Abstract
In this letter, we reported thorough the structural and molecular dynamics studies on 1,6-anhydro-β-D-glucose, the second compound reported so far that is capable to form rotator and supercooled liquid phases. In contrast to the data presented for ethanol, temperature dependences of structural dynamics in both phases are very comparable. On the other hand, X ray measurements revealed unusually long range ordering/correlations between molecules in the ODIC (d ≈ 95 Å) and supercooled phases (d ≈ 30–40 Å) of this carbohydrate. Our consideration clearly demonstrated that the interplay between length scales of static range ordering and dynamical heterogeneities as well as internal molecular arrangement seem to be the key to understanding the molecular dynamics of different materials characterized by varying degree of disorder in the vicinity of the glass transition temperature.
Similar content being viewed by others
Introduction
The molecular origin of the glass transition phenomenon seems to be one of the most challenging issue of condensed matter physics. The special effort is put to understand the microscopic nature of this peculiar phase transition as well as to explain enormous increase of the viscosity or relaxation times in the close vicinity of the glass transition temperature, Tg, probed by different spectroscopies, including mechanical, dielectric or dynamic light scattering ones1,2,3,4. To get deeper insight into nature of this characteristic feature of supercooled liquids, systematic theoretical and experimental studies on various kinds of materials characterized by different degree of disorder and intermolecular interactions have been performed5,6,7. In this context, it is worthwhile to mention Orientationally Disordered Crystals (ODIC) that seem to be very promising materials, having centers of mass of molecules fixed like in the crystalline lattice. Simultaneously, they are characterized by the orientational disorder. Interestingly, the current studies show that in many aspects plastic crystals behave in the same way as it was reported for the ordinary glass formers8,9,10. Just to mention that both kinds of materials reveal clear deviation from the Arrhenius-like behavior of the temperature dependence of structural relaxation times. Although, a degree of deviation, as quantified by the fragility index, (m), is much smaller in the case of plastic crystals. The best illustration of this relationship is ethanol, the only liquid that can form PC and SL phases at the same thermodynamic conditions. Over the years, this alcohol became a canonical reference material for the discussion of the data measured for other plastic crystals. Moreover, taking into account the behavior of ethanol, low fragility of plastic crystals was related to the reduced density of minima in the potential energy. However, it is worthwhile to recall that there are some controversy concerning assignments of the main structural process observed in this compound. Consequently, previously derived conclusions might be biased.
Very recently Michl et al. have proposed a new, alternative explanation of low fragility of plastic crystals11. They clearly demonstrated that the temperature dependence of the number of correlated molecules (Nc) is much weaker in such materials with respect to the ordinary glasses. What is more, it was also shown that even for the large Nc, the energy barrier for the reorientational motions is pretty low in cyclooctanols, the prototypical ODICs. They rationalized that the lattice strain, which underlined molecular correlations, decreases the effective barrier for the reorientation of the neighboring molecules. However, it should be stressed that authors compared materials differing in chemical structures and intermolecular interactions. That seems to be an important aspect preventing formulation of more general conclusion about the behavior of plastic crystals and ordinary glass formers.
Herein, we report thorough the structural and molecular dynamics studies on 1,6-anhydro-β-D-glucose (levoglucosan), a second compound capable to form ODIC, orientational glass, supercooled liquid and ordinary glass at the same thermodynamic conditions. Herein, it should be mentioned that the molecular dynamics and calorimetric studies on this compound have been reported previously by some of us12, Rocha et al.13 and Tombari et al.14,15. However, the possibility of creation of various degree of disorder by this carbohydrate has not been discussed in any of these articles. Furthermore, the direct comparison of the data obtained for ethanol and levoglucosan provided us an unique opportunity of better understanding a fundamental issue related to the molecular interpretation of the difference between molecular dynamics of materials characterized by various degree of disorder.
X-ray diffraction measurements were carried out for various forms of levoglucosan, namely polycrystalline, plastic crystal, orientational glass, liquids and glass using a laboratory X-ray Rigaku-Denki D/MAX RAPID II-R powder diffractometer attached with a rotating silver anode, an incident beam graphite monochromator and an image plate as two dimensional detector. The experimental set up and the data processing are described in details in refs 16,17. The temperature was controlled using the Oxford Cryostream Plus and Compact Cooler. The dielectric (BDS) measurements were carried out at various temperatures (T = 163 K–298 K), in a frequency range from 10−2 to 106 Hz, using an Alpha spectrometer (Novocontrol). The sample of 99% purity was obtained from Aldrich.
