Abstract
The glass transition of an amorphous material is a fundamental property characterized by an abrupt change in viscosity. Its very knowledge was a conundrum as no satisfying theory existed at the molecular level. We herein relate this complex phenomenon to events occurring at the molecular scale. By studying conformational transitions in the carbon-chain polymer of polyethylene, we clearly establish a relation between local dynamics and the classical dihedral potential energy diagram of a carbon-carbon bond. This methodology is applied to a carbon-chain polymer with a side-group, polystyrene. A direct link is proved between activation energy and glass transition temperature. This work thus provides the cornerstone for linking molecular structure to macroscopic polymer properties, and in particular, the glass transition temperature.
Similar content being viewed by others
Introduction
Numerous systems such as polymers exhibit a glass transition. As the temperature is decreased they turn from an amorphous state of low viscosity to a supercooled liquid with very high viscosity1. This transition is characterized by the glass transition temperature (Tg). Despite being known for a very long time it remains a thrilling domain of research as its very nature is not fully solved2. The main reason is that no experiments or theories can grasp its entire domain of dynamic ranging from nanoseconds to years (ageing). These recent years, molecular simulation became an additional and powerful technique to complement existing data or current theories3. Among the different simulation tools, molecular dynamics simulation is of special interest as it can probe local dynamics. In 1988, Rigby and Roe, in their seminal work, showed that Tg can be extracted for polymers from full-atomistic simulation4. Since then, a huge number of simulations dealing with the glass transition of polymers have been reported5,6,7. However, despite nice linear relationship between simulated and experimental Tg, as our group showed for instance8, the extremely rapid simulated cooling rate, in order of 1011 times more rapid than usual experiments, raise questions on its meaning. We thus propose, in this study, to shed some light on this issue by exploring the energy landscape, a powerful approach to deal with the glass transition. Consequently, a chemical picture of this tricky transition will emerge.
For decades, Tg was defined by the temperature at which the viscosity reaches 1013 poise1,9. Nowadays, Differential Scanning Calorimetry (DSC) remains the most common technique to measure it by detecting change in the heat capacity of the sample occurring at the glass transition10. Dynamical Mechanical Analysis (DMA) is also greatly employed since it measures differences in viscoelasticity with temperature. Regardless the experimental technique, the temperature domain associated with the glass transition is in order of 3 to 5 K10. The most used simulation method to locate Tg is the dilatometry technique4. It consists in reporting the specific volume with respect to the temperature. The intersection between two linear fits at low and high temperatures gives Tg. However, we recently showed that due to the extremely rapid cooling rate, molecular dynamics (MD) simulation leads to a spreading of the glass transition domain, in order of 160 K11. It was shown that the heat capacity and the coefficient of thermal expansion vary gradually between two limit temperatures, the lower \(({T}_{g}^{l})\) and the upper \(({T}_{g}^{u})\) transition temperatures. A comparison was then proposed with ultra-fast camera leading to the so-called overcrancked effect where molecular features should be unveiled. Accordingly, behind the measurement of Tg from MD, the meaning of these two extra temperatures during the glass transition process must also be investigated. To unravel their significance, computing the activation energy (Ea) is a very attracting avenue as it depicts local dynamics12,13. Moreover, it can be related to the energy landscape introduced by Angell14, and developed by Debenedetti and Stillinger15, one of the prevailing current theories to untangle the glass transition mystery.
In the energy landscape picture, a glass is trapped in a specific basin. The height of its barrier is directly related to the glass properties16. However, despite the very interesting outlook it brings, its depiction remains a challenging task. The activation energy corresponds to the energy necessary to go from one potential energy minimum to another inherent structure15. In polymers, it is numerically computed from an Arrhenius plot where frequencies of transitions between different rotameric states are reported with respect to the inverse of temperature13,17,18,19. Nevertheless, the very interpretation of Ea stemming from MD simulation was source of interrogation20. More specifically, an Arrhenius depiction of such relaxation remains surprising. It was suggested to be in relation with the computed potential energy barrier computed for the rotation of a single bond, in addition to be directly linked to Tg20. As pointed out by Boyd and Smith, “The resolution of these questions follows and leads to considerable insight into the nature of glass formation in polymer melts”20. We thus propose to address the relationship between Ea and the temperatures associated with the glass transition, Tg, and the two temperature limits, through a description of a relevant portion of the potential energy landscape15. For this, two polymers are considered, one with the simplest architecture, polyethylene (PE), and one with a side-chain, polystyrene (PS). We then argue that an atomistic representation of the glass transition emerges.
