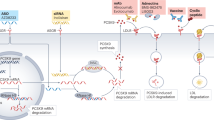
These lipoproteins can be reduced using novel gene-silencing approaches (antisense oligonucleotide inhibition and small interfering RNA technology) by targeting proteins that have an important role in lipoprotein production or removal
-
Using gene-silencing approaches, plasma levels of LDL, remnant lipoproteins, lipoprotein(a), and triglyceride-rich lipoproteins can be reduced by 50–90% when treatment is administered 2–12 times yearly
-
Evidence of long-term safety and cardiovascular disease reduction is needed before the widespread use of gene-silencing therapies; however, these drugs might have a role in patients with rare diseases
Abstract
New treatment opportunities are emerging in the field of lipid-lowering therapy through gene-silencing approaches. Both antisense oligonucleotide inhibition and small interfering RNA technology aim to degrade gene mRNA transcripts to reduce protein production and plasma lipoprotein levels. Elevated levels of LDL, remnant lipoproteins, and lipoprotein(a) all cause cardiovascular disease, whereas elevated levels of triglyceride-rich lipoproteins in some patients can cause acute pancreatitis. The levels of each of these lipoproteins can be reduced using gene-silencing therapies by targeting proteins that have an important role in lipoprotein production or removal (for example, the protein products of ANGPTL3, APOB, APOC3, LPA, and PCSK9). Using this technology, plasma levels of these lipoproteins can be reduced by 50–90% with 2–12 injections per year; such dramatic reductions are likely to reduce the incidence of cardiovascular disease or acute pancreatitis in at-risk patients. The reported adverse effects of these new therapies include injection-site reactions, flu-like symptoms, and low blood platelet counts. However, newer-generation drugs are more efficiently delivered to liver cells, requiring lower drug doses, which leads to fewer adverse effects. Although these findings are promising, robust evidence of cardiovascular disease reduction and long-term safety is needed before these gene-silencing technologies can have widespread implementation. Before the availability of such evidence, these drugs might have roles in patients with unmet medical needs through orphan indications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1151–1210 (2017).
Baigent, C. et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 (2005).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Baigent, C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Ridker, P. M. et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N. Engl. J. Med. 376, 1527–1539 (2017).
Ginsberg, H. N. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
The HPS3/TIMI55–REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med. 377, 1217–1227 (2017).
Ridker, P. M. et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Brunzell, J. D. & Deeb, S. S. in The Metabolic & Molecular Bases of Inherited Disease (eds Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D.) 2789–2816 (McGraw-Hill, New York, 2001).
Sullenger, B. A. & Nair, S. From the RNA world to the clinic. Science 352, 1417–1420 (2016).
McClorey, G. & Wood, M. J. An overview of the clinical application of antisense oligonucleotides for RNA-targeting therapies. Curr. Opin. Pharmacol. 24, 52–58 (2015).
Geyer, P. E., Holdt, L. M., Teupser, D. & Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol. 13, 942 (2017).
Nordestgaard, B. G. et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints — a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 62, 930–946 (2016).
Havel, J. H. & Kane, J. P. in The Metabolic & Molecular Bases of Inherited Disease (eds Scriver C. R., Beaudet, A. L., Sly, W. S. & Valle, D.) 2705–2716 (McGraw-Hill, New York, 2001).
Nordestgaard, B. G. A. Test in Context: Lipid Profile, Fasting Versus Nonfasting. J. Am. Coll. Cardiol. 70, 1637–1646 (2017).
Nordestgaard, B. G. & Varbo, A. Triglycerides and cardiovascular disease. Lancet 384, 626–635 (2014).
Nordestgaard, B. G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ. Res. 118, 547–563 (2016).
Pedersen, S. B., Langsted, A. & Nordestgaard, B. G. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern. Med. 176, 1834–1842 (2016).
Nordestgaard, B. G., Benn, M., Schnohr, P. & Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298, 299–308 (2007).
Varbo, A. et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 61, 427–436 (2013).
Varbo, A., Benn, M., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 128, 1298–1309 (2013).
