Abstract
Optical thermometry has been widely studied to achieve an inaccessible temperature measurement in submicron scale and it has been reported that the temperature sensitivity depends mainly on host types. In this work, we propose a new method to improve the optical temperature sensitivity of Yb3+-Er3+ co-doped CaWO4 phosphors by doping with Li+, Sr2+, and Mg2+ ions and by controlling excitation powers of 980 nm laser. It is found that the thermometric parameters such as upconversion emission intensity, intensity ratio of green-to-red emission, fluorescence color, emission intensity ratios of thermally coupled levels (2H11/2/4S3/2), and relative and absolute temperature sensitivity can be effectively controlled by doping with Li+, Sr2+, and Mg2+ ions in the Yb3+-Er3+ co-doped CaWO4 system. Moreover, the relative sensitivity SR and the absolute sensitivity SA are proved to be dependent on the pump power of 980 nm laser. The sensitivities of SR and SA in Yb3+-Er3+ co-doped CaWO4 increase about 31.5% and 12%, respectively, by doping with 1 mol% Sr2+.
Similar content being viewed by others
Introduction
In recent years, more and more research works have been focused on detecting temperature in submicron scale through optical thermometry, due to wide application in nano-devices1,2,3. Optical thermometry is a method to calculate the temperature of phosphors by fitting the fluorescence intensity ratios (FIR) of thermally coupled levels at different temperate points4. Compared with conventional temperature measurement contact methods, the non-contact FIR technique overcomes some limitations of spatial resolution and accuracy of detection. The thermally coupled levels 2H11/2 and 4S3/2 of Er3+ ion were reported as one of preferable choice to achieve optical thermometry, due to their large energy gap and less overlap of two emission peaks between the 2H11/2 and 4S3/2 levels1,5,6. Three comprehensive reviews summarized the optical thermometry based on upconversion emissions of Er3+ doped phosphors, such as oxide crystals, glasses, core-shell heterojunction nanoparticles, and transparent glass ceramics containing fluoride nanocrystals7,8,9. It was reported that the optical temperature sensitivity of Er3+ doped phosphors depends mainly on host types irrespective of the other conditions, such as excitation powers, doping concentrations, and sizes and shapes of samples. The main weakness of the previous research works is the failure to address the question: If the power of excitation source changes from low to high, how the changes in power affect the optical temperature sensitivity? Rakov and Maciel observed the optical temperature sensitivity is inconsistent between continuous wave and pulsed (~5 ns) laser excitations in the Er3+-Yb3+ doped Y2SiO5 system10. Chen et al. reported that the intensity ratio of green and red emissions depends strongly on the excitation power density11. Thus, it is necessary to study the influence of pump power on optical temperature sensitivity.
If the excitation power is fixed, how to enhance the optical temperature sensitivity without changing the category of hosts? According to the crystal-field theory, the emissions from the 2H11/2 and 4S3/2 thermally coupled levels are inevitably affected by the crystal field around Er3+ ions in hosts12,13,14. In some works, the tuning of the upconversion emissions of Er3+ were realized by tailoring the crystal field around Er3+ ions. For examples, the enhancement of the upconversion luminescence was achieved in Yb3+/Er3+-codoped Y2O3 nano-crystals by tri-doping with Li+ ions15. Qin et al. reported and discussed a strategy to enhance upconversion emission in β-NaLuF4:Yb3+, Er3+ nano-crystals by tri-doping with La3+ ions16. The lattice distortion of hosts is induced by dopant ions with small ionic radius and affects strongly the crystal-field environment around Er3+ ions leading to increase in radiative transition probability from the excited state to the ground state of 4I15/217. The doping of the ions with small ionic radius may be a preferable method to improve the optical temperature sensitivity.
