Abstract
We herein investigated the role of the STAT signaling cascade in the production of pro-inflammatory cytokines and cisplatin ototoxicity. A significant hearing impairment caused by cisplatin injection was observed in Balb/c (wild type, WT) and STAT4−/−, but not in STAT6−/− mice. Moreover, the expression levels of the protein and mRNA of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, were markedly increased in the serum and cochlea of WT and STAT4−/−, but not STAT6−/− mice. Organotypic culture revealed that the shape of stereocilia bundles and arrays of sensory hair cell layers in the organ of Corti from STAT6−/− mice were intact after treatment with cisplatin, whereas those from WT and STAT4−/− mice were highly distorted and disarrayed after the treatment. Cisplatin induced the phosphorylation of STAT6 in HEI-OC1 auditory cells, and the knockdown of STAT6 by STAT6-specific siRNA significantly protected HEI-OC1 auditory cells from cisplatin-induced cell death and inhibited pro-inflammatory cytokine production. We further demonstrated that IL-4 and IL-13 induced by cisplatin modulated the phosphorylation of STAT6 by binding with IL-4 receptor alpha and IL-13Rα1. These findings suggest that STAT6 signaling plays a pivotal role in cisplatin-mediated pro-inflammatory cytokine production and ototoxicity.
Similar content being viewed by others
Introduction
Cisplatin is a chemotherapeutic agent that is used extensively for the treatment of a broad spectrum of tumors. However, progressive irreversible side effects of cisplatin, including nephrotoxicity and ototoxicity, greatly impair the patient's quality of life and frequently result in the need to lower the dosage during treatment or to discontinue the treatment. Cisplatin ototoxicity primarily occurs in the cochlea, and generally consists of apoptosis-induced damage to the outer hair cells (OHCs), spiral ganglion cells and the marginal cells of the stria vascularis 1, 2. Multiple studies have demonstrated that the inner ear has the capacity to generate an active immune response 3, thereby resulting in hearing loss in some individuals 4. Yoshida et al. reported that inflammatory response mediators, such as IL-6, TNF-α, MCP-1, KC, MIP-2, sICAM-1 and VEGF, were produced by spiral ligament fibrocytes in response to stimulation with pro-inflammatory cytokines such as IL-1β and TNF-α 5. Consequently, inflammatory response mediators induce the infiltration of inflammatory cells, which may prolong the inflammatory response and lead to fibrocyte damage and cochlear malfunction. In a previous study, we also demonstrated that pro-inflammatory cytokines play a critical role in cisplatin-induced cochlear injury 6. TNF-α plays an important role in this process because inhibition of the action of TNF-α significantly attenuates the expression of other pro-inflammatory cytokines following cisplatin injection. Furthermore, we demonstrated that cisplatin-induced pro-inflammatory cytokine production and ototoxicity involved the up-regulation of NF-κB signaling 1, 6.
STAT proteins are transcription factors that provide a direct link between the cytokine receptors and cytokine-induced gene transcription. STAT4 and STAT6 are essential in mediating responses to IL-12 and IL-4, respectively. Consequently, mice deficient in STAT6 have impaired Th2 cytokine production and Th2-type responses, whereas STAT4 deficiency results in the lack of IL-12-induced IFN-γ production and Th1 differentiation. STAT activation stimulates development, proliferation, differentiation, cell migration and apoptotic cell death depending on the type of stimuli and cells. Additionally, close cross-talk between the STAT and NF-κB pathways has been reported, and production of several inflammatory mediators, such as TNF-α and IL-1β, is known to be regulated by the STAT pathway 7, 8. Thus, in the present study, we examined the role of the STAT pathway in the generation of pro-inflammatory cytokines and cisplatin ototoxicity by using STAT gene knockout (KO) mice in vivo and HEI-OC1 auditory cells in vitro. Our results indicate that STAT6 signaling is required for the production of pro-inflammatory cytokines and ototoxicity in response to cisplatin, and that IL-4 and IL-13 function as additional effector cytokines for cisplatin ototoxicity via STAT6, which is independent of the NF-κB pathway.
Results
Cisplatin-induced hearing loss and production of pro-inflammatory cytokines were markedly suppressed in STAT6−/− mice
We previously demonstrated that pro-inflammatory cytokines, including TNF-α and IL-1β, play a critical role in cisplatin-induced cochlear injury 6. Production of inflammatory mediators, including TNF-α and IL-1β, is known to be regulated by the STAT pathway 7, 8. To examine the involvement of STAT signaling in the generation of pro-inflammatory cytokines and cisplatin ototoxicity, wild type (WT), STAT4−/−, and STAT6−/− mice were given intraperitoneal (i.p.) injections of cisplatin. First, to confirm the identity of STAT4−/− and STAT6−/− mice, we examined the expression levels of STAT proteins by western blot in the cochlea of STAT4−/− or STAT6−/− mice. STAT4−/− or STAT6−/− mice do not express their STAT4 or STAT6 proteins, respectively. However, other STAT proteins were not changed in the cochlea of these mice (Supplementary information, Figure S1). Cisplatin ototoxicity was observed by the auditory brain stem response (ABR) threshold shift of WT and STAT4−/− mice at 4, 8, 16, and 32 kHz (Figure 1). In the phosphate-buffered saline (PBS)-treated control group, there were no significant ABR threshold changes at these frequency ranges. However, the ABR threshold shifts at 16 and 32 kHz were predominant in both WT and STAT4−/− mice. Interestingly, the ABR threshold shift in STAT6−/− mice after cisplatin injection was significantly lowered at 4, 16, and 32 kHz when compared to WT and STAT4−/− mice (Figure 1A). There was no significant difference in the threshold shifts at 4, 8, 16, and 32 kHz between WT and STAT4−/− mice. We next examined the secretion and mRNA expression of pro-inflammatory cytokines in vivo following cisplatin injection to determine if they are modulated in STAT KO mice. As shown in Figure 1B, the serum levels of TNF-α, IL-1β, and IL-6 were significantly increased in response to cisplatin injection in WT and STAT4−/− mice when compared with PBS-injected control mice. However, cisplatin did not increase the serum levels of TNF-α, IL-1β, and IL-6 in STAT6−/− mice. In fact, the serum levels of these cytokines were significantly decreased when compared to those of WT or STAT4−/− mice. The mRNA expression of TNF-α, IL-1β, and IL-6 was markedly increased in the cochlea of WT and STAT4−/− mice after cisplatin injection (Figure 1C). Conversely, the mRNA expression of pro-inflammatory cytokines following cisplatin injection was reduced in the cochlea of STAT6−/− mice. These data suggest that STAT6-mediated signaling may be critically required in the cisplatin-induced production of pro-inflammatory cytokines.
