Abstract
Here, this study reports single-band red upconversion emission in β-Ba2ScAlO5: Yb3+/Er3+ phosphor by doping Mn2+. The optimum concentration of Mn2+ ions in β-Ba2ScAlO5: Yb3+/Er3+ phosphor was 0.20. The intensity of red and green emissions is increased by 27.4 and 19.3 times, respectively. Compared with the samples without Mn2+ ions, the red-green integral strength ratio of β-Ba2ScAlO5: Yb3+/Er3+/Mn2+ sample was significantly increased by 28.4 times, reaching 110.9. The UCL mechanism was explored by analyzing the down-conversion luminescence spectra, absorption spectra, UCL spectra, and upconversion fluorescence lifetime decay curves of Yb3+/Er3+/Mn2+ co-doped β-Ba2ScAlO5. The enhancement of upconversion red light is achieved through energy transfer between defect bands and Er3+ ions, as well as energy transfer between Mn2+ ions and Er3+ ions. In addition, the Mn2+ doped β-Ba2ScAlO5: Yb3+/Er3+ red UCL phosphors have great potential for ambient temperature sensing in the 298–523 K temperature range. The maximum sensitivity of β-Ba2ScAlO5: Yb3+/Er3+/Mn2+ phosphor as a temperature sensor at 523 K is 0.0247 K−1.
Similar content being viewed by others
Introduction
In recent years, rare-earth-doped upconversion luminescence (UCL) materials have been widely used in optical anticounterfeiting, optical temperature measurement, and illumination display due to their stable physicochemical properties, low toxicity, and long fluorescence lifetime1,2,3,4,5,6. At present, the UCL efficiency is still one of the focuses of research, but the single-color UCL has gradually become one of the focuses of research at this stage, especially to achieve the single UCL of red and near-infrared light7,8. In the UCL process, rare-earth Yb3+ ions are widely used as sensitizers due to their strong absorption cross section near 980 nm. Meanwhile, Er3+ ions have abundant step energy states that can be coupled with sensitizers and become commonly used as activators9,10. Upconversion materials co-doped with Yb3+ and Er3+ ions tend to emit strong green light and relatively weak red light11. Unfortunately, the poor penetration of green luminescence into biological tissues limits the biomedical applications of this class of materials, whereas light located in the red to near-infrared wavelength band (650–1350 nm) has a strong biological tissue penetration capability12,13. Therefore, red UCL materials are very interesting for optical temperature measurement, bio-imaging, and medical diagnostics14,15,16. Optical temperature measurement technology, due to its non-contact measurement, fast response and high sensitivity, has shown great application potential in daily life, medical treatment, scientific research and other fields17,18. Optical temperature sensing is a technology to obtain temperature information by measuring the change of optical properties of objects, including fluorescence intensity, fluorescence ratio and decay lifetime19,20. The single emission temperature measurement is based on the change of the relationship between a certain luminous intensity and temperature in the luminous center of the material. The application of upconversion green emission luminescence technology in optical temperature measurement has been studied, but the penetration of green light in complex environments and biological tissues is poor21,22. Therefore, it is urgent to develop upconversion red light emission materials for optical temperature measurement.
Various techniques have been employed to modulate the UCL performance and single-colour UCL, such as substrate selection, rare-earth ion doping type, concentration, crystal field modulation, surface plasmon, and doping of transition metals23,24,25,26,27. Wang et al. synthesized a novel K3(Y0.88Yb0.10Er0.02)Si2O7 phosphor, which enhanced the UCL intensity and thus increased the absolute and relative sensitivity28. Lin and colleagues designed a novel spindle probe with an adjustable aspect ratio for mitochondrial imaging and coated gold nanoparticles (SPS@Au) layer by layer on the surface of the probe, which has the property of enhancing the red UCL and can be used for synergistic immune-photodynamic anticancer therapy29. Bai et al. gave the CeO2: Yb/Er samples stronger UCL by doping transition metal Fe3+ ions, which enhanced the photoelectric conversion efficiency30. In addition, Bi et al. achieved single-band red light emission and improved the red to green ratio (R/G) by adjusting the Mn2+ content in NaLnF4: Er/Mn31. Recently, we found that a new β-Ba2ScAlO5: 0.3Yb3+/0.03Er3+ (BASO: Y/E) phosphor could achieve red upconversion emission32. However, the upconversion red emission intensity of BASO: Y/E phosphors is still low and cannot satisfy the optical temperature measurement. Therefore, we need to further increase the intensity of red light emission to meet current needs.