Results and Discussion
To get numerical value of Tg, the simulated dilatometry technique is employed4. The specific volume is reported with respect to the temperature, as it is shown in Fig. 1a and b, for PE and PS, respectively. Values of Tg are 254 K and 469 K for PE and PS, respectively. The actual display of a discontinuity in the linear behavior of the specific volume with respect to the temperature is the sign of a change in the molecular behavior. However, as we recently reported, due to the very rapid cooling rate (in order of 1011 times more rapid that the experimental rate), this discontinuity is spread between two temperatures, a lower \({T}_{g}^{l}\) and upper \({T}_{g}^{u}\), as revealed by the behavior of the thermal expansion coefficient (Fig. 1), the heat capacity4, or the bulk modulus21. It was argued that MD simulation acts as an ultra-fast camera leading to overcranking effects, enabling very slow-motion to be captured. This spread occurs between 160 K and 320 K, for PE (Fig. 1a), and 380 K and 540 K, for PS (Fig. 1b). For both polymers (Fig. 1), no changes in the behavior of the coefficient of thermal expansion are detected at Tg. The local dynamics is then investigated in the whole glass transition domain bounded by \({T}_{g}^{l}\) and \({T}_{g}^{u}\). For this, the activation energy associated with the backbone motion is computed.
Specific volume ( ) and thermal expansion coefficient (★) with respect to the temperature (K) for PE (a), and PS (b). The glass transition domain is limited by vertical dot lines at 160 K \({T}_{g}^{l}\) and 320 K \({T}_{g}^{u}\), and 380 K \({T}_{g}^{l}\) and 540 K \({T}_{g}^{u}\) for PE (a) and PS (b), respectively.
) and thermal expansion coefficient (★) with respect to the temperature (K) for PE (a), and PS (b). The glass transition domain is limited by vertical dot lines at 160 K \({T}_{g}^{l}\) and 320 K \({T}_{g}^{u}\), and 380 K \({T}_{g}^{l}\) and 540 K \({T}_{g}^{u}\) for PE (a) and PS (b), respectively.
To establish the link between Ea extracted from an Arrhenius diagram, and the values of energies in the dihedral potential energy curve associated with the carbon-carbon bond, a reference is needed. We propose to use the following equation:
With the trans state is at 0 deg, and c1, c2, and c3, are coefficients whose values are 0.7055, −0.1355, and 1.5722 kcal/mol respectively19,22. The energetical diagram is displayed in Fig. 2. Three reference energies are indicated therein: energy of the gauche (g or g-) state, 0.8 kcal/mol, barrier heights to pass from the trans to gauche states, 3.3. kcal/mol, and to exchange the gauche states, 4.5 kcal/mol. The barrier height to go from a gauche state to the trans state is also indicated. Comparison with Ea stemming from the description of the polymer local dynamics can now be proceeded.
Dihedral potential energy for a carbon-carbon bond21.
To compute Ea, the number of transitions between two rotameric states must first be established. It must be pointed out that all the reported data correspond to averages over eight configurations. The way a transition is registered was discussed in previous publications and summarized in the simulation methods paragraph13. Arrhenius diagrams for all transitions along the backbone chain in PE and PS are shown in Fig. 3.