Jorgensen, A. B. et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 34, 1826–1833 (2013).
Varbo, A., Freiberg, J. J. & Nordestgaard, B. G. Extreme nonfasting remnant cholesterol versus extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin. Chem. 61, 533–543 (2015).
Varbo, A. et al. Remnant cholesterol, low-density lipoprotein cholesterol, and blood pressure as mediators from obesity to ischemic heart disease. Circ. Res. 116, 665–673 (2015).
Helgadottir, A. et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat. Genet. 48, 634–639 (2016).
Wurtz, P. et al. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J. Am. Coll. Cardiol. 67, 1200–1210 (2016).
Jepsen, A. M. et al. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin. Chem. 62, 593–604 (2016).
Khera, A. V. et al. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA 317, 937–946 (2017).
Varbo, A., Freiberg, J. J. & Nordestgaard, B. G. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen General Population Study. Clin. Chem. 64, 219–230 (2018).
Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15, 1512–1531 (1995).
Stary, H. C. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 20, 1177–1178 (2000).
Brown, R. A., Shantsila, E., Varma, C. & Lip, G. Y. Current understanding of atherogenesis. Am. J. Med. 130, 268–282 (2017).
Gronholdt, M. L. et al. Macrophages are associated with lipid-rich carotid artery plaques, echolucency on B-mode imaging, and elevated plasma lipid levels. J. Vasc. Surg. 35, 137–145 (2002).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Batt, K. V. et al. Differential effects of low-density lipoprotein and chylomicron remnants on lipid accumulation in human macrophages. Exp. Biol. Med. 229, 528–537 (2004).
Takahashi, S. et al. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J. Atheroscler. Thromb. 11, 200–208 (2004).
Nordestgaard, B. G., Stender, S. & Kjeldsen, K. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 8, 421–428 (1988).
Nordestgaard, B. G. & Zilversmit, D. B. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol-fed rabbits. J. Lipid Res. 29, 1491–1500 (1988).
Nordestgaard, B. G., Tybjaerg-Hansen, A. & Lewis, B. Influx in vivo of low density, intermediate density, and very low density lipoproteins into aortic intimas of genetically hyperlipidemic rabbits. Roles of plasma concentrations, extent of aortic lesion, and lipoprotein particle size as determinants. Arterioscler. Thromb. 12, 6–18 (1992).
Shaikh, M. et al. Quantitative studies of transfer in vivo of low density, Sf 12–60, and Sf 60–400 lipoproteins between plasma and arterial intima in humans. Arterioscler. Thromb 11, 569–577 (1991).
Nordestgaard, B. G., Wootton, R. & Lewis, B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 15, 534–542 (1995).
Nielsen, L. B., Nordestgaard, B. G., Stender, S., Niendorf, A. & Kjeldsen, K. Transfer of lipoprotein(a) and LDL into aortic intima in normal and in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 15, 1492–1502 (1995).
Nielsen, L. B., Gronholdt, M. L., Schroeder, T. V., Stender, S. & Nordestgaard, B. G. In vivo transfer of lipoprotein(a) into human atherosclerotic carotid arterial intima. Arterioscler. Thromb. Vasc. Biol. 17, 905–911 (1997).
Nordestgaard, B. G. & Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J. Lipid Res. 57, 1953–1975 (2016).
Boffa, M. B. & Koschinsky, M. L. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J. Lipid Res. 57, 745–757 (2016).
Nielsen, L. B., Stender, S., Kjeldsen, K. & Nordestgaard, B. G. Specific accumulation of lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circ. Res. 78, 615–626 (1996).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2472 (2017).
Chapman, M. J. et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32, 1345–1361 (2011).
Hegele, R. A. et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2, 655–666 (2014).
Nordestgaard, B. G. et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur. Heart J. 31, 2844–2853 (2010).
Kronenberg, F. & Utermann, G. Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273, 6–30 (2013).
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Berglund, L. et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 2969–2989 (2012).
Miller, M. et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011).
Catapano, A. L. et al. 2016 ESC/EAS guidelines for the Management of Dyslipidaemias. Eur. Heart J. 37, 2999–3058 (2016).