ABR threshold shift and the production of pro-inflammatory cytokines after cisplatin injection were significantly alleviated in STAT6−/− mice. (A) The changes in ABR threshold at 4, 8, 16, and 32 kHz were measured. Representative ABR signals at 16 kHz before and after cisplatin injection are shown in the left column. The data represent the means ± SD of triplicate analyses. *p < 0.05 as determined by one-way ANOVA when compared with each WT group of mice. (B) The animals in all groups were killed under anesthesia on the day following the final cisplatin injection. Prior to sacrifice, blood samples were collected and pro-inflammatory cytokines were then measured by ELISA. *, #P < 0.05, by one-way ANOVA when compared with the WT (*) or STAT4−/− (#) group. (C) Total RNA was isolated from whole cochleas and cDNA was synthesized by reverse transcriptase. TNF-α, IL-1β, IL-6, and β-actin cDNA sequences were amplified using mouse-specific primers.
Cytotoxic effect of cisplatin on sensory hair cells was attenuated in the organ of Corti from STAT6−/− mice
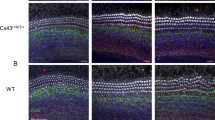
To examine whether cisplatin caused direct damage to the stereocilia on the organ of Corti, the organ of Corti explants were prepared from WT, STAT4−/−, and STAT6−/− mice treated with PBS or cisplatin (4 mg/kg) for 4 consecutive days and then labeled with TRITC-conjugated phalloidin. As shown in Figure 2, treatment with cisplatin markedly destroyed most of the stereocilia bundles of the hair cells and resulted in disarray of the stereocilia of the organ of Corti from WT and STAT4−/− mice. However, loss of stereocilia by cisplatin was nearly undetectable in the organ of Corti explants from STAT6−/− mice treated with cisplatin.
Cisplatin cytotoxicity on hair cells from the organ of Corti was attenuated in STAT6−/− mice. The half middle turn of the organ of Corti explants from cisplatin-injected adult mice (P50) of WT, STAT4−/−, and STAT6−/− experimental groups was isolated. The explants were stained with TRITC-conjugated phalloidin and observed under a fluorescent microscope.
In vivo expression of pro-inflammatory cytokines and NF-κB after cisplatin injection was significantly attenuated in the cochlea of STAT6−/− mice
To investigate the expression profiles of pro-inflammatory cytokines and NF-κB, immunohistochemical analysis of mice injected with cisplatin was performed. TNF-α, IL-1β, and NF-κB p65 expression was broadly observed throughout the stria vascularis, spiral ligament, and spiral ganglion neuron, as well as the sensory hair cell layers in the organ of Corti from cisplatin-injected WT and STAT4−/− mice (Figure 3B, 3D, 3H, 3J, 3T, and 3V), respectively. Conversely, only very slight expression of IL-6 was detected in the spiral limbus, spiral ligament, and spiral modulus of cisplatin-injected WT and STAT4−/− mice (Figure 3N and 3P). It is important to note that, in cisplatin-injected STAT6−/− mice, the protein expression of these three pro-inflammatory cytokines and NF-κB p65 was barely detectable (Figure 3F, 3L, 3R, and 3X). In PBS-control mice from each experimental group, expression of these pro-inflammatory cytokines and NF-κB was also barely detectable. In addition, we examined the apoptotic cell death in the cochlea by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining. Histological sections from the cisplatin-injected WT and STAT4−/− mice exhibited TUNEL-positive cells in the stria vascularis, spiral ligament, spiral limbus, and the organ of Corti (Figure 3β and 3δ). However, TUNEL-positive cells were nearly undetectable in the whole cochlea of STAT6−/− mice (Figure 3ϕ).
In vivo expression of pro-inflammatory cytokines and NF-κB after cisplatin injection and the number of TUNEL-positive cells were significantly alleviated in the cochlea of STAT6−/− mice. Apoptotic cells were identified by TUNEL staining and examination under a fluorescent microscope. The TUNEL-positive nuclei were visualized as green. Counterstaining was conducted with propidium iodide and nuclei were visualized as red. SLig: spiral ligament; SV: stria vascularis; SGN: spiral ganglion neuron; SLim: spiral limbus; OC: organ of Corti.
STAT6, but not STAT4, was required for the secretion of pro-inflammatory cytokines stimulated by cisplatin in HEI-OC1 cells
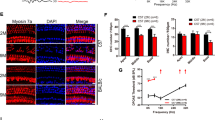
As found that STAT6 is critical for cisplatin-mediated pro-inflammatory cytokine production and ototoxicity in vivo, we next determined whether in vitro exposure of cisplatin to HEI-OC1 auditory cells affected the tyrosine phosphorylation of STAT4 or STAT6. Phosphorylation of STAT4 or STAT6 was analyzed by immunoblot analysis using phosphotyrosine-specific antibodies for STAT4 or STAT6, respectively. As shown in Figure 4A, exposure of the cells to cisplatin did not induce phosphorylation of STAT4, whereas it led to increased tyrosine phosphorylation of STAT6 in a time-dependent manner. The maximal intensity of the immunoreactive band of phosphorylated STAT6 was observed 8 h after cisplatin treatment. We further confirmed tyrosine phosphorylation of STAT6 by immunocytochemical analysis using a phosphotyrosine-specific STAT6 antibody. Tyrosine phosphorylation of STAT6 was clearly elevated in the nucleus as well as the cytoplasm after cisplatin exposure for 8 h (Figure 4B). Next, to confirm that cisplatin cytotoxicity is indeed mediated by STAT6, siRNA constructs targeting STAT4 and STAT6 were transfected into HEI-OC1 cells. Transfection with STAT6 siRNA resulted in a substantial suppression of cisplatin-induced cell death. However, transfection with either STAT4 siRNAs or control siRNAs did not attenuate the cisplatin-induced cell death (Figure 4C). We also confirmed that transfection with STAT4 or STAT6 siRNAs reduced the cognate protein expression level (Supplementary information, Figure S1). Next, we examined the role of STAT4 and STAT6 in the cisplatin-induced production of pro-inflammatory cytokines by HEI-OC1 cells via transfection with validated STAT4- and STAT6-specific siRNAs. Knockdown of STAT6 significantly reduced the cisplatin-induced secretion of TNF-α, IL-1β and IL-6 when compared with the cisplatin-alone group (Figure 4D). However, the control or STAT4 siRNAs led to only a slight decrease (not significant) in the cisplatin-induced production of these pro-inflammatory cytokines. Similarly, expression of the mRNA encoding these pro-inflammatory cytokines after cisplatin exposure was clearly decreased by transfection with STAT6-specific siRNAs (Figure 4E). Conversely, transfection with control or STAT4 siRNAs did not affect the mRNA expression of cisplatin-induced pro-inflammatory cytokines. These data indicate that STAT6 signaling, but not STAT4, may provide one aspect of cisplatin cytotoxicity by modulating the pro-inflammatory cytokine production in HEI-OC1 cells.