Recently, the Yb3+-Er3+ co-doped CaWO4 phosphors were reported as one of excellent materials to achieve the optical thermometry based on the green emissions from the thermally coupled 2H11/2 and 4S3/2 levels of Er3+ ions18,19,20. The CaWO4 crystal has tetragonal structure with distorted [WO6] octahedral clusters containing two quadrilateral biconical units21. This asymmetric crystal structure can be efficiently distorted by doping with small ions giving rise to change in fluorescence intensity ratio of the 2H11/2 and 4S3/2 levels. It means that the optical temperature sensitivity can be improved by changing crystal-field environment around Er3+ ions. In this work, we study the influences of the tri-doping with Li+, Sr2+, and Mg2+ ions and excitation powers on optical thermometry in Yb3+-Er3+ co-doped CaWO4. The relative and absolute emission sensitivities can be controlled effectively by doping with Li+, Sr2+, and Mg2+ ions in Yb3+-Er3+ co-doped CaWO4 and by changing the pump power of 980 nm laser.
Results
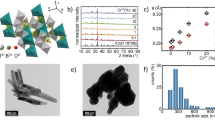
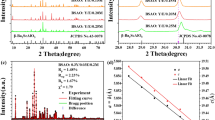
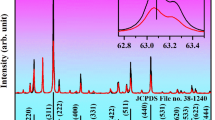
Figure 1 presents the XRD patterns of as-synthesized CaWO4 doped with Yb3+-Er3+, Yb3+-Er3+-0.1%Li+, Yb3+-Er3+-1%Sr2+, and Yb3+-Er3+-0.5%Mg2+. The position and relative intensity of all the diffraction peaks can be readily indexed to pure Scheelite-type tetragonal CaWO4 according to the PDF card (No. 77-2236). The sharp and well defined diffraction peaks in the patterns suggest that the synthesized phosphors are well crystallized. The raw materials could not be detected in the as-synthesized samples confirming that Yb3+, Er3+, Li+, Sr2+ and Mg2+ are doped into the CaWO4 lattices. Figure 1(b) shows the crystal structure and atomic arrangement along the a-axis direction in CaWO4 which has tetragonal symmetry (ICSD 60550) with octahedral quadrilateral biconical crystals21. The tetragonal CaWO4 belongs the I41/aZ space group, and contains 4 formula units in each unit cell. In the unit cell of CaWO4, there are 4b Ca and 4a W cation sites. The Sr and Mg ions occupy the same 4b Ca cation sites, and the Li ion is located at an interstitial site in the CaWO4 lattice. In order to study the influence of the Li+, Sr2+ and Mg2+ doping on the morphology change, the scanning electron microscope (SEM) images are given in Figs S1, S2 and S3. One can find that the average size of Yb3+-Er3+ doped CaWO4 increases with the increase of Li+, Sr2+ and Mg2+ concentration, and its morphology becomes more irregular and agglomerated. The shape and size modifications are attributed to the surface charge redistribution of the crystal nucleus induced by inner electron charge transfer between the dopant ions and lattice cations22.
The upconversion emission spectra of CaWO4:Yb3+, Er3+, Li+ excited by 980 nm infrared radiation are shown in Fig. 2(a). Three emission bands of Er3+ are observed at 528, 550, and 660 nm due to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 transitions, respectively. No spectral shifts of the emission bands occur for various Li+ concentrations in CaWO4:Yb3+, Er3+, Li+. Compared with CaWO4:Yb3+, Er3+, the red emission intensity of CaWO4:Yb3+, Er3+, Li+ increases sharply with increasing Li+ concentration, while the green emission intensity changes irregularly with increasing Li+ concentration, as shown in Fig. 2(b). The total emission intensity increases sharply with increasing Li+ concentration from 0 to 0.5 mol%, as shown in Fig. 2(c). Figure 2(d) shows the intensity ratio of the red/green emissions (IR/IG) which is changed by changing Li+ concentration and the ratio reaches a maximum at 0.1 mol%. The CIE chromaticity in the inset of Fig. 2(d) shows that the emission color is tunable from greenish-yellow to green with increasing Li+ concentration. The spectrum modulation of CaWO4:Yb3+, Er3+ can also be achieved by doping with Sr2+ and Mg2+ ions, as shown in Figs S4 and S5. Similar to the Li+ ions doped in CaWO4:Yb3+, Er3+, the codoping with Sr2+ ions increases the total emission intensity of Er3+ ions (Fig. S4(c)). But for the Mg2+ ions, the total emission intensity of Er3+ ions rather decreases at higher concentration of 1.5 mol% (Fig. S5(c)). Consequently, the changes in total intensity and the intensity ratio of red to green emissions are not consistent in three kind of dopant ions (Li+, Sr2+ and Mg2+). Nevertheless, the results show that the spectrum modulation can be induced by doping of Li+, Sr2+ and Mg2+ as previously reported in Li+-doped Y2O3:Er3+ 15.