This study achieved an intense single-band red UCL by doping Mn2+ in BASO: Y/E samples. The study results show that the red emission was enhanced in the optimum sample of 0.20 Mn2+, and the red to green integral intensity ratio reaches a high value of 110.9. The UCL mechanism was explored by analyzing the down-conversion luminescence spectra, absorption spectra, UCL spectra, and upconversion fluorescence lifetime decay curves of β-Ba2ScAlO5: Yb3+/Er3+/Mn2+ (BSAO: Y/E/M) phosphors. The enhancement of upconversion red light is achieved through energy transfer between defect bands and Er3+ ions, as well as energy transfer between Mn2+ ions and Er3+ ions. The BSAO: Y/E/M phosphors have a wide range of applications in optical temperature measurement.
Experimental
Materials
BSAO: Y/E/M phosphors were synthesized using a solid-phase method at high temperatures32. Primary raw materials, including high-purity BaCO3, Sc2O3, Al(OH)3, Mn2O3, Yb2O3, and Er2O3, were directly purchased from Aladdin Corporation without additional refinement.
Synthesis
Unprocessed materials (BaCO3, Sc2O3, Al(OH)3, Mn2O3, Yb2O3, and Er2O3) were weighed in a beaker according to stoichiometry. The mixture was then placed in a blender with anhydrous ethanol and stirred for 1 h to create a well-mixed suspension. After standing at ambient temperature for 2 h and incubating at 70 °C for 24 h, the dried raw materials were ground in an agate bowl and pressed into tablets. The samples were subjected to a high-temperature reaction furnace: temperature was raised to 900 °C for 30 min, then to 1700 °C for 1.5 h33,34. Finally, the temperature was lowered to ambient temperature. The samples were ground in an agate bowl to obtain BSAO: Y/E/M phosphors.
Instruments
X-ray diffractograms of prepared compounds were obtained using a diffractometer (BRUKER D8 ADVANCED, MA, Germany, Cu target Kα radiation, λ = 1.54184 Å) in the 2θ range of 8° to 75° at steps of 0.02°. Shape and size of prepared materials were examined using a Japan JEOL JSM6701F field emission scanning electron microscope (FESEM). Elemental composition was analyzed using a JEOL JED-2300 energy dispersive spectrometer (EDS) attached to a FESEM. A fluorescence spectrophotometer (Acton Spectra Pro 2300i) with a 980 nm near-infrared laser emitter was used to test the 300–800 nm spectral range by Princeton Instruments, USA. The absorption spectrum was characterized using the Lambda 750 absorption spectrometer by Perkin Elmer, USA35,36.
Results and discussion
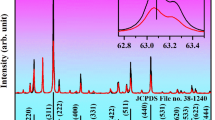
Figure 1a shows the XRD diffraction spectra of BASO: Y/E phosphor doped with different concentrations of xMn2+ (x = 0.05, 0.10, 0.15, 0.20 and 0.25) and β-Ba2ScAlO5 standard card (JCPDS No. 43-0078). The diffraction peaks of all samples agree with the diffraction pattern of pure phase β-Ba2ScAlO5 of JCPDS No. 43–0078 [space group P63/mmc (194)]37. This result confirms the effective doping of Mn2+, Yb3+ and Er3+ ions in β-Ba2ScAlO5 phosphor. All the diffraction peaks of the XRD of the samples were shifted to a high angle with increasing Mn2+ ion doping concentration (see Fig. 1b). Rietica program was used to perform Rietveld refinement calculations on cell parameters of BSAO: Y/E/M, and the results are shown in Fig. 1c,d. The unit parameters and atomic coordinates after refinement are shown in Table 1. The results show that the ionic radius of Mn2+ (0.67 Å, CN = 4) is smaller than that of Ba2+ (1.34 Å, CN = 4), and the parameters a and c decrease with the increase of Mn2+ content. The refinement of atomic coordinates and position occupation reveal the changes of internal structure of Mn2+ doped crystals: (1) Mn2+ ions occupy Ba2+ 2a, 4f. and Al3+ 4e sites simultaneously; (2) The oxygen vacancy content of the doped Mn2+ sample was 6.2%, which was significantly higher than that of the undoped Mn2+ sample (1.3%). Structural changes usually result in the optical properties of the material33,34.