STAT6, but not STAT4, was required for cisplatin-induced cell death and the secretion of pro-inflammatory cytokines in HEI-OC1 cells. (A) Western blotting with the indicated antibodies. (B) Fluorescent microscopic image of the cells treated with 20 μM cisplatin for 8 h. (C) MTT assay for testing the viability of the cells transfected with STAT4, STAT6, and scrambled control siRNAs. *P < 0.05, by one-way ANOVA when compared with cisplatin-only treated cells. (D) Pro-inflammatory cytokines in the supernatants measured by ELISA. *P < 0.05, by one-way ANOVA when compared with cisplatin-only treated cells. (E) mRNA expression levels of TNF-α, IL-1β, IL-6, and GAPDH.
IL-4 and IL-13 were involved in cisplatin-mediated cytotoxicity and STAT6 phosphorylation
Many studies have shown that STAT6 signaling is activated by IL-4 and IL-13 9, but not by TNF-α, IL-6 or IL-1β. Accordingly, we examined the expression profiles of a variety of cytokines that might be responsible for the STAT6 activation in cisplatin-treated HEI-OC1 cells. As shown in Figure 5A, exposure of the cells to cisplatin markedly increased the transcriptional activation of IL-4, IL-5, and IL-13, with peak levels being observed between 12 and 18 h. The mRNA expression of other cytokines such as IFN-γ, IL-2, IL-10, and IL-12 was not observed in cisplatin-treated HEI-OC1 cells. To determine the roles of IL-4, IL-5, and IL-13 in cisplatin cytotoxicity, cells were pretreated with various doses of anti-IL-4, anti-IL-5, and anti-IL-13 antibodies for 30 min before cisplatin exposure to neutralize the endogenously produced cytokines. As shown in Figure 5B, neutralization with an anti-IL-4 or IL-13 antibody significantly attenuated the cisplatin-induced cytotoxicity in a dose-dependent manner. However, pretreatment with anti-IL-5 (Figure 5B) did not show any notable cytoprotective effect. We next determined which cytokines were involved in STAT6 phosphorylation induced by cisplatin in HEI-OC1 auditory cells. Cells were pretreated with various doses of anti-TNF-α, anti-IL-4, anti-IL-5, and anti-IL-13 antibodies for 30 min, after which they were further treated with cisplatin for 8 h. As shown in Figure 5C, neutralization of TNF-α and IL-5 did not block the cisplatin-induced tyrosine phosphorylation of STAT6. However, neutralization of IL-4 and IL-13 by their specific antibodies markedly attenuated the cisplatin-induced tyrosine phosphorylation of STAT6 in a dose-dependent manner. These results indicate that both IL-4 and IL-13 act as important mediators of cisplatin cytotoxicity in HEI-OC1 cells via STAT6 phosphorylation. IL-4 and IL-13 signaling occurs via binding to their respective subunits of a receptor complex comprised of the IL-4 receptor alpha (IL-4Rα) and IL-13 receptor alpha 1 (IL-13Rα1), with subsequent activation of STAT6 9. Therefore, we determined whether cisplatin induced the association between the cytokine receptors and phosphorylated STAT6 in HEI-OC1 cells. Briefly, cells were stimulated with cisplatin for various lengths of time, after which the cell lysates with equal amounts of protein were immunoprecipitated with antibodies for the TNF receptor 1 (TNFR1), IL-5 receptor (IL-5R), IL-4Rα, and IL-13Rα1. As shown in Figure 6A and 6B, phosphorylated STAT6 was not detected in either the TNFR1 or the IL-5R immunoprecipitates. However, cisplatin induced a marked increase in phosphorylated STAT6 in the immunoprecipitates of IL-4Rα or IL-13Rα1 in a time-dependent manner (Figure 6C and 6D). Taken together, these results suggest that STAT6 activation in cisplatin-treated cells occurs through direct binding of either IL-4 to IL-4Rα or IL-13 to IL-13Rα1, which presumably leads to phosphorylation of the cytoplasmic domain of each receptor and subsequent recruitment of STAT6.
IL-4 and IL-13 were involved in cisplatin-mediated cytotoxicity and STAT6 phosphorylation in HEI-OC1 cells. (A) Cells were treated with cisplatin for the indicated periods. The total RNA was then isolated by TRIzol and cDNA was synthesized by reverse transcription. IFN-γ, IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, and GAPDH cDNAs were then amplified using specific primer sets. (B) Cells were pretreated with the indicated doses of neutralizing antibodies including anti-IL-4, anti-IL-5, and anti-IL-13 for 30 min, after which they were further maintained in 20 μM cisplatin for 24 h. The cell viability was then measured by MTT assay. *P < 0.05 as determined by one-way ANOVA when compared with cisplatin-only treated cells. (C) Cells were pretreated with the indicated doses of neutralizing antibodies including anti-TNF-α, anti-IL-4, anti-IL-5, and anti-IL-13 for 30 min and then further maintained in 20 μM cisplatin for 8 h. The cell extracts were then subjected to 12% SDS-PAGE, after which they were immunoblotted with antibodies specific for phospho-STAT6 and STAT6.