The enhancement of visible emissions induced by doping can be explained as follows: CaWO4 has octahedral quadrilateral biconical structure for which the Ca ion is coordinated with eight oxygen ions in the CaWO4 lattice, as shown in Fig. 1(b). Er3+ (eight coordination r = 1.004 Å) as an optical active center has a local structure with the ErO8 unit when the Er3+ is doped in CaWO4 lattice (eight coordination Ca2+, r = 1.12 Å). When the Li+ ions (eight coordination r = 0.92 Å) are doped into CaWO4:Er3+, they compensate the excess charge of 1+ between Er3+ and Ca2+. The Li+ ions may occupy the sites in or near the ErO8 units or diffuse to various interstitial sites in the CaWO4 lattice. The Sr2+ and Mg2+ ions (eight coordination rSr = 1.26 Å, rMg = 0.89 Å) substitute for the Ca2+ sites in CaWO4:Er3+ leading to the distortion of the Er-O bonds. As a result, the local symmetry of the crystal field around Er3+ lowers with the change of Coulomb interaction17. The forbidden intra-4f electronic transitions are partially allowed with increase of the intra-4f electronic transitions probability of the Er3+ ions12,17. Thus, the visible emissions can be enhanced by tailoring the local environments of the Er3+ ions by doping with Li+, Sr2+ and Mg2+ ions.
Based on upconversion emissions centered at 528 nm (2H11/2 → 4I15/2) and 550 nm (4S3/2 → 4I15/2) of Er3+ ions, the optical temperature sensing of Yb3+-Er3+ co-doped CaWO4 was studied by Li et al. by analyzing the temperature dependent fluoresce intensity ratio (FIR)19. Figure 3 shows the influence of doping with Li+, Sr2+, and Mg2+ on optical temperature sensing of Yb3+-Er3+ doped CaWO4. According to previous works4,19, the FIR of two thermally coupled levels of 2H11/2 and 4S3/2 can be fitted by the Boltzmann distributing law

where A is a constant, ΔE is the energy difference between thermally coupled levels, k is the Boltzmann constant, and T is the absolute temperature. The relative sensitivity SR and the absolute sensitivity SA are defined as


Figure 3 shows the FIR as a function of temperature for various doping systems. The solid lines in Fig. 3 are best fit results obtained using Eq. (1). All the data are well fitted to Eq. (1). It is obvious that the fit results depend strongly on the types of dopant ions. Compared with the fit result for Yb3+-Er3+ co-doped CaWO4, the fit results vary much more for Yb3+-Er3+-Mn+ (Mn+ = Li+, Sr2+, and Mg2+) tri-doped CaWO4. The values of ΔE can be calculated from the data points of luminescence intensity ratios at different temperatures using Eq. (1). This indicates that the different types of dopant ions cause different values of ΔE. The peaks of green emissions at 528 and 550 nm do not shift with temperature for all the samples. However, the experimental value of ΔE should be unique for thermometry. As observed in Fig. 4 the changes in error also occur between the best-fit and experimental values of ΔE depending on the types of dopants and their concentrations. The error δ between ΔEf and ΔEm is expressed as:

where ΔEf is the fit value of ΔE, and ΔEm is experimental value of ΔE from the spectrum9. The experimental value of ΔE is 757.6 cm−1 from the spectra in Fig. 2. The errors δ for Yb3+-Er3+ co-doped and Yb3+-Er3+-Mn+ (Mn+ = Li+, Sr2+, and Mg2+) tri-doped CaWO4 are calculated using Eq. (4) and the results are shown in Fig. 4. One can find that the values of error δ depend strongly on the types of dopant ions. All the values of error δ are more than 20%, and are too large to be ignored. Moreover, the value of error δ for Yb3+-Er3+ co-doped CaWO4 exceeds 30%. The large values of δ are attributed to the inconsistency between the restriction of Eq. (1) and luminescence mechanism at high temperature. As a theoretical model, Eq. (1) was proposed only by considering the Boltzmann distribution and the radiative transitions in the thermally coupled levels23. The temperature dependent nonradiative relaxation and energy transfer between the host and the rare-earth ions were not be considered at all. In fact, the nonradiative relaxation and energy transfer become active at high temperature24 giving rise to the large errors of ΔE in the optical temperature sensing process. The large errors of ΔE were also observed in more than fifty kinds of materials9 as well as Yb3+-Er3+ co-doped CaWO. This means that the ΔE is an unreasonable parameter to evaluate the optical temperature sensitivity SR and SA. Thus, Eqs (2) and (3) should be modified to be reasonable for optical thermometry.