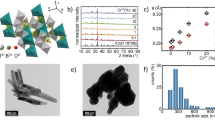
The morphology and elemental composition of the BSAO: Y/E/0.2 M sample were characterized using FESEM and EDS. The mass fractions of Ba, Mn, Sc, Al, O, Yb and Er elements are very close to the theoretical values of 58.38%, 5.06%, 6.94%, 6.17%, 18.37%, 11.92%, and 1.16%, respectively, in Fig. 2a. The EDS spectra show that they are uniformly distributed in the phosphor in Fig. 2b–i. The results show that Yb3+, Er3+ and Mn2+ ions were successfully doped into the β-Ba2ScAlO5 lattice, reducing concentration quenching and improving UCL efficiency.
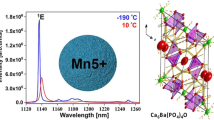
Figure 3 shows the UCL spectra of the BSAO: Y/E/xM (x = 0–0.25) phosphor under 980 nm laser excitation. Weak green and strong red emission peaks appeared at 530 nm, 566 nm and 664 nm, respectively. The green (530 nm and 566 nm) and red (664 nm) upconversion emission of BSAO: Y/E/M samples corresponds to the radiative transitions of the Er3+ energy levels 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2, respectively. The increase of Mn2+ concentration enhances the upconversion red emission intensity of the sample. With the increase of Mn2+ concentration, the luminous intensity of the upconversion red emission was first enhanced and then weakened. When the Mn2+ concentration was 0.20, the red emission reached the strongest. The intensity of red and green emissions is increased by 27.4 and 19.3 times, respectively. At a doping concentration of 0.20, the ratio reaches 110.9, an increase of 28.4 compared to the undoped Mn2+ sample, which has a ratio of 82.532. A single red upconversion emission was achieved for BSAO: Y/E/0.20 M.
In order to explore the luminescence mechanism of BSAO: Y/E/xM (x = 0–0.25) phosphor, the power dependence of BSAO: Y/E/0.2 M under 980 nm laser excitation was investigated. In Fig. 4a, the upconversion red-green luminescence intensity of BSAO: Y/E/0.2 M phosphor is enhanced with increased excitation power. Meanwhile, the mathematical relationship between the UCL intensity (I) and the pump power (P) is given by38: I ∝ Pn. The exponent n denotes the number of photons required for the upconversion process. The double logarithmic coordinate plots from the above equation are shown in Fig. 4b, and the slopes of the fitted curves indicate that the quantum numbers n required for UCL at 664 nm and 566 nm are 1.52 and 1.71, respectively. The n values are close to 2, which suggests that the 4S3/2 and 4F9/2 energy levels of the Er3+ ion are involved in the two-photon process. The numerical coefficients n associated with the red and green UCL of BSAO: Y/E are 1.46 and 1.57, respectively32. Doping of the Mn2+ ion increases the number of photons emitted by red light from 1.46 to 1.52 and that emitted by green light from 1.57 to 1.71. Interestingly, although the number of photons increases in both cases, the number of photons for the green light is significantly larger than that for the red light, suggesting that the doping of Mn2+ ions may have changed the UCL mechanism39,40.