IL-4 and IL-13 were involved in cisplatin-mediated STAT6 phosphorylation through their receptor binding. Cells were treated with cisplatin for the indicated periods, after which the cell lysates were immunoprecipitated with anti-TNFR1, anti-IL-5Rα, anti-IL-4Rα, and anti-IL-13Rα1, respectively. Immunoprecipitated proteins were subjected to 12% SDS-PAGE and then immunoblotted with antibodies specific for phospho-STAT6, TNFR1 (A), IL-5Rα (B), IL-4Rα (C), and IL-13Rα1 (D), respectively.
Neither IL-4 nor IL-13 signaling was involved in NF-κB activation in cisplatin-treated HEI-OC1 cells
In our previous study, we demonstrated that cisplatin induces the nuclear translocation of NF-κB and that the secretion of pro-inflammatory cytokines, especially TNF-α, is directly involved in NF-κB activation in cisplatin-treated cells 6. To examine the effect of endogenously produced cytokines, especially IL-4 and IL-13, on NF-κB activity, HEI-OC1 cells were transfected with an NF-κB luciferase reporter plasmid. At 36 h after transfection, these cells were treated with cisplatin for 12 h in the presence of neutralizing antibodies for TNF-α, IL-1β, IL-4, IL-6, and IL-13, respectively. As shown in Figure 7A, the increase in NF-κB luciferase activity induced by cisplatin was significantly attenuated by neutralizing antibodies for TNF-α and IL-6. However, neutralizing antibodies for IL-4 and IL-13 did not affect the cisplatin-induced NF-κB activation. These results suggest that both IL-4 and IL-13 signaling were not involved in NF-κB activation in cisplatin-treated HEI-OC1 cells. We further investigated whether the knockdown of STAT6 could affect the cisplatin-induced nuclear translocation of NF-κB and degradation of cytoplasmic IκB. As shown in Figure 7B, knockdown of STAT6 by transfection with STAT6-specific siRNAs did not affect the cisplatin-induced nuclear translocation of NF-κB and degradation of cytoplasmic IκB.
Neither IL-4 nor IL-13 signaling was involved in NF-κB activation in cisplatin-treated HEI-OC1 cells. (A) Luciferase activity of the NF-κB-Luc reporter gene constructs. *P < 0.05, by one-way ANOVA when compared with the only-cisplatin treated group. (B) Cells were transfected with 100 nM STAT4, STAT6, and control siRNAs, after which they were incubated for 36 h. The cells were then further treated with 20 μM cisplatin for 2 h. Next, cytosolic and nuclear fractions were prepared as described in Materials and Methods. The extracts were then subjected to 12% SDS-PAGE and immunoblotted with antibodies specific for NF-κB p65, IκB, lamin, and β−actin.
Discussion
Ototoxicity following cisplatin therapy is very common and occurs in the OHCs of the organ of Corti 10, 11, 12, 13. In fact, cisplatin has been shown to induce apoptosis of OHCs in the organ of Corti explants 12 and in vivo 13, 14. Many recent studies have suggested that cochlear and vestibular functions such as hearing and balance are influenced by immune responses in the inner ear 15, 16. Although immune function in the inner ear is very important for protection against infectious diseases such as labyrinthitis, immune-related inflammatory responses also cause damage to the delicate tissues of the inner ear compartments and can often lead to cochlear degeneration and permanent hearing loss 3, 17, 18. Previously, we found that inflammatory responses that occurred through pro-inflammatory cytokine production are involved in the pathogenesis of cisplatin ototoxicity 1, 6. One of the major signaling events triggered by cytokines or growth factors is mediated by the STAT pathway. A large array of cytokines and growth factors use the specific STAT network to transduce their cognate signals to the nucleus. For example, IL-6 and IL-10 cytokines induce STAT3 activity, while IFNs predominantly use STAT1 and STAT2, and IL-4 and IL-13 primarily activate STAT6 19, 20. Some members of the STAT family, such as STAT1, STAT3, and STAT5, are activated in numerous cell types by a wide variety of cytokines and are involved in mediating general signals for cell growth and survival. Others, including STAT2, STAT4, and STAT6, are more restricted in their expression, are activated in response to a small number of cytokines and play a distinct role in IFN-γ signaling and the development of T-cells 21, 22. STAT6 activation is known to be closely related to the inhibition of cell growth and the induction of apoptosis in human cancer cells 23, normal hepatocytes 24, and endothelial cells 25. Recently, Schmitt et al. demonstrated that STAT1 is required for cisplatin-induced vestibular hair cell death 26. However, no study has evaluated the involvement of STAT signaling in cochlear damage and hearing loss. Therefore, the role of STAT signaling in pro-inflammatory cytokine production and hearing loss caused by cisplatin must be elucidated.
Here, we clearly demonstrated that the activation of STAT6 is involved in the production of pro-inflammatory cytokines and the cytotoxic effect of cisplatin. Specifically, cisplatin increased the phosphorylation of STAT6 in HEI-OC1 auditory cells. Furthermore, transfection with STAT6 siRNAs markedly suppressed the production of pro-inflammatory cytokines and the cytotoxic effect of cisplatin. STAT6 activation in auditory systems was absolutely dependent on the production of IL-4 and IL-13 induced by cisplatin, because neutralization of these cytokines by using specific neutralizing antibodies prevented cisplatin-induced cytotoxicity and tyrosine phosphorylation of STAT6. Immunoprecipitation analysis revealed a direct link between the association of IL-4, IL-13, and their receptors and the subsequent activation of STAT6 signaling in cisplatin-treated HEI-OC1 cells. Specific binding of TNF-α to its receptor did not directly recruit and activate STAT6. In addition, cisplatin-induced pro-inflammatory cytokine production is dependent on STAT6 activation. This was demonstrated by the failure of cisplatin to induce pro-inflammatory cytokine production in the cochlear regions of STAT6−/− mice.