Considering the nonradiative relaxation and energy transfer at high temperature, Eq. (1) is modified as

where a is constant dependent on the materials10. The b is a correction term for the comprehensive population of thermally coupled energy levels induced by not only the thermal population but also the nonradiative relaxation and energy transfer. The relative sensitivity SR and the absolute sensitivity SA are defined as


where a and b are from Eq. (5). Figure 5 shows the temperature dependent FIR of 528 and 550 nm green emissions in CaWO4 doped with different types of dopant ions. One can find that the experimental points can be fitted well with a linear mode. The slopes of the fit results depend on the types of dopant ions and concentrations. It means that the FIR of thermally coupled levels (2H11/2/4S3/2) are susceptible to the ion doping. Notably, all the a values in Fig. 5 are not consistent with those values of ΔE/k from Eq. (1) as shown in Fig. 3. In general, no difference in the a value is found between in Eqs (1) and (5), if considering only the thermal population. This deviation proves that it is unreasonable to use the ΔE to evaluate the optical temperature sensitivities SR and SA. Figure 6 shows temperature dependent sensitivities SR and SA of CaWO4 doped with different types of dopant ions. All the sensitivity curves exhibit maxima with increasing temperature. The maximum SR value of all the tri-doped CaWO4 systems changes non-monotonically with increasing dopant concentration, but the maximum SR value of the Yb3+-Er3+ co-doped CaWO4 system are enhanced obviously by doping with Li+, Sr2+ and Mg2+ ions in optimum dopant concentrations. The maximum SR value of Yb3+-Er3+ co-doped CaWO4 is estimated to be 0.0073 K−1 at 380 K, while 0.0076 K−1 at 380 K for Yb3+-Er3+-Li+ (0.1 mol%), 0.0096 K−1 at 429 K for Yb3+-Er3+-Sr2+ (1.0 mol%), and 0.0093 K−1 at 386 K for Yb3+-Er3+-Mg2+ (0.5 mol%) systems. The corresponding SA values decrease with increasing temperature as shown in Fig. 6(d). The SA value of Yb3+-Er3+ co-doped CaWO4 is enhanced by doping with Sr2+ ions. The values of SR and SA indicate that the resulting Yb3+-Er3+-Sr2+ tri-doped CaWO4 might be a promising candidate for optical temperature sensors.
The thermal stability of emission bands plays a key role in the optical temperature sensors. In order to explore the thermal stability, the temperature dependent log–log plots of emission intensity and pump power need to be investigated. The upconversion emission intensity I and excitation power P is expressed as follows

where n is the number of photons absorbed to pump the population in particular level in upconversion process13. Theoretically, the n values of green and red emissions of Er3+ ions excited by 980 nm are close to 224. The temperature dependent log–log plots of emission intensity and pump power for green and red emissions are shown in Fig. S6. The slopes of the fit results for green and red emissions of Yb3+-Er3+ co-doped CaWO4 depend on temperature, and show that the upconversion process involves two photons to contribute green and red emissions. The slopes of the red emissions are much smaller than 2, which means that the red emission is not stable at high temperature24. Compared with the slopes of Yb3+-Er3+ co-doped CaWO4, those of the best-fit results for green and red emissions of Yb3+-Er3+-1.0%Sr2+ tri-doped CaWO4 are closer to 2. It means that Yb3+-Er3+-1.0%Sr2+ tri-doped CaWO4 has high thermal stability in emission bands than Yb3+-Er3+ co-doped CaWO4.