In order to investigate the upconversion red luminescence enhancement mechanism of BSAO: Y/E/M phosphor, its absorption spectra were characterized as shown in Fig. 5. Figure 5a shows the absorption spectra of BSAO: Y/E/xM (x = 0, 0.2). The two samples have six identical absorption bands at the same location: one broad band (main peak at 300 nm) and five peaks (380, 488, 530, 664, and 980 nm). The absorption band near 300 nm is the intrinsic absorption of the Ba2ScAlO5 main matrix. The absorption peaks at 488, 530 and 664 nm are the characteristic absorption of the rare-earth Er3+ ions. The absorption peak at 980 nm is from the rare-earth Yb3+ ion. The absorption peak at 380 nm is from the Yb2+ ion. The change in the valence state of the Yb ion from + 3 to + 2 results in a decrease in the number of positive charges in the crystal41. Therefore, the increase in oxygen vacancies is necessary to maintain the electroneutrality of the material42. Furthermore, a broad absorption band from 470 to 820 nm is observed in BSAO: Y/E/M doped with 0.2 Mn2+ ions compared to the undoped Mn2+ sample. The center wavelength is about 640 nm. The broad absorption band ranging from 470 to 820 nm is due to oxygen vacancy defects caused by doping with 0.2 Mn2+. Figure 5b shows the correspondence between (αhυ)2 and hυ for BSAO: Y/E/xM (x = 0, 0.2) samples. The calculated results show that the band gap of Mn2+ doped is 3.55 eV, and that of undoped Mn2+ is 3.57 eV43,44,45. Therefore, the band gap can be reduced by doping Mn2+ ions, which confirms the above conclusion.
The upconversion excitation processes mainly include multiphonon relaxation (MPR), ground state absorption (GSA), and energy transfer (ET) processes46. Figure 6a shows the conversion mechanism of BSAO: Y/E/M phosphor, demonstrating its energy transfer paths. Based on a previous report, we demonstrated that the main excitation pathway for the red upconversion emission is GSA + MPR + ET132. In the BSAO: Y/E/0.2 M phosphor, the broad absorption band from 470 to 820 nm is due to oxygen vacancy defects. As shown in Fig. 6a, the defect bands coincide precisely with the emission levels in red (4F9/2) and green (2H11/2 and 4S3/2), which leads to the energy transfer process between the defect bands and the corresponding energy levels of Er3+ ions (4F7/2, 4F9/2, 2H11/2 and 4S3/2). The first energy transfer pathway for enhanced UCL: the energy of the 4F7/2 energy level of Er3+ is transferred to the defect band through energy transfer, and then the defect band is transferred to the 2H11/2, 4S3/2, and 4F9/2 energy levels through energy transfer to emit the corresponding green (530 and 566 nm) and red (664 nm) light. Since the 4F9/2 energy level is located at the edge of the absorption band, the defect band transfers more energy to the 4F9/2 energy level47. This finding is consistent with the significant enhancement of red light in the UCL spectrum (Fig. 3). The second energy transfer pathway for enhanced UCL: the non-radiative energy transfer of Er3+ to Mn2+ (2H 11/2/4S3/2 → 4T1), followed by the back energy transfer to Er3+ (4T1 → 4F9/2)48. As the content of Mn2+ increases, the weak green radiation of Er3+ hardly changes, while the red radiation is greatly enhanced, indicating that the energy transfer between Er3+ and Mn2+ is very efficient. As a result, higher R/G ratios can be achieved.
Figure 6b shows the down-conversion luminescence spectra of BSAO: Y/E and BSAO: Y/E/0.2 M. A broad emission band centered at 485 nm is observed for both samples beside the characteristic emission peaks of rare earth Yb3+ and Er3+. This emission band corresponds to the conduction band to the valence band leap of the two samples. As can be seen from the black line in Fig. 6b, the BSAO: Y/E phosphor exhibits a strong green emission under 365 nm excitation with a green–red ratio of 2.97. After Mn2+ doping, BSAO: Y/E/0.2 M has a green emission peak and a red emission peak [red line in Fig. 6b] with a green–red ratio of 0.95. Under 365 nm laser excitation, BSAO: Y/E/0.2 M directly leaps the energy of Er3+ in the 4F7/2 energy level to the 2H11/2, 4S3/2, and 4F9/2 excited states via MPR. The emission of red light indicates that more photons relax to the 4F9/2 excited state, while very few photons go to the 2H11/2 and 4S3/2 energy levels, so the red light is stronger and the green light is weaker. The BSAO: Y/E shows red light under 980 nm excitation with a red-green ratio of about 82.532. For the BSAO: Y/E/0.2 M sample, red emissions were enhanced under 980 nm laser excitation, and a high R/G ratio of 110.9 was observed. This shows that the upconversion emission excitation path of BSAO: Y/E/0.2 M does not change compared with BSAO: Y/E, only two energy transfer paths are added to increase the number of electrons at the luminous level, thus enhancing the upconversion emission (Fig. 6a).