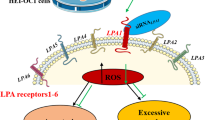
NF-κB activation is pivotal in the expression of pro-inflammatory cytokines and other mediators involved in acute inflammatory responses. Several studies have recently reported that STATs regulate NF-κB activity directly or indirectly. STAT1 and STAT5 interact with an overlapping site in the p300 cofactor, which also interacts with the NF-κB p65 subunit to regulate NF-κB-mediated signaling 27. STAT activation can inhibit or activate NF-κB depending on the stimuli and STAT factors involved. For example, STAT1 enhances and STAT5b inhibits NF-κB signaling to the interferon regulatory factor-1 promoter. Additionally, STAT2 inhibits NF-κB signaling by binding to a specific domain of p300, which also binds to the p65 subunit of NF-κB, thereby suppressing cytokine-induced HIV gene expression 28. Interestingly, STAT6 does not bind to the p300 cofactor. Shen and Stavnezer reported that NF-κB and tyrosine-phosphorylated STAT6 could directly bind to each other in vitro and in vivo. They showed that these two transcription factors also cooperatively bind their cognate DNA-binding sites and synergistically activate IL-4-induced transcription 29. However, Abu-Amer reported that IL-4 inhibited nuclear translocation and activation of NF-κB in a STAT6-dependent manner 30. These data suggest that STAT6 may also act as a transcriptional inhibitor of NF-κB target genes, including NF-κB itself. In the present study, we demonstrated that, unlike TNF-α, IL-4 and IL-13 were not involved in cisplatin-induced NF-κB activation, even though direct binding of either IL-4 to IL-4Rα or IL-13 to IL-13Rα1 was critical for cisplatin-mediated STAT6 activation. Furthermore, knockdown of STAT6 by transfection of STAT6-specific siRNAs did not affect the cisplatin-induced nuclear translocation of NF-κB and degradation of cytoplasmic IκB. These findings suggest that cisplatin induces ototoxicity through two different pathways – one that is mediated by IL-4 and IL-13 signaling and subsequent STAT6 activation and the other that is mediated primarily by the TNF-α cytokine which activates NF-κB signaling (Figure 8). STAT6-deficient mice showed impaired response to both IL-4 and IL-13 31, 32. In our in vivo experiment, the expression of various cytokines, including TNF-α, IL-4, and IL-13, as well as NF-κB in the cochleas of STAT6−/− mice following cisplatin injection was clearly suppressed when compared to WT and STAT4−/− mice. These results indicate that there is a positive feedback loop of STATs, NF-κB and various cytokines involved in cisplatin ototoxicity. Specifically, STAT6 is activated by IL-4 and IL-13, which are secreted soon after cisplatin exposure, and activation of STAT6 enhances the production of various cytokines, including IL-4/IL-13 and TNF-α, which subsequently activates NF-κB. Furthermore, the activation of NF-κB enhances de novo synthesis of pro-inflammatory cytokines, thereby aggravating the cisplatin-mediated damage to the cochlea. Consistent with our hypothesis, many studies have shown that the promoter regions of both TNF-α and IL-4 contain NF-κB and STAT6 binding sites 33, 34.
Proposed mechanisms in cisplatin-induced auditory cell damage. Cisplatin induces ototoxicity through two different pathways. One is mediated by IL-4 and IL-13 cytokine signaling and subsequent STAT6, but NF-κB-independent pathway. Another is mediated primarily by TNF-α cytokine, which activates NF-κB signaling.
To conclude, our findings suggest that cisplatin-mediated ototoxicity and synergistic pro-inflammatory cytokine productions result from enhanced activation and interaction of the NF-κB and STAT6 signaling pathways. In addition, our results revealed a previously unknown function of the STAT pathway in cisplatin ototoxicity that occurs through the production of cytokines. Elucidation of the role of STAT6 signaling in cisplatin-induced damage to the inner ear may provide new opportunities for pharmacotherapy with regard to cisplatin-induced and immune-mediated disorders of hearing and balance.
Materials and Methods
Reagents
Cisplatin and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma Chemical Co (Sigma, St Louis, MO, USA). The plastic culture materials were bought from Becton Dickinson and Company (Franklin Lakes, NJ, USA). Dulbecco's modified essential medium (DMEM), fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD, USA), and other tissue culture reagents were obtained from Life Technologies Inc. (Gaithersburg, MD, USA). Recombinant mouse IL-4 and IL-13 protein, antibodies against IL-4, IL-13, TNF-α, IL-1β, IL-6, and enzyme-linked immunosorbent assay (ELISA) kits (QuantikineR) for cytokines were purchased from R&D Systems Inc. (Minneapolis, MN, USA). These antibodies were used for immunohistochemistry at a concentration of 1:200. Antibodies to p-STAT4, STAT4, p-STAT6, STAT6, NF-κB (p65), and IκB were purchased from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA).
Cell culture and viability
The establishment and characterization of the conditionally immortalized HEI-OC1 auditory cells was described in our previous report 1. Expression of OHC-specific markers such as Math1 and Myosin 7a suggests that HEI-OC1 cells represent OHC precursors. HEI-OC1 cells were maintained in high-glucose DMEM (Gibco BRL) containing 10% FBS. For the experiments described below, HEI-OC1 cells were cultured under the following permissive conditions: 33 °C and 5% CO2 in DMEM supplemented with 10% FBS. Cells (3 × 104 cells/well of a 24-well plate) were incubated with 20 μM cisplatin for 24 h. To determine the cell viability, MTT (0.25 mg) was added to a 1-ml cell suspension for 4 h. After washing the cells, and three washes with PBS (pH 7.4), the insoluble formazan product was dissolved in DMSO. The optical density (OD) of each culture well was then measured using a Microplate reader (Titertek Multiskan, Flow Laboratories) at 590 nm. The OD of the control cells was taken to indicate 100% viability. To examine the effects of various cytokines, neutralizing antibodies against cytokines were added to the cultures for 30 min, after which they were cultured with 20 μM cisplatin for 24 h.