As discussed above, Rocha et al.13 and Tombari et al.14 postulated that 1,6-anhydro-β-D-glucose is a ninth molecule that forms ODIC phase. Interestingly, this state can be created very easily at T = 383 K upon heating of the crystal or cooling of the liquid. However, it was also concluded that it is not possible to obtain structural glass in this carbohydrate. We decided to continue their research and found that after application of very fast cooling (molten sample has been directly immersed in liquid nitrogen) levoglucosan supercools and forms a structural glass. Therefore, it is just a second compound that can be prepared as a crystal, plastic crystal, orientational glass, supercooled liquid and ordinary glass.
In Fig. 1, the diffraction data for all investigated phases of levoglucosan measured at indicated temperatures are shown. It should be mentioned that obtained diffractograms enabled us to identify orthorhombic structure of crystal of commercial levoglucosan. In addition, their pair distribution functions are presented in Fig. 2. According to the literature, the diffraction data for disordered materials are usually presented as the structure factor  , where the scattering vector
, where the scattering vector  , 2θ is the scattering angle, λ is the wavelength,
, 2θ is the scattering angle, λ is the wavelength,  ,
,  , n indicates the number of atomic species in the investigated sample, ci is the concentration and fi is the atomic scattering factor of the ith element, respectively. The diffraction data were then converted to a real space representation in the form of the pair distribution function
, n indicates the number of atomic species in the investigated sample, ci is the concentration and fi is the atomic scattering factor of the ith element, respectively. The diffraction data were then converted to a real space representation in the form of the pair distribution function  , where
, where  indicates the maximum value of Q, achieved in the experiment (here 20 Å−1) and
indicates the maximum value of Q, achieved in the experiment (here 20 Å−1) and  is used in computation in order to minimize truncation oscillations.
is used in computation in order to minimize truncation oscillations.
The comparison of the pair distribution functions, PDF(r), computed from the data displayed in Fig. 1 for the stable levoglucosan crystal at 295 K (a), the plastic phase at 450 K (b), the orientational glass at 230 K (c), the liquid at 470 K (d), and the glass at 230 K (e). The open circles on the right part represent the Fourier transforms of the structure factors computed for the Q-range from 1.0 Å to 1.5 Å.
As shown in Fig. 1, the structure factors of the plastic crystal, orientational glass, liquid and glass samples exhibit almost the same behavior in the Q range from 4 to 20 Å−1. For the levoglucosan crystal, sharp diffraction peaks are superimposed on the broad features seen for the other forms of saccharide. The long-period oscillations seen at high Q-values can be related to intra-molecular correlations in the atomic arrangement leading to very similar features of the pair distribution functions, PDFs, in the range of 0–4.5 Å. These results show that the intra-molecular structure of the levoglucosan molecule remains practically unchanged in the crystal, plastic crystal, orientational glass, liquid and glass states. It is important to note that the PDFs for the plastic crystal and orientational glass phases exhibit oscillatory behavior, which extends to about 100 Å indicating longer range ordering of these forms of 1,6-anhydro-β-D-glucose (see right parts of Fig. 2b and c). On the other hand, the oscillations are completely attenuated for the liquid and glass phases at 30 Å and 40 Å, respectively, as it is shown in Fig. 2d and e. The Fourier transforms of the structure factors, computed from the diffraction data limited to the Q-range from 1.0 to 1.5 Å, are superimposed on the PDFs obtained from the data in the Q-range 0–20 Å, as it is presented in Fig. 2b–e. A very good agreement between both curves indicates that in the case of investigated materials the first diffraction peaks are in a direct relationship with longer range inter-molecular correlations. Additional information about a spatial extent of correlations or coherence length can be obtained from the position of the first diffraction peak Q1 and its width ΔQ1. The quantity 2π/ΔQ1 allows estimation of the coherence length for the plastic crystal and orientational glass to be 95 Å on the average of three peaks, that is in agreement with the plots of the PDFs displayed in Fig. 2b and c. As it can be clearly seen, the beat-like behavior of the PDFs shown in Fig. 2b and c is due to summation of three components with very close frequencies. The coherence lengths obtained for the liquid and glass phases are 30 Å and 39 Å, respectively being also in agreement with decay of the PDFs as plotted in Fig. 2d and c. In this context, one should refer to the structural studies reported for ethanol. In this compound, coherence lengths obtained for supercooled and plastic phases are around 10 A and 25 A, indicating that the range of inter-molecular correlations in ethanol and 1,6-anhydro-β-D-glucose is completely different. Another important difference between ethanol and levoglucosan emerged from structural studies. Previously, it was shown that there is noticeable decrease in orientational correlations during passing from the disordered crystal to the glass and liquid in ethanol18. On the other hand, such pattern of behavior is not observed for levoglucosan.