Together with the thermal stability of emission bands, the excitation power dependence of the luminescence spectra was investigated in the temperature range from 298 to 573 K by using a 980 nm laser with pump powers of 85.2 and 322.4 mW/mm2, as shown in Fig. S7. The luminescence spectra contain green and red emission bands, which are assigned to the 4I15/2 → 2H11/2 (528 nm), 4I15/2 → 4S3/2 (550 nm), and 4I15/2 → 4F9/2 (660 nm) transitions of Er3+ ion. One can find that the intensities of 550 and 660 nm emission bands greatly decrease as temperature increases, while the intensity of 528 nm emission bands greatly increase as temperature increases. No peak shift of the emission bands induced by the doping and excitation powers is observed. The LnFIR as a function of 1/T for various excitation powers are illustrated in Fig. 7. The slopes of the fit results at low excitation powers are different from those at high excitation powers. Moreover, the slopes of Yb3+-Er3+ co-doped CaWO4 are different from those of Yb3+-Er3+-1%Sr2+ tri-doped CaWO4 at the same excitation power. It means that the FIR of thermally coupled levels (2H11/2/4S3/2) are also susceptible to excitation powers like the dopant ions described above. The excitation power dependences of SA and SR are illustrated in Fig. 8. One can find that the values of SA and SR change non-monotonically with increasing excitation powers. The large value of SA and SR appear at the excitation power of 322.4 mW/mm2. The values of SA and SR of Yb3+-Er3+ co-doped CaWO4 are more susceptible to excitation powers than those of Yb3+-Er3+-1%Sr2+ tri-doped CaWO4. Thus, the Yb3+-Er3+-1%Sr2+ tri-doped CaWO4 is a better candidate for optical temperature sensors than Yb3+-Er3+ co-doped CaWO4 by considering the stabilities induced by temperature and excitation powers.
Conclusions
In this work, a series of Yb3+-Er3+ co-doped and Yb3+-Er3+-Mn+ (Mn+ = Li+, Sr2+, and Mg2+) tri-doped CaWO4 phosphors were synthesized by using the high-temperature solid-state reaction method. The structural property of resulting powder is investigated by the X-ray diffraction. The upconversion emission intensity, green-to-red emission intensity ratio, fluorescence color of the Yb3+/Er3+ co-doped sample are controlled by doping Li+, Sr2+ and Mg2+ ions. The fluorescence intensity ratios of thermally coupled levels (2H11/2/4S3/2) and optical temperature sensitivity are observed to be dependent on dopant ions and excitation powers. It is found that the temperature sensitivity of CaWO4:5%Yb3+, 0.3%Er3+, 1.0%Sr2+ can reach a maximum relative sensitivity of 0.0111 K−1 at 456 K and absolute sensitivity of 949.8/T2, which is much higher than the reported temperature sensors based on Er3+ green luminescence. This work opens a new method to increase the optical temperature sensitivity of rare earth ions doped materials.
Methods
All starting materials are CaCO3 (AR), WO3 (99.99%), Er2O3 (99.99%), Yb2O3 (99.99%), Li2CO3 (AR), SrO (AR) and MgO (AR). All the chemicals were used as received without any further purification.