To further understand the effect of Mn2+ doping on the upconversion of BSAO: Y/E, Fig. 7a,b shows the upconversion green (566 nm) and red (664 nm) decay curves of BSAO: Y/E/xM (x = 0, 0.2) fluorescent material. BSAO: Y/E/xM (x = 0, 0.2) decay curves for both red (4F9/2 → 4I15/2) and green (4S3/2 → 4I15/2) light show biexponential decay curves, which are in good agreement with the second-order exponential decay mode. Since the curves exhibit double-exponential decay characteristics, the average fluorescence decay lifetime can be determined by the following equation49: \(\tau =({A}_{1}{\tau }_{1}^{2}+{A}_{2}{\tau }_{2}^{2})/({A}_{1}{\tau }_{1}+{A}_{2}{\tau }_{2})\). A1 and A2 are constants, τ represents the decay time, and τ1 and τ2 represent the fast and slow exponential components. As can be seen from Fig. 7a,b, the fluorescence decay lifetime of 0.2Mn2+ ions doped with BSAO: Y/E is longer than that of BSAO: Y/E. The results show that the doping of Mn2+ increases the population number of Er3+ excited states. It is confirmed that the energy of the oxygen-vacancy induced defect band in the BSAO: Y/E/M phosphor is transferred to the excited state of the Er3+ ion50,51. Thus, the number of electrons in Er3+ ion transition from excited state to ground state is increased, and the UCL is enhanced.
BSAO: Y/E/0.2 M phosphor has a strong red UCL, making it an ideal candidate for single-parameter temperature sensors. In order to investigate the temperature sensitivity of BSAO: Y/E/0.2 M samples, the temperature dependence of the red UCL of the samples under 980 nm laser irradiation in the temperature range of 298–523 K is measured in Fig. 8a. The intensity of the red UCL decreases with increasing temperature. Figure 8b shows the temperature dependence of the red UCL integral intensity for BSAO: Y/E/0.2 M, where T and I664 are the absolute temperature and red integral intensity of the BSAO: Y/E/0.2 M samples, respectively, which can be fitted with T = 543.8−I664/53.4. The sensitivity (S) of the material is generally defined as S = (∂I664/∂T)/I66452. The maximum sensitivity of BSAO: Y/E/0.2 M phosphor as a temperature sensor at 523 K is 0.0247 K−1. The Mn2+ doped BSAO: Y/E red UCL phosphor has excellent potential for temperature sensing and can be used for practical non-contact sensing applications in harsh environments.
Conclusion
This study successfully employed the solid-phase reaction strategy to synthesize a triple-doped BSAO: Y/E/M phosphor. Optimally doped with 0.20 Mn2+ ions, the sample showed a single upconversion red emission with a red-to-green integrated intensity ratio of 110.9, a 28.4-fold enhancement over the undoped BSAO: Y/E phosphor with Mn2+ ions. The intensity of red and green emissions is increased by 27.4 and 19.3 times, respectively. There are two pathways for red light enhancement. The first enhancement pathway: the energy of the 4F7/2 energy level of Er3+ is transferred to the defect band through energy transfer, and then the defect band is transferred to the 2H11/2, 4S3/2, and 4F9/2 energy levels through energy transfer to emit the corresponding green (530 and 566 nm) and red (664 nm) light. The second enhancement pathway: the non-radiative energy transfer of Er3+ to Mn2+ (2H 11/2/4S3/2 → 4T1), followed by the back energy transfer to Er3+ (4T1 → 4F9/2). The maximum sensitivity of BSAO: Y/E/0.2 M phosphor as a temperature sensor at 523 K is 0.0247 K−1. The BSAO: Y/E/M phosphor has a wide range of applications in optical thermometry.