Furthermore, the molecular dynamics of 1,6-anhydro-β-D-glucose was investigated by means of BDS. In Fig. 3 dielectric loss spectra obtained in the ODIC and supercooled phases of levoglucosan are presented. In both cases, one dominant structural process, governing glass transition phenomenon, shifting towards lower frequencies with decreasing temperature is observed. In the inset to Fig. 3a, we compared the shape of the structural process measured at the same temperature for carbohydrate prepared in the supercooled liquid (SL) and plastic crystal (PC) phases. The α-loss peak of SL sample was shifted horizontally and vertically to superpose at the maximum with the one obtained for PC. As it can be seen, the distribution of the relaxation times is not the same for both samples. It is slightly broader at the high frequency side for the supercooled 1,6-anhydro-β-D-glucose suggesting most likely more heterogeneous dynamics in this system. In this context, one can recall studies on ethanol where the shape of the most dominant process remained unchanged in ODIC and supercooled states. However, it should be stressed that over the years a lot of controversy arose concerning molecular interpretation of this process. Just to mention that the most intense process detected in this alcohol, might be related to the dynamics of H bonds rather than structural process19.
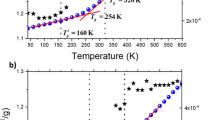
Dielectric loss spectra measured in plastic crystal (a) and supercooled phase (b); Solid lines represent HN fits. In the inset to panel (a) a comparison of the shape of the structural process measured in both phases and is shown. In the inset to panel (b) heat capacity jump at the glass transition temperature obtained from DSC measurements for SL and PC forms of saccharide. The temperature dependence of the structural relaxation times (c), where solid lines are the best VFT fits; The inset presents relaxation times obtained by Benkhof et al.19 for ethanol in different phases. The number of dynamically correlated molecules is plotted vs temperature in panel (d), while the inset demonstrates the temperature evolution of length scale of dynamic heterogeneity ξ in both phases of levoglucosan.
To gain deeper insight into dynamics of PC and SL phases, the measured loss spectra were fitted to Havriliak-Negami (HN) function20. In upper panels of Fig. 3 the representative HN fits (solid lines) to the loss spectra measured at both states of levoglucosan are shown. Next, using well known relationship between τmax and HN parameters21 the former ones have been determined and plotted as a function of inverse temperature in panel (c) to Fig. 3. Dependencies τα(T) were described by the Vogel-Fulcher-Tammann (VFT equation22,23,24. In addition, relaxation times determined for ethanol are also shown in the inset to Fig. 3(c). As observed, there is a remarkable difference in dynamics of supercooled and plastic phases in alcohol and carbohydrate. Just to mention that in the latter compound just slight difference in the structural dynamics of both considered phases, that vanishes upon approaching the glass transition temperature (Tg ≈ 245 K), can be detected. Just to mention that herein Tg was defined as temperature at which τα = 100 s. On the other hand, the dynamics of dominant relaxation process in ethanol is much different in ODIC and supercooled phases. Although similarly as in the case of 1,6-anhydro-β-D-glucose, the transition to the glass and orientational glass occurs at the same temperature Tg = 97 K19. In this context, studies on binary mixtures of succinonitrile with glutaronitrile should be reminded25. In this complex system, the glass transition temperature of SL and PC also occurs at the same temperature emphasizing universality of observation of independency of Tg to the degree of disorder in given material.