The solid-state reaction method is used to synthesize CaWO4:Er3+/Yb3+ and CaWO4:Er3+/Yb3+/Mn+ (Mn+ = Li+, Sr2+ and Mg2+) phosphors. The molar ratio of CaWO4:Er3+/Yb3+ sample is as follows: 48.7CaCO3-48.7WO3-0.15Er2O3-2.45Yb2O3. The molar ratios of CaWO4:Er3+/Yb3+/Li+ samples are as follows: 48.7CaCO3-48.7WO3-0.15Er2O3-2.45Yb2O3-xLi2CO3, x = 0.05, 0.10, 0.15, and 0.25; The molar ratios of CaWO4:Er3+/Yb3+/Sr2+ samples are as follows: (48.7-y)CaCO3-48.7WO3-0.15Er2O3-2.45Yb2O3-ySrO, y = 0.50, 1.00, 1.50 and 2.00. The molar ratios of CaWO4: Er3+/Yb3+/Ca2+ samples are as follows: (48.7-z)CaCO3-48.7WO3-0.15Er2O3-2.45Yb2O3-zMgO, z = 0.25, 0.50, 1.00, and 1.50. The starting materials are entirely mixed and ground with alcohol for 1 hour. The powder is subsequently sintered in a furnace for 10 h at 1350 °C. Finally, a series of the required samples in the form of white powder are obtained after cooling down naturally.
Structures of the samples were investigated by X-ray diffraction (XRD) using a X’TRA (Switzerland ARL) equipment provided with Cu tube with Kα radiation at 1.54056 Å. The size and shape of the samples were observed using a scanning electron microscope (LEO-1550). Luminescence spectra were obtained by the Acton SpectraPro Sp-2300 Spectrophotometer with a photomultiplier tube equipped with 980 nm laser as the excitation sources. Different temperature spectra were obtained by using an INSTEC HCS302 Hot and Cold System.
Additional Information
How to cite this article: Wang, X. et al. Influence of Doping and Excitation Powers on Optical Thermometry in Yb3+-Er3+ doped CaWO4. Sci. Rep. 7, 43383; doi: 10.1038/srep43383 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Vetrone, F., Naccache, R., Zamarrón, A. & Capobianco, J. A. Temperature sensing using fluorescent nanothermometers. ACS Nano 4, 3254–3258 (2010).
Brites, C. D. S., Xie, X., Debasu, M. L. & Carlos, L. D. Instantaneous ballistic velocity of suspended Brownian nanocrystals measured by upconversion nanothermometry. Nature Nanotechnology 11, 851–856 (2016).
Carrasco, E., Rosal, B. D. & Jaque, D. Intratumoral Thermal Reading During Photo-Thermal Therapy by Multifunctional Fluorescent Nanoparticles. Advanced Functional Materials 25, 615–626 (2015).
Wade, S. A., Collins, S. F. & Baxter, G. W. Fluorescence intensity ratio technique for optical fiber point temperature sensing. J. Appl. Phys. 94, 4743 (2003).
LeÓn-Luis, S. F. & Lavín, V. Effects of Er3+ concentration on thermal sensitivity in optical temperature fluorotellurite glass sensors. Sens. Actuators B 176, 1167–1175 (2013).
Du, P., Luo, L. H., Li, W. P., Yue, Q. Y. & Chen, H. B. Optical temperature sensor based on upconversion emission in Er-doped ferroelectric 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3) TiO3 ceramic. Appl. Phys. Lett. 104, 152902 (2014).
Jaque, D. & Vetrone, F. Luminescence nanothermometry. Nanoscale 4, 4301–4326 (2012).
Brites, C. D. S., Lima, P. P., Silva, N. J. O. & Carlos, L. D. Thermometry at the nanoscale. Nanoscale 4, 4799–4829 (2012).
Wang, X. F., Liu, Q., Bu, Y. Y. & Yan, X. H. Optical temperature sensing of rare-earth ion doped phosphors. RSC Advances 5, 86219–86236 (2015).
Rakov, N. & Maciel, G. S. Three-photon upconversion and optical thermometry characterization of Er3+:Yb3+ co-doped yttrium silicate powders. Sens. Actuators B 164, 96–100 (2012).
Chen, G., Ohulchanskyy, T. Y., Kachynski, A., Agren, H. & Prasad, P. N. “Intense visible and near-infrared upconversion photoluminescence in colloidal LiYF4:Er3 + nanocrystals under excitation at 1490 nm”. ACS Nano 5(6), 4981–4986 (2011).
Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 104, 139–174 (2004).
Dong, H., Sun, L. D. & Yan, C. H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 44, 1608–1634 (2015).