Ea is directly extracted from the slope in the Arrhenius diagrams displayed in Fig. 3. The linear regression is made between Tg and \({T}_{g}^{u}\). A value of 3.2 kcal/mol for Ea for PE is supported by previous simulations and experimental data23. Moreover, this value was usually suspected to be correlated with the actual potential energy barrier necessary for a bond to go from the trans to gauche states, as shown in Fig. 2 24,25,26. It remains surprising that cooperative effects can be integrated in a value that corresponds to the simple barrier height between trans and gauche (Fig. 2). However, for PS, Ea is equal to 5.6 kcal/mol (Fig. 3b). This value should transcribe the cooperativity between side-chain and backbone motion as it is different from the potential energy height of a carbon-carbon bond (Fig. 2). It is in agreement with published data of 6 kcal/mol27. Nevertheless, these values of Ea stemming from MD have been claimed by several authors including ourselves to be correlated with Tg13,25. These data have thus been inserted in the graph of Ea (Tg) previously derived from polyvinyldifluoride (PVDF) and its derivatives with different percentages of regioisomerism defects, in Fig. 4 13. The additional data fit perfectly the linear regression arising from the previous data. The new linear regression is:
with R2 of 99.9%. The only difference with previous fitting equation lies in the ordinate at the origin, before \(0.64\,\mathrm{kcal}/\mathrm{mol}\). Such a fit confirms the intimate relationships between Tg and Ea.
Activation energy (Ea in kcal/mol) with respect to the Tg of PVDFs (■)13, PE ( ) and PS (
) and PS ( ).
).
The linear relationship between Ea and Tg (Fig. 4) is of the utmost importance as it gives a molecular perspective of the nature of the glass structure. Accordingly, knowing Ea for each kind of backbone fragments of a polymer can lead to the computation of Tg stemming from simulated dilatometry, as we showed for E-PTFE13. Moreover, since Ea depicts local dynamics, it can be argued that Tg from MD is relevant to describe the backbone motion underlying the glass transition. 5.6 kcal/mol thus represents the activation energy associated with the backbone motion of the PS chain. However, in the case of PE, Ea is deduced from the simple barrier height between trans and gauche rotameric states (Fig. 2). This simple picture does not ultimately estimate cooperative effects28. In fact, transitions reported in Fig. 3 include all kinds of transitions whatever the transition involves cooperative transition or local changes in the position of neighboring atoms. Separation between these two categories must be carried out.
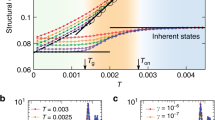
Local dynamics is mainly governed by two types of motion: i) rotational transitions of backbone bonds from one rotameric state, i.e. trans, gauche, and gauche-, to another, and ii) librational motions about rotameric minima and fluctuations in bond lengths and bond angles29. Intramolecular jumps in bond rotations associated with the backbone motion are actually due to librational motions29,30. However, configurational transition referred as triggering transition31, can take place without resulting in large displacement of the whole polymer chain by two main mechanisms12. (1) Cooperative transitions (CT) correspond to coupling of transition of closely-neighboring bonds along the chain. As a classical example, the crankshaft motion belongs to this category32,33. (2) Isolated transitions (IT) do not lead to other dihedral transitions but distort surrounding atoms31,34. Such transitions mainly occur at low temperatures35. Their particularity is that they do not lead to a chain rearrangement, but a local alteration of atomic positions. Root means square displacements after an IT are shown in Figure of the Supporting Information, at 200 K, and agree published data35. Percentages of these two transitions are then shown in Fig. 5 for both studied polymers.
Tg is set following an established protocol (Fig. 1). It does not correspond to a change in the coefficient of thermal expansion behavior. In Fig. 5, it can be observed that neither Tg nor \({T}_{g}^{u}\) are indicative of any changes in the transition percentage. Conversely, \({T}_{g}^{l}\) is the evidence of a change in the chain dynamics. Below this temperature, the number of both transitions remain roughly constant. While for PE, only isolated transitions occur in this domain, some cooperative transitions are detected for PS. The complexity of PS due to the presence of the side-chain should be accountable for their presence. Nevertheless, we can argue that \({T}_{g}^{l}\) coincides with the beginning of cooperative motions as temperature is raised. Cooperative transitions below this transition temperature will not be considered in the rest of the text, in agreement with published data35,36. As a result, the stemming activation energies should reflect the entire set of phenomena resulting in the occurrence of the glass transition process, giving insight into the chain dynamics, as outlined by de Gennes37. Activation energy for the two kinds of transition can now be investigated.
Since there is clear variation in physical properties (coefficient of thermal expansion, specific volume and heat capacity) throughout