Different temperature dependences of the structural relaxation times in ODIC and SL states indicate the difference in the fragility, m, of examined carbohydrate as well as ethanol prepared in both forms. In fact, using the following definition of fragility:

we obtained m to be equal to 92, 102 and 40, 6019 for Orientationally Disordered Crystalline and supercooled phases of levoglucosan and alcohol, respectively. Recently, Michl et al.11 claimed that lower fragility of the plastic crystals can be due to lower number of dynamically correlated molecules as well as to the weaker temperature variation in Nc. To verify this experimental finding and seek for a closer relation between long range ordering (revealed from structural investigations), dynamical heterogeneities and molecular dynamics, we decided to utilize two methods proposed by Berthier et al.26,27,28 and Donth et al.29,30. Briefly, according to the former approach, the approximation of four point susceptibility χ4(t) related to the four point correlation function G4(r, t) can be done with the use of derivative of the two point correlation function. Once KWW function [exp(−(t/τα)βKWW)] is applied to describe Φ(t), the number of molecules involved in correlated motions Nc can be calculated directly from the formula given by Capaccioli et al.31:

where: ΔCp is a difference between heat capacity of the supercooled liquid or plastic crystal phase and glass or orientational glass at given T, kB is a Boltzmann constant and βKWW is a stretched parameter. It should be added that ΔCp (=0.696 J/gK and 0.688 J/gK for SL and PC phase of 1,6-anhydro-β-D-glucose, respectively) were estimated from Differential Scanning Calorimetry measurements (see inset to panel (b) of Fig. 3). Moreover, the last term in equation 2 was calculated from VFT fits (red solid lines) to temperature evolution of structural relaxation times.
The temperature dependences of the number of dynamically correlated molecules are presented in Fig. 3d. As shown, Nc evolves with temperature in a similar way in both phases reaching value 105 ± 12 and 102 ± 12 at the glass transition for the supercooled and ODIC levoglucosan respectively. Hence, the observed difference seems to be within the experimental uncertainty. It should be mentioned that very similar Nc (88 ± 9 and 96 ± 9) were determined for leveglucosan prepared in both phases from Donth approach.
Additionally, we also calculated the size of dynamical heterogeneities, ξ, which provides information about the length scale of dynamically correlated molecules characterized by the same dynamics or relaxation time. For that purpose following equation that links four point dynamic susceptibility χ4(t) (equation 2) with correlation volume has been used26:

where: a (diameter) is equal to 5 Å and d defines the shape of the dynamically correlated regions. One can mention that in literature cooperatively rearranging regions of different compact or fractal geometries are considered32,33,34,35. In the majority of investigations, d varies in the range 2–4. Although, d = 3, which reflects cubic or spherical geometry of the dynamically correlated regions is generally taken into account. Due to this reason we assume d = 3. In the inset to Fig. 3d, the temperature evolution of ξ is presented. It is well seen that ξ ≈ 2 nm at the glass transition temperature for levoglucosan prepared in both SL and PC phases As a complementary, this parameter was also evaluated for ethanol ξ = 1.8 nm at Tg using Nc = 195 molecules at Tg26 and a = 3 Å. Our results indicated unquestionably that in contrast to the conclusion derived by Michl et al.11 Nc as well as ξ evolve in the same manner in PC and SL phases at the same compound. Moreover, taking into account structural and dynamical studies carried out for 1,6-anhydro-β-D-glucose and ethanol, we have an unique opportunity to understand the real molecular mechanism that underlies difference in dynamics of plastic and ordinary glasses appeared. As demonstrated above, the static long range correlations between molecules oscillates around 3–4 nm and 9 nm for supercooled and ODIC states of 1,6-anhydro-β-D-glucose, respectively. What is more, the molecular arrangement in both studied phases is fairly the same. Hence, by simple comparison of length scales of static long range order and dynamical heterogeneities in PC and SL carbohydrate, one can find that the latter quantity is few times lower than the former in both phases. Hence, the length scale of static long range ordering, exceeding significantly ξ, seems to be a major factor controlling molecular dynamics of supercooled and plastic phase in levoglucosan. Completely different situation is noted for ethanol. In this alcohol, the length scale of dynamical heterogeneity is almost twice larger than the static intermolecular long range correlations between molecules. Moreover, the significant decrease in orientational correlations was reported when passing from plastic crystal to supercooled state in this material. Therefore, it can be concluded that not heterogeneity alone but rather interplay between length scales of static long range ordering and dynamical heterogeneities as well as internal molecular arrangements and correlations are the most important factors influencing molecular dynamics of supercooled and orientationally disordered crystal states.