Wang, F. & Liu, X. G. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 38, 976–989 (2009).
Liang, H. J., Chen, G. Y., Liu, H. C. & Zhang, Z. G. Ultraviolet upconversion luminescence enhancement in Yb3+/Er3+-codoped Y2O3 nanocrystals induced by tridoping with Li+ ions. J. Lumin. 129, 197–202 (2009).
Xiang, G. T., Zhang, J. H., Hao, Z. D. & Zhao, H. F. Transition to cubic phase and enhancement of green upconversion emission by adding La3+ ions in hexagonal NaLuF4:Yb3+/Er3+ nanocrystals. Cryst. Eng. Comm. 16, 2499–2507 (2014).
Burns, G. Shielding and Crystal Fields at Rare-Earth Ions. Phys. Rev. 128, 2121 (1962).
Xu, W., Zhang, Z. G. & Cao, W. X. Excellent optical thermometry based on short-wavelength upconversion emissions in Er3+∕Yb3+ codoped CaWO4. Opt. Lett. 37, 4865–4867 (2012).
Xu, W., Gao, X. Y., Zheng, L. J., Wang, P., Zhang, Z. G. & Cao, W. X. Optical Thermometry through Green Upconversion Emissions in Er3+/Yb3+-Codoped CaWO4 Phosphor. Appl. Phys. Express 5, 072201 (2012).
Li, L. P., Zheng, L. J., Xu, W., Liang, Z., Zhou, Y., Zhang, Z. G. & Cao, W. X. Optical thermometry based on the red upconversion fluorescence of Er3+ in CaWO4:Yb3+/Er3+ polycrystalline powder. Opt. Lett. 41, 1458–1461 (2016).
Hazen, R. M., Finger, L. W. & Mariathasan, J. W. E. High-pressure crystal chemistry of scheelite-type tungstates and molybdates. J. Phys. Chem. Solids 46, 253–263 (1985).
Wang, X. F., Bu, Y. Y., Xiao, Y., Kan, C. X., Lu, D. & Yan, X. H. Size and shape modifications, phase transition, and enhanced luminescence of fluoride nanocrystals induced by doping. J. Mater. Chem. C 1, 3158–3166 (2013).
Liu, G. K. & Chen, X. Y. Chapter 233 Spectroscopic properties of lanthanides in nanomaterials. Handbook on the Physics and Chemistry of Rare Earths 37, 99–169 (2007).
Suyver, J. F., Aebischer, A., García-Revilla, S., Gerner, P. & Güdel, H. U. Anomalous power dependence of sensitized upconversion luminescence. Phys. Rev. B 71, 125123 (2005).
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC) (11404171, 11374162, 51651202), the Six Categories of Summit Talents of Jiangsu Province of China (2014-XCL-021), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013RA1A2009154).
Author information
Authors and Affiliations
Contributions
X.W., X.Y., and H.S. developed the idea and supervised the project. Y.W. did all the synthetic experiments and performed measurements. Y.B., J.W., P.C., and T.V. analyzed the structure and spectra properties. All authors discussed the results and contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, X., Wang, Y., Bu, Y. et al. Influence of Doping and Excitation Powers on Optical Thermometry in Yb3+-Er3+ doped CaWO4. Sci Rep 7, 43383 (2017). https://doi.org/10.1038/srep43383
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43383
This article is cited by
-
Up-conversion luminescence performance of Yb3+, Er3+/Ho3+ co-doped Y2W3O12 phosphors
Journal of Materials Science: Materials in Electronics (2023)
-
Novel YBO3 Phosphors Doped with Ln3+ (Ce3+, Tb3+, Eu3+) Ions with Tunable Color for WLED Applications
Electronic Materials Letters (2022)
-
Rare-earth (Gd3+,Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging
Scientific Reports (2019)
-
Near infrared emission properties of Er doped cubic sesquioxides in the second/third biological windows
Scientific Reports (2018)
-
Improving luminescence properties of submicron-sized spherical di-strontium magnesium silicate phosphors through morphology control
Journal of Materials Science: Materials in Electronics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.