Animals
STAT4−/− (backcross generation N10), STAT6−/− (backcross generation N6), and WT BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The homozygous STAT4 and STAT6 KO mice were identified by PCR. The KO mice did not show any developmental abnormalities. Experiments were performed in 6-week-old mice, and all mice were age-matched within 3 days. Mice were fed a standard commercial diet while housed at an ambient temperature of 20–22 °C and a relative humidity of 50 ± 5% under a 12:12 h light:dark cycle in a specific pathogen-free facility. All animal studies were approved by the Animal Care and Use Committee at Wonkwang University School of Medicine.
Luciferase reporter assay
Cells were transiently transfected with NF-κB luciferase reporter plasmid using the transfection reagent, Lipofectamine 2000. After 36 h of incubation, the cells were treated with cisplatin for 12 h in the presence of neutralizing cytokine antibodies. The cells were then washed twice with PBS buffer and subsequently lysed in reporter lysis buffer (Promega, Madison, WI, USA). A 20-μl aliquot of the lysate was then mixed with 100 μl of luciferase assay reagent, after which the emitted light intensity was measured using a luminometer AutoLumat LB953 (EG and G Berthold, Bad Wildbad, Germany). Finally, the luciferase activity was measured in triplicate, averaged, and then normalized against the β-galactosidase activity using the galactosidase assay system (Galacto-Light, Tropix Inc., MA, USA) according to the manufacturer's instruction.
Transfection with siRNA constructs
Predesigned siRNAs against mouse STAT4, STAT6, and control scrambled siRNA were purchased from Santa Cruz Biotechnology. The sense strands of siRNAs against STAT4 and STAT6 are as follows. The STAT4 siRNAs construct is a pool of three sequences of siRNA as follows: Duplex 1 Sense Strand: 5′-GUA GCU GUG GUA AUU UCA A-3′, Duplex 2 Sense Strand: 5′-CUA CCU UCC UGA UCU ACA A-3′, and Duplex 3 Sense Strand: 5′-CUG UCG UGA UGA UUU CUA A-3′ (mRNA accession number: NM_011487). The STAT6 siRNAs construct is a pool of three sequences of siRNA as follows: Duplex 1 Sense Strand: 5′-GAU GCU UUC UGU UAC AAC A-3′, Duplex 2 Sense Strand: 5′-CUA GCC UUC UCC UCA AUG A-3′, and Duplex 3 Sense Strand: 5′-GUC UCU ACU ACU AUC AAG A-3′ (mRNA accession number: NM_009284). Cells were transiently transfected with 100 nM of siRNA constructs in X-tremeGENE siRNA transfection reagent (Roche Applied Science, Penzberg, Germany) according to the manufacturer's protocol. After incubation at 33 °C and 5% CO2 for 36 h, the cells were further treated with cisplatin for 24 h. The samples were then prepared and analyzed for viability or Western blot analysis. The interference of expression was confirmed by immunoblot analysis.
Measurement of pro-inflammatory cytokines by ELISA
To measure the secretion of pro-inflammatory cytokines from the cisplatin-treated cells, culture supernatants were harvested at each time point and the levels of secreted proinflammatory cytokines were then determined by ELISA (Quantikine Cytokine Kits; R&D Systems Inc.) according to the manufacturer's instruction.
Preparation of cytosolic and nuclear extracts
Cells were washed with ice-cold PBS, scraped and centrifuged at 1 000× g for 5 min at 4 °C. The cell pellet was then resuspended in 200 μl of lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol) and then incubated on ice for 15 min. At the end of incubation, 10 μl of 10% NP-40 was added and the tube was vortexed for 10 s. After centrifugation at 13 000× g for 1 min at 4 °C, the supernatant (cytosolic extract) was collected and stored at −80 °C, whereas the pellet was further processed to obtain the nuclear extracts. The pellet was then resuspended in extraction buffer (5 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.5 mM phenylmethylsulfonyl fluoride, 0.2 mM EDTA, 0.5 mM dithiothreitol and 25% (vol/vol) glycerol) and incubated for 30 min at 4 °C. Nuclear extracts were isolated by centrifugation at 13 000× g for 30 min at 4 °C. The supernatant was then removed and stored at −80 °C until used for western blot analysis. Finally, the protein concentration was determined by the Lowry method.
Western blot analysis
Western blot analysis was performed as follows. Briefly, the cells were harvested and washed twice with ice-cold PBS. The total and nuclear/cytosolic fractionated lysates were then subjected to electrophoresis on 12% SDS-polyacrylamide gels for 3 h at 20 mA, after which they were transferred to nitrocellulose. The membrane was then incubated in 5% (wt/vol) dried milk protein in PBS containing 0.05% (vol/vol) Tween-20 (PBS-T) for 1 h, after which they were washed in PBS-T, and then further reacted with primary antibody (1:1 000) for 1 h. Next, the membrane was extensively washed with PBS-T and then incubated with anti-rabbit IgG antibody conjugated to HRP (1:3 000) for 1 h. After extensive washes, protein bands on the membrane were visualized using chemiluminescent reagents according to the manufacturer's instructions (Supersignal Substrate; Pierce, Rockford, IL, USA).
In vivo experiment of cisplatin ototoxicity
All mice were randomly divided into two groups of six mice each. Group 1 animals, which were regarded as a control group, received intraperitoneal injection of PBS. Group 2 animals were administered cisplatin (4 mg/kg body weight) by intraperitoneal injection for 4 consecutive days. The animals were then killed under anesthesia using CO2 gas on the day following the final cisplatin injection, after which temporal bone of the right ear was removed.
Measurement of ABR
For further analysis of the auditory threshold, the ABR was measured before and 24 h after the final treatment of cisplatin. The ABR threshold changes between pre-treatment and post-treatment were then compared. Mice were anesthetized using a cocktail of ketamine (40 mg/kg) and xylazine (10 mg/kg) and kept warm with a heating pad during ABR recording. A subdermal (active) needle electrode was inserted at the vertex, while ground and reference electrodes were inserted subdermally in the loose skin beneath the pinnae of opposite ears. Test stimuli consisted of alternating phase tone bursts at frequencies of 4, 8, 16, and 32 kHz. Signals were generated using Tucker Davis Technologies (TDT, Gainesville, FL, USA) SigGen software. Each tone burst (1 ms duration) was gated through a Blackmann window, and had a 0.5-ms rise-fall time with no plateau. The stimuli were calibrated prior to each testing session, by recording the output of the speaker with a microphone placed at the animals' head level. ABR waveforms were averaged in response to 300 tone bursts at each tested frequency. At each frequency, the amplitude of the signal was automatically attenuated in 10-dB steps from 90 dB SPL until the ABR waves disappeared in traces recorded with filter settings of 100-3 000 Hz. Judgment of the threshold was made off-line by two independent, experimentally blind observers based on the ABR records.