In this letter, the molecular dynamics and structural studies were performed on 1,6-anhydro-β-D-glucose, an unique material that form both supercooled liquid and plastic phases at the same thermodynamic conditions. Our combined investigations indicated that the interplay between length scales of static long range inter-molecular correlations and dynamical heterogeneities as well as internal molecular arrangements determines degree of deviation of molecular dynamics of PC and SL phases. This finding is crucial for better understanding of the relationship between varying disorder, static range correlations and molecular dynamics in various materials in the close vicinity of the glass transition temperature.
Additional Information
How to cite this article: Madejczyk, O. et al. Interplay between the static ordering and dynamical heterogeneities determining the dynamics of rotation and ordinary liquid phases in 1,6-anhydro-β-D-glucose. Sci. Rep. 7, 42103; doi: 10.1038/srep42103 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Adrjanowicz, K. et al. Molecular dynamics of the supercooled pharmaceutical agent posaconazole studied via differential scanning calorimetry and dielectric and mechanical spectroscopies. Mol. Pharm. 10, 3934 (2013).
Comez, L. et al. Light-scattering study of a supercooled epoxy resin. Phys. Rev. E. 60, 3086 (1999)
Schmidtke, B., Petzold, N., Kahlau, R. & Rössler, E. A. Reorientational dynamics in molecular liquids as revealed by dynamic light scattering: from boiling point to glass transition temperature. J. Chem. Phys. 139, 084504 (2013).
Adrjanowicz, K. et al. Communication: Slow supramolecular mode in amine and thiol derivatives of 2-ethyl-1-hexanol revealed by combined dielectric and shear-mechanical studies. J. Chem. Phys. 143, 181102 (2015)
Swiety-Pospiech, A. et al. High pressure study of molecular dynamics of protic ionic liquid lidocaine hydrochloride. J. Chem. Phys. 136, 224501 (2012).
Roland, C. M., Hensel-Bielowka, S., Paluch, M. & Casalini, R. Supercooled dynamics of glass-forming liquids and polymers under hydrostatic pressure. Rep. Prog. Phys. 68, 1405 (2005).
Kaminski, K., Kaminska, E., Paluch, M., Ziolo, J. & Ngai, K. L. The true Johari-Goldstein β-relaxation of monosaccharides. J. Phys. Chem. B. 110, 25045 (2006).
Brand, R., Lunkenheimer, P. & Loidl, A. Relaxation dynamics in plastic crystals. J. Chem. Phys. 116, 10385 (2002).
Ngai, K. L. Relaxation and Diffusion in Complex Systems. Springer. New York (2011).
Winsor, P. A. & Gray, G. W. In Liquid Crystals Plastic Crystals. Wiley. Vol. 1, p. 48. New York (1974).
Michl, M., Bauer, Th., Lunkenheimer, P. & Loidl, A. Cooperativity and Heterogeneity in Plastic Crystals Studied by Nonlinear Dielectric Spectroscopy. Phys. Rev. Lett. 114, 067601 (2015).
Kaminski, K. J. et al. Origin of the commonly observed secondary relaxation process in saccharides. Phys. Chem. B. 114, 11272 (2010).
Rocha, M. et al. Thermodynamics, Thermochemistry, and Thermal Properties. J. Chem. Eng. Data. 58, 1813 (2013).
Tombari, E. & Johari, G. P. Structural fluctuations and orientational glass of levoglucosan-High stability against ordering and absence of structural glass. J. Chem. Phys. 142, 104501 (2015).
Righetti, M. C., Tombari, E. & Johari, G. P. J. Aging kinetics of levoglucosan orientational glass as a rate dispersion process and consequences for the heterogeneous dynamics view. J. Chem. Phys 145, 054501 (2016).