Data availability
All data generated or analysed during this study are included in this published article.
References
Yang, J. et al. Site-selective occupancy of Mn2+ enabling adjustable red/near-infrared multimode luminescence in olivine for dynamic anticounterfeiting and encryption. ACS Appl. Electron. Mater. 4(2), 831–841 (2022).
Yan, B. et al. Red-tunable LuAG garnet phosphors via Eu3+→ Mn4+ energy transfer for optical thermometry sensor application. Inorgan. Chem. Front. 8(3), 746–757 (2021).
Rajendran, M. & Vaidyanathan, S. High performance red/deep-red emitting phosphors for white LEDs. New J. Chem. 44(14), 5354–5365 (2020).
Wang, S. et al. Sensitive Ho3+, Yb3+ co-doped mixed sesquioxide single crystal fibers thermometry based on upconversion luminescence. J. Alloy. Compd. 891, 162062 (2022).
Bai, Y. et al. Low toxicity, high resolution, and red tissue imaging in the vivo of Yb/Tm/GZO@SiO2 core-shell upconversion nanoparticles. ACS Omega 5(10), 5346–5355 (2020).
Sun, G. et al. Lanthanide upconversion and downshifting luminescence for biomolecules detection. Nanoscale Horizons 6(10), 766–780 (2021).
Zhu, Y. et al. Stable and efficient upconversion single red emission from CsPbI3 perovskite quantum dots triggered by upconversion nanoparticles. Inorgan. Chem. 60(4), 2649–2655 (2021).
Liu, Y. et al. Highly efficient upconversion single red emission of hollow cubic α-NaErF4 nanoparticles by Mn/Yb heavy doping. J. Lumin. 228, 117637 (2020).
Cheng, X. et al. Recent development in sensitizers for lanthanide-doped upconversion luminescence. Chem. Rev. 122(21), 15998–16050 (2022).
Chen, T. et al. Activators confined upconversion nanoprobe with near-unity forster resonance energy transfer efficiency for ultrasensitive detection. ACS Appl. Mater. Interfaces 14(17), 19826–19835 (2022).
Voiculescu, A. M. et al. Optical thermometry through infrared excited green upconversion emissions of Er3+-Yb3+ co-doped LaAlO3 phosphors. J. Lumin. 242, 118602 (2022).
Zhang, J. X. et al. Upconversion red emission and near-infrared quantum-cutting persistent luminescence of Nd3+-activated Ca2SnO4 induced by Yb3+. J. Phys. Chem. C 124(36), 19774–19780 (2020).
He, X. et al. Enhanced red and near-infrared upconversion luminescence properties in CaSc2O4 microcrystals. Chem. Phys. Lett. 749, 137425 (2020).
Xiang, G. et al. Deep-tissue temperature sensing realized in BaY2O4: Yb3+/Er3+ with ultrahigh sensitivity and extremely intense red upconversion luminescence. Inorgan. Chem. 59(15), 11054–11060 (2020).
Li, W. et al. Enhanced red up-conversion of β-NaYF4: Er3+, Tm3+ microcrystals for bio-imaging applications. J. Alloys Compd. 926, 166743 (2022).
Zhou, J. et al. Ratio-adjustable upconversion luminescence nanoprobe for ultrasensitive in vitro diagnostics. Anal. Chem. 93(27), 9299–9303 (2021).
Cao, L. et al. Enhanced up-conversion luminescence and temperature sensing performance of NaBiF4: Er3+, Yb3+, Al3+. J. Lumin. 270, 120556. https://doi.org/10.1016/j.jlumin.2024.120556 (2024).
Guo, J. et al. A multi-mode optical thermometry based on the up-conversion La2MgGeO6: Er3+, Yb3+ phosphor. J. Lumin. 266, 120331 (2024).
Mukhopadhyay, L. & Rai, V. K. Colloidal stability and optical thermometry in mesoporous silica coated phosphate based upconverting nanoparticles. J. Alloys Compd. 878, 160351 (2021).