Piepoli, M. F. et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
Lindkvist, B., Appelros, S., Regner, S. & Manjer, J. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology 12, 317–324 (2012).
Murphy, M. J., Sheng, X., MacDonald, T. M. & Wei, L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern. Med. 173, 162–164 (2013).
Havel, R. J. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv. Intern. Med. 15, 117–154 (1969).
Saharia, P., Margolis, S., Zuidema, G. D. & Cameron, J. L. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery 82, 60–67 (1977).
Nordstoga, K., Sorby, R., Olivecrona, G., Smith, A. J. & Christophersen, B. Pancreatitis in hyperlipemic mink (Mustela vison). Vet. Pathol. 49, 557–561 (2012).
Crick, F. Central dogma of molecular biology. Nature 227, 561–563 (1970).
Bennett, C. F. & Swayze, E. E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 50, 259–293 (2010).
Burnett, J. C. & Rossi, J. J. RNA-based therapeutics: current progress and future prospects. Chem. Biol. 19, 60–71 (2012).
Watts, J. K. & Corey, D. R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 226, 365–379 (2012).
Huang, Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol. Ther. Nucleic Acids 6, 116–132 (2017).
Prakash, T. P. et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 42, 8796–8807 (2014).
Nair, J. K. et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958–16961 (2014).
Gaudet, D. & Brisson, D. Gene-based therapies in lipidology: current status and future challenges. Curr. Opin. Lipidol. 26, 553–565 (2015).
Wierzbicki, A. S. & Viljoen, A. Anti-sense oligonucleotide therapies for the treatment of hyperlipidaemia. Expert. Opin. Biol. Ther. 16, 1125–1134 (2016).
Lee, R. G., Crosby, J., Baker, B. F., Graham, M. J. & Crooke, R. M. Antisense technology: an emerging platform for cardiovascular disease therapeutics. J. Cardiovasc. Transl Res. 6, 969–980 (2013).
Reyes-Soffer, G. et al. Complex effects of inhibiting hepatic apolipoprotein B100 synthesis in humans. Sci. Transl Med. 8, 323ra12 (2016).
Panta, R., Dahal, K. & Kunwar, S. Efficacy and safety of mipomersen in treatment of dyslipidemia: a meta-analysis of randomized controlled trials. J. Clin. Lipidol. 9, 217–225 (2015).
Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010).
Duell, P. B. et al. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J. Clin. Lipidol. 10, 1011–1021 (2016).
Hashemi, N. et al. Liver histology during Mipomersen therapy for severe hypercholesterolemia. J. Clin. Lipidol. 8, 606–611 (2014).
Cuchel, M. et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 35, 2146–2157 (2014).
Wiegman, A. et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur. Heart J. 36, 2425–2437 (2015).
Jorgensen, A. B., Frikke-Schmidt, R., Nordestgaard, B. G. & Tybjaerg-Hansen, A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371, 32–41 (2014).
Crosby, J. et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371, 22–31 (2014).
Graham, M. J. et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res. 112, 1479–1490 (2013).
Gaudet, D. et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 373, 438–447 (2015).
Gaudet, D. et al. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 371, 2200–2206 (2014).
Digenio, A. et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care 39, 1408–1415 (2016).
Crooke, S. T. et al. The effects of 2′-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 27, 121–129 (2017).
Dewey, F. E. et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 377, 211–221 (2017).
Graham, M. J. et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 377, 222–232 (2017).
Gaudet, D. et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 377, 296–297 (2017).
Kamstrup, P. R., Tybjaerg-Hansen, A., Steffensen, R. & Nordestgaard, B. G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 301, 2331–2339 (2009).
Clarke, R. et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361, 2518–2528 (2009).
Thanassoulis, G. et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368, 503–512 (2013).
Kamstrup, P. R., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 63, 470–477 (2014).
Graham, M. J., Viney, N., Crooke, R. M. & Tsimikas, S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J. Lipid Res. 57, 340–351 (2016).
Tsimikas, S. et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386, 1472–1483 (2015).