Hawelek, L. et al. The Atomic Scale Structure of Nanographene Platelets Studied by X-Ray Diffraction, High-Resolution Transmission Electron Microscopy and Molecular Dynamics. Diam. Rel. Mat. 35, 40 (2013).
Jurkiewicz, K. et al. Conversion of Natural Tannin to Hydrothermal and Graphene-Like Carbons Studied by Wide-Angle X-ray Scattering. J. Phys. Chem. A. 119, 8692 (2015).
Bermejo, F. et al. Structural correlations in disordered matter: An experimental separation of orientational and positional contributions. Phys. Rev. B. 56, 11536 (1997).
Benkhof, S., Kudlik, A., Blochowicz, T. & Rössler, E. Two glass transitions in ethanol: a comparative dielectric relaxation study of the supercooled liquid and the plastic crystal. J. Phys. Cond Matter. 10, 8155 (1998).
Havriliak, S. & Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. 14, 99 (1966).
Kremer, F. & Schönhals, A. Broadband Dielectric Spectroscopy Springer, Berlin (2002).
Vogel, H. Das Temperaturabhangigkeitsgestz der Viskositat von Flussigkeiten. Physikalische Zeitschrift. 22, 645–646 (1921).
Fulcher, G. J. Analisis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 8, 339–355 (1925).
Tammann, G. & Hesse, W. Die Abhangigkeit der Viscositat von der Temperatur bie unterkuhlten Flussigkeiten. Zeitschrift für anorganische und allgemeine Chemie. 156, 245–257 (1926).
Götz, M., Bauer, Th., Lunkenheimer, P. & Loidl, A. Supercooled-liquid and plastic-crystalline state in succinonitrile- glutaronitrile mixtures. J. Chem. Phys. 140, 094504 (2014).
Berthier, L. et al. Direct experimental evidence of a growing length scale accompanying the glass transition. Science 310, 1797 (2005).
Berthier, L., Biroli, G., Bouchaud, J.-P., Cipeletti, L. & van Saarloos, W. Eds. Dynamical heterogeneities in glasses, colloids and granular media. Oxford University Press, Oxford (2011).
Berthier, L. Trend Dynamic Heterogeneity in Amorphous Materials. Physics. 4, 42 (2011).
Donth, E. Characteristic length of the glass transition. J. Polym. Sci. Part B. 34, 2881 (1996).
Donth, E. The Glass Transition. 1st ed. Springer, Berlin (2001).
Capaccioli, S., Ruocco, G. & Zamponi, F. Dynamically correlated regions and configurational entropy in supercooled liquids. J. Phys. Chem. B. 112, 10652 (2008).
Whitelam, S., Berthier, L. & Garrahan, J. P. Dynamic Criticality in Glass-Forming Liquids. Phys. Rev. Lett. 92, 185705 (2004).
Garrahan, J. P. & Chandler, D. Geometrical Explanation and Scaling of Dynamical Heterogeneities in Glass Forming Systems. Phys. Rev. Lett. 89, 035704 (2002).
Toninelli, C., Wyart, M., Biroli, G., Berthier, L. & Bouchaud, J. P. Dynamical susceptibility of glass formers: Contrasting the predictions of theoretical scenarios. Phys. Rev. E. 71, 041505 (2005).
Biroli, G. & Bouchaud, J. P. Diverging length scale and upper critical dimension in the Mode-Coupling Theory of the glass transition. Europhys. Lett. 67, 21 (2004).
Acknowledgements
K.K. and O.M. acknowledges financial assistance from National Center of Science within OPUS project based on decision DEC-2015/17/B/ST3/01195.
Author information
Authors and Affiliations
Contributions
O.M. co-author of the manuscript, data analysis and measurement. K.K. wrote the main manuscript text and supervised the measurement, data analysis and discussed the results. E.K. wrote the main manuscript text. K.J. measurements with X ray and analysis of data. M.T. data analysis and measurement. A.B. measurements with X ray and analysis of data. M.P. supervised the measurement, data analysis and discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Madejczyk, O., Kaminski, K., Kaminska, E. et al. Interplay between the static ordering and dynamical heterogeneities determining the dynamics of rotation and ordinary liquid phases in 1,6-anhydro-β-D-glucose. Sci Rep 7, 42103 (2017). https://doi.org/10.1038/srep42103
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42103
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.