Zheng, W. et al. Low power high purity red upconversion emission and multiple temperature sensing behaviors in Yb3+, Er3+ codoped Gd2O3 porous nanorods. ACS Sustain. Chem. Eng. 8(25), 9578–9588 (2020).
Uji, M. et al. Visible-to-UV photon upconversion: Recent progress in new materials and applications. Angew. Chem. 135(25), e202301506 (2023).
Tong, Y. et al. Na2YMg2(VO4)3: Er3+, Yb3+ phosphors: up-conversion and optical thermometry. Ceram. Int. 47(2), 2600–2606 (2021).
Zhou, L. et al. Ultralong-lived up-conversional room-temperature afterglow materials with a polyvinyl alcohol substrate. Polymers 14(12), 2414 (2022).
Zhao, T. et al. New perspectives to trigger and modulate circularly polarized luminescence of complex and aggregated systems: Energy transfer, photon upconversion, charge transfer, and organic radical. Accounts Chem. Res. 53(7), 1279–1292 (2020).
Mohanty, S. & Kaczmarek, A. M. Unravelling the benefits of transition-metal-co-doping in lanthanide upconversion nanoparticles. Chem. Soc. Rev. 51(16), 6893–6908. https://doi.org/10.1039/D2CS00495J (2022).
Bi, W. et al. Dye sensitization and local surface plasmon resonance-enhanced upconversion luminescence for efficient perovskite solar cells. ACS Appl. Mater. Interfaces 12(22), 24737–24746 (2020).
Torres-García, S. et al. Tailoring luminescent patterns with rare-earth photonic materials for anti-counterfeiting applications: A lightkey. Ceram. Int. 49(14), 24390–24394 (2023).
Wang, J. et al. A novel K3(Y0.88Yb0.10Er0.02)Si2O7 silicate phosphor for multi-mode thermometry of high sensitivity through up-conversion luminescence. Ceram. Int. 49(16), 27464–27472. https://doi.org/10.1016/j.ceramint.2023.06.020 (2023).
Lin, B. et al. Enhanced upconversion luminescence-guided synergistic antitumor therapy based on photodynamic therapy and immune checkpoint blockade. Chem. Mater. 32(11), 4627–4640 (2020).
Bai, J. et al. Upconversion luminescence enhancement by Fe3+ doping in CeO2: Yb/Er nanomaterials and their application in dye-sensitized solar cells. RSC Adv. 10(32), 18868–18874 (2020).
Bi, S. et al. NIR-II responsive upconversion nanoprobe with simultaneously enhanced single-band red luminescence and phase/size control for bioimaging and photodynamic therapy. Adv. Mater. 35(7), 2207038 (2023).
Hu, J. et al. Strong red upconversion luminescence and optical thermometry of Yb3+/Er3+ Co-doped β-Ba2ScAlO5 phosphor. J. Alloys Compd. 895, 162692 (2022).
Hu, J. et al. Single red upconversion luminescence in β-Ba2ScAlO5: Yb3+/Er3+ phosphor assisted by Ce3+ ions. J. Lumin. 246, 118832 (2022).
Hu, J. et al. Enhanced upconversion luminescence and single-band red emission in β-Ba2ScAlO5: Yb3+/Er3+ phosphor through doping Cu2+. J. Lumin. 263, 120053 (2023).
Hu, J. et al. Intense red upconversion luminescence and optical thermometry of a novel Yb3+/Er3+ co-doped Ba3Sc2WO9 phosphor. Mater. Res. Bull. 171, 112633 (2024).
Hu, J. et al. Giant enhancement in upconversion luminescence of β-Ba2ScAlO5: Yb3+/Er3+ phosphor by the intermediate band through Ca2+ doping. Chem. Mater. 34(7), 3089–3098 (2022).
Rath, M. & Müller-Buschbaum, H. Ein Beitrag zur Kristallstruktur von Ba2ScAlO5 und Sr2Sc0.5Al1.5O5. J. Alloys Compd. 189(1), 127–130 (1992).
Liao, J. et al. Helix shape power-dependent properties of single upconversion nanoparticles. J. Phys. Chem. Lett. 11(8), 2883–2890 (2020).