Viney, N. J. et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 388, 2239–2253 (2016).
Mora, S. et al. Lipoprotein(a) and risk of type 2 diabetes. Clin. Chem. 56, 1252–1260 (2010).
Kamstrup, P. R. & Nordestgaard, B. G. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 1, 220–227 (2013).
Ding, L. et al. Serum lipoprotein (a) concentrations are inversely associated with T2D, prediabetes, and insulin resistance in a middle-aged and elderly Chinese population. J. Lipid Res. 56, 920–926 (2015).
Ye, Z. et al. The association between circulating lipoprotein(a) and type 2 diabetes: is it causal? Diabetes 63, 332–342 (2014).
Tolbus, A. et al. Kringle IV type 2, not low lipoprotein(a), as a cause of diabetes: a novel genetic approach using SNPs associated selectively with lipoprotein(a) concentrations or with Kringle IV type 2 repeats. Clin. Chem. 63, 1866–1876 (2017).
Langsted, A., Kamstrup, P. R. & Nordestgaard, B. G. High lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: a Mendelian randomization study. Clin. Chem. 63, 1714–1723 (2017).
Ishikawa, S. et al. Inverse association between serum lipoprotein(a) and cerebral hemorrhage in the Japanese population. Thromb. Res. 131, e54–e58 (2013).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Cohen, J. C., Boerwinkle, E., Mosley, T. H. Jr & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Benn, M. & Nordestgaard, B. G., Grande, P., Schnohr, P. & Tybjaerg-Hansen, A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 55, 2833–2842 (2010).
Nicholls, S. J. et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 316, 2373–2384 (2016).
Stein, E. A. et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366, 1108–1118 (2012).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
Jackson, A. L. et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 12, 1197–1205 (2006).
Garber, K. Alnylam terminates revusiran program, stock plunges. Nat. Biotechnol. 34, 1213–1214 (2016).
Mullard, A. RNAi hits another rut. Nat. Rev. Drug Discov. 15, 738 (2016).
Author information
Authors and Affiliations
Contributions
All the authors contributed to researching data for the article, discussion of its content, writing the article, and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.G.N. is a consultant for Amgen, AstraZeneca, Denka Seiken, Dezima Pharma, Ionis Pharmaceuticals, Kowa, Merck, Regeneron Pharmaceuticals, and Sanofi. S.J.N. has received research funding from Amgen, Anthera Pharmaceuticals, AstraZeneca, Cerenis Therapeutics, Eli Lilly, InfraReDx, LipoScience, Novartis, Regeneron Pharmaceuticals, Resverlogix, Roche, Sanofi, and The Medicines Company, and is a consultant for Anthera Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion Therapeutics, Merck, Omthera Pharmaceuticals, Regeneron Pharmaceuticals, Resverlogix, Sanofi, and Takeda. K.K.R. has received research funding from Amgen, Merck, Pfizer, Regeneron, and Sanofi, and is a consultant for AbbVie, Algorithm, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis Therapeutics, Cipla, Eli Lilly, Esperion, Ionis Pharmaceuticals, Kowa, Merck, Novo Nordisk, Pfizer, Regeneron, Resverlogix, Sanofi, Takeda, and The Medicines Company. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Nordestgaard, B., Nicholls, S., Langsted, A. et al. Advances in lipid-lowering therapy through gene-silencing technologies. Nat Rev Cardiol 15, 261–272 (2018). https://doi.org/10.1038/nrcardio.2018.3
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2018.3
This article is cited by
-
Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: a population-based prospective cohort study
Diabetologia (2023)
-
A method for lipoprotein (a) Isolation from a small volume of plasma with applications for clinical research
Scientific Reports (2022)
-
A Tale of Two New Targets for Hypertriglyceridaemia: Which Choice of Therapy?
BioDrugs (2022)
-
A pathophysiological compass to personalize antianginal drug treatment
Nature Reviews Cardiology (2021)
-
The Role of RNA-Targeted Therapeutics to Reduce ASCVD Risk: What Have We Learned Recently?
Current Atherosclerosis Reports (2021)