Surface preparation of the organ of Corti explants
All mice were killed on the day after final cisplatin injection. The temporal bone was dissected out and fixed in 4% paraformaldehyde for 16 h at 4 °C, and rinsed with 0.1 M PBS. The temporal bone was further decalcificated with 10% EDTA in PBS for 3 days. The cochlea was carefully dissected out. Next, the stria vascularis and spiral ligament were dissected away, leaving the organ of Corti. The middle turn of the cochlea was immersed in TRITC-labeled phalloidin (Sigma P1951, 1:100) in PBS for 20 min. After three washes with PBS, the specimen was examined under a fluorescence microscope using appropriate filters for TRITC (excitation: 510–550 nm, emission: 590 nm).
Immunohistochemical staining and TUNEL assay
The removed temporal bone was fixed in 4% paraformaldehyde for 16 h and then decalcificated with 10% EDTA in PBS for 2 weeks, after which it was dehydrated and embedded in paraffin wax. Sections of 5 μm were deparaffinized in xylene and rehydrated through graded concentrations of ethanol. For the immunohistochemistry study, an immunohistochemistry kit (DAKO LSAB Universal K680, Carpinteria, CA, USA) was used and procedures were conducted out according to the manufacturer's instructions. The endogenous peroxidase was then blocked with 3% hydrogen peroxide for 5 min at room temperature (RT). After the sections were washed in PBS, nonspecific binding was blocked with 1% bovine serum albumin for 1 h. Primary antibodies (1:200 diluted) were then added to the slides, after which the incubation proceeded for 1 h. After repeated washes with PBS, the sections were incubated with biotinylated secondary antibody for 30 min and then covered for 30 min with a secondary antibody containing horseradish peroxidase. Finally, the sections were stained in a freshly prepared substrate solution (3 mg of 3-amino-9-ethylcarbazole in 10 ml of sodium acetate buffer (pH 4.9), 500 μl of dimethylformamide, 0.03% hydrogen peroxide) for 5 min. The nuclei of the immuno-stained cells were then counterstained with Mayer's hematoxylin (Sigma-Aldrich Co.). Apoptotic cells were detected in situ utilizing the TUNEL assay (TUNEL POD kit, Roche Molec Biochemic, Mannheim, Germany). Briefly, a section was deparaffinized and rehydrated. After incubation with 20 μg/ml proteinase K (Boehringer Mannheim, Mannheim, Germany), the endogenous peroxidase was blocked by incubating the samples in 2% H2O2 in methanol for 30 min at RT. Next, the tissue sections were washed in PBS and incubated with labeling solution for 1 h at 37 °C. The nuclei were then counterstained with propidium iodide (0.5 μg/ml, Molecular Probes) for 10 min at RT. After washes with PBS, the specimen was examined under a fluorescence microscope.
Reverse transcriptase-PCR amplification
For the cochlear PCR, the left ear temporal bone was quickly harvested and immersed in RNAlater (Ambion, Inc., Austin, TX, USA) till use at −20 °C. Then, whole cochleae was dissected out and used to extract total RNA by the use of TRIzol (Invitrogen) according to the manufacturer's protocols. After extraction of the total RNA using Trizol (Invitrogen), single-stranded cDNA was synthesized from the total RNA. Next, PCR with Taq DNA polymerase (Takara, Takara Shuzo, Japan) was performed by subjecting the samples to 30 cycles of 95 °C for 40 s, 58 °C for 40 s, and 72 °C for 50 s. Ten microliters of the PCR products was then separated on 1.2% agarose gel and visualized under UV light. The sequences of the primers used for PCR amplification were as follows: GAPDH (forward, 5′-GGG TGT GAA CCA CGA GAA AT-3′, reverse, 5′-GTC ATG AGC CCT TCC ACA AT-3′), TNF-α (forward, 5′-CAG GGG CCA CCA CGC TCT TC-3′, reverse, 5′-CTT GGG GCA GGG GCT CTT GAC-3′), IL-1β (forward, 5′-AAG GAG ACC AAG CAA CGA C-3′, reverse, 5′-GAG ATT GAG CTG TCT GCT CA-3′), IL-2 (forward, 5′-GTC AAC AGC GCA CCC ACT TCA AGC-3′, reverse, 5′-GCT TGT TGA GAT GAT GCT TTG ACA-3′), IL-4 (forward, 5′-ACG GAG ATG GAT GTG CCA AAC GTC-3′, reverse, 5′-CGA GTA ATC CAT TTG CAT GAT GC-3′), IL-5 (forward, 5′-GCT CCT TCA GGA ATC TGT TC-3′, reverse, 5′-GGC TCA TGTACT TTC ATG AG-3′), IL-6 (forward, 5′-TTG CCT TCT TGG GAC TGA TGC-3′, reverse, 5′-TTG GAA ATT GGG GTA GGA AGG A-3′), IL-10 (forward, 5′-AGA CTT TCT TTC AAA CAA AGG ACC AGC TGG A-3′, reverse, 5′-CCT GGA GTC CAG CAG ACT CAA TAC ACA CTG C-3′), IL-12 (forward, 5′-ACC TCA GTT TGG CCA GGG TC-3′, reverse, 5′-GTC ACG ACG CGG GTG GTG AAG-3′), and IFN-γ (forward, 5′-TAC TGC CAC GGC ACA GTC ATT GAA-3′, reverse, 5′-GCA GCG ACT CCT TTT CCG CTT CCT-3′).
Statistical analysis
Each experiment was performed at least three times, and all values reported represent the means ± SD of triplicate analyses. Statistical multivariate analysis was performed by analysis of variance and Duncan tests, using the SPSS 11 (Chicago, IL, USA) statistical software. Two-way ANOVA and/or one-way ANOVA were used to determine the significance of the results. The statistical results were reviewed by a masters-level biostatistician. Values of P < 0.05 were considered to be statistically significant.
References
So H, Kim H, Kim Y, et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol 2008; 9:290–306.
Kim HJ, Lee JH, Kim SJ, et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci 30:3933–3946.
Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM . Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol 2003; 4:139–147.
McCabe BF . Autoimmune sensorineural hearing loss. 1979. Ann Otol Rhinol Laryngol 2004; 113:526–530.
Yoshida K, Ichimiya I, Suzuki M, Mogi G . Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hear Res 1999; 137:155–159.
So H, Kim H, Lee JH, et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol 2007; 8:338–355.
Teng X, Zhang H, Snead C, Catravas JD . Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am J Physiol Cell Physiol 2002; 282:C144–C152.
Ohmori Y, Schreiber RD, Hamilton TA . Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem 1997; 272:14899–14907.
Jiang H, Harris MB, Rothman P . IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 2000; 105 (Part 1):1063–1070.
Feghali JG, Liu W, Van De Water TR . L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 2001; 111:1147–1155.
Huang T, Cheng AG, Stupak H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci 2000; 18:259–270.
Kopke RD, Liu W, Gabaizadeh R, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol 1997; 18:559–571.
Watanabe KC, Jinnouchi K, Hess A, et al. Carboplatin induces less apoptosis in the cochlea of guinea pigs than cisplatin. Chemotherapy 2002; 48:82–87.
Alam SA, Ikeda K, Oshima T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res 2000; 141:28–38.
Ma C, Billings P, Harris JP, Keithley EM . Characterization of an experimentally induced inner ear immune response. Laryngoscope 2000; 110 (Part 1):451–456.
Rahman MU, Poe DS, Choi HK . Autoimmune vestibulo-cochlear disorders. Curr Opin Rheumatol 2001; 13:184–189.
Ryan AF, Harris JP, Keithley EM . Immune-mediated hearing loss: basic mechanisms and options for therapy. Acta Otolaryngol Suppl 2002:38–43.
Stone JH, Francis HW . Immune-mediated inner ear disease. Curr Opin Rheumatol 2000; 12:32–40.
Gao B . Cytokines, STATs and liver disease. Cell Mol Immunol 2005; 2:92–100.
Campbell IL . Cytokine-mediated inflammation, tumorigenesis, and disease-associated JAK/STAT/SOCS signaling circuits in the CNS. Brain Res Brain Res Rev 2005; 48:166–177.
Tan JC, Rabkin R . Suppressors of cytokine signaling in health and disease. Pediatr Nephrol 2005; 20:567–575.
Alas S, Bonavida B . Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res 2003; 9:316–326.
Gooch JL, Christy B, Yee D . STAT6 mediates interleukin-4 growth inhibition in human breast cancer cells. Neoplasia 2002; 4:324–331.
Aoudjehane L, Podevin P, Scatton O, et al. Interleukin-4 induces human hepatocyte apoptosis through a Fas-independent pathway. FASEB J 2007; 21:1433–1444.
Nishimura Y, Nitto T, Inoue T, Node K . STAT6 mediates apoptosis of human coronary arterial endothelial cells by interleukin-13. Hypertens Res 2008; 31:535–541.
Schmitt NC, Rubel EW, Nathanson NM . Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J Neurosci 2009; 29:3843–3851.
Pfitzner E, Jahne R, Wissler M, Stoecklin E, Groner B . p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the transactivation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol 1998; 12:1582–1593.
Hottiger MO, Felzien LK, Nabel GJ . Modulation of cytokine-induced HIV gene expression by competitive binding of transcription factors to the coactivator p300. EMBO J 1998; 17:3124–3134.
Shen CH, Stavnezer J . Interaction of stat6 and NF-kappaB: direct association and synergistic activation of interleukin-4-induced transcription. Mol Cell Biol 1998; 18:3395–3404.
Abu-Amer Y . IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. J Clin Invest 2001; 107:1375–1385.
Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996; 380:630–633.
Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S . Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol 1996; 157:3220–3222.
Chappell VL, Le LX, LaGrone L, Mileski WJ . Stat proteins play a role in tumor necrosis factor alpha gene expression. Shock 2000; 14:400–402; discussion 402–403.
Curiel RE, Lahesmaa R, Subleski J, et al. Identification of a Stat-6-responsive element in the promoter of the human interleukin-4 gene. Eur J Immunol 1997; 27:1982–1987.
Acknowledgements
This work was supported by the Ministry of Science and Technology (MoST)/Korea Science and Engineering Foundation (KOSEF) through the Vestibulocochlear Research Center (VCRC) at Wonkwang University in 2009 (R13-2002-055-00000-0).
Author information
Authors and Affiliations
Corresponding authors
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
(A) Expression of STAT proteins by transfection of STAT4- or STAT6-siRNA into HEI-OC1 auditory cells. (PDF 130 kb)
Rights and permissions
About this article
Cite this article
Kim, HJ., Oh, GS., Lee, JH. et al. Cisplatin ototoxicity involves cytokines and STAT6 signaling network. Cell Res 21, 944–956 (2011). https://doi.org/10.1038/cr.2011.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2011.27
Keywords
This article is cited by
-
2-Hydroxypropyl-β-cyclodextrin Ototoxicity in Adult Rats: Rapid Onset and Massive Destruction of Both Inner and Outer Hair Cells Above a Critical Dose
Neurotoxicity Research (2020)
-
Cisplatin-induced ototoxicity involves interaction of PRMT3 and cannabinoid system
Archives of Toxicology (2019)
-
The Role of Tumor Necrosis Factor Alpha (TNFα) in Hearing Loss and Vestibular Schwannomas
Current Otorhinolaryngology Reports (2018)
-
QTL Mapping of Endocochlear Potential Differences between C57BL/6J and BALB/cJ mice
Journal of the Association for Research in Otolaryngology (2016)
-
Loss of STAT1 protects hair cells from ototoxicity through modulation of STAT3, c-Jun, Akt, and autophagy factors
Cell Death & Disease (2015)