Saidi, K., Chaabani, W. & Dammak, M. Highly sensitive optical temperature sensing based on pump-power-dependent upconversion luminescence in LiZnPO4: Yb3+–Er3+/Ho3+ phosphors. RSC Adv. 11(49), 30926–30936 (2021).
Wen, S. et al. Power-dependent optimal concentrations of Tm3+ and Yb3+ in upconversion nanoparticles. J. Phys. Chem. Lett. 13(23), 5316–5323 (2022).
Verma, R. K. et al. Up and down conversion fluorescence studies on combustion synthesized Yb3+/Yb2+: MO-Al2O3 (M= Ca, Sr and Ba) phosphors. J. Lumin. 130(7), 1248–1253 (2010).
Mantella, V. et al. Synthesis and size-dependent optical properties of intermediate band gap Cu3VS4 nanocrystals. Chem. Mater. 31(2), 532–540 (2018).
Dimitriev, O. & Slominskii, Y. Impact of the thermal properties of the environment on the hot-band absorption-assisted single-photon upconversion. Phys. Chem. Chem. Phys. 24(44), 27423–27431 (2022).
Liu, Y. et al. Promising lanthanide-doped BiVO4 phosphors for highly efficient upconversion luminescence and temperature sensing. Dalton Trans. 50(3), 960–969 (2021).
Qian, B. et al. Comparative study on the morphology, growth mechanism and luminescence property of RE2O2S: Eu3+ (RE= Lu, Gd, Y) phosphors. J. Alloys Compd. 870, 159273 (2021).
Trejgis, K., Bednarkiewicz, A. & Marciniak, L. Engineering excited state absorption based nanothermometry for temperature sensing and imaging. Nanoscale 12(7), 4667–4675 (2020).
Prasad, M. & Rai, V. K. Photon avalanche assisted upconversion via customizing the green emission. RSC Adv. 13(29), 20342–20350 (2023).
Zeng, S. et al. Simultaneous realization of phase/size manipulation, upconversion luminescence enhancement, and blood vessel imaging in multifunctional nanoprobes through transition metal Mn2+ doping. Adv. Funct. Mater. 24(26), 4051–4059 (2014).
Zhang, J., Chen, J. & Jin, C. The effect of dopant concentration and pump power on sensitivities of optical thermometry in LiLa9Si6O26: Yb3+-Er3+/Ho3+ phosphors based on upconversion luminescence. J. Alloys Compd. 846, 156397 (2020).
Zhu, M., Hao, H. & Li, L. Effect of excitation condition and Mn2+ doping on the red-to-green emission ratio in NaYF4: Er3+/Yb3+ phosphors. J. Mater. Sci. Mater. Electron. 34(10), 869 (2023).
Nayak, P. et al. Yb-Mn dimer tailored upconversion luminescence in CaWO4: Er3+/Yb3+/Mn2+ green phosphors for thermometry and optical heating. Opt. Laser Technol. 159, 108990 (2023).
Cadiau, A. et al. Ratiometric nanothermometer based on an emissive Ln3+-organic framework. ACS Nano 7(8), 7213–7218 (2013).
Acknowledgements
This work was supported by the Natural Science Foundation of Sichuan Province (2023NSFSC0334, 2023NSFSC0909 and 2022NSFSC1947) and Sichuan Provincial Science and Technology Plan Project (2021YJ0518).
Author information
Authors and Affiliations
Contributions
Y.L. is involved in conceptualization, methodology, validation, characterization, formal analysis, investigation, writing – original draft, visualization. B.D. is involved in conceptualization, methodology, characterization, formal analysis, data curation, writing-review and editing. L.Z. is involved in investigation, methodology. Y.W. is involved in investigation, methodology. F.W. is involved in writing – review & editing. C.D. is involved in writing – review & editing, funding acquisition. J.H. is involved in writing – review & editing, conceptualization, methodology, project administration, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Duan, B., Zhou, L. et al. Large enhancement of red upconversion luminescence in beta Ba2Sc0.67Yb0.3Er0.03AlO5 phosphor via Mn2+ ions doping for thermometry. Sci Rep 14, 8893 (2024). https://doi.org/10.1038/s41598-024-59732-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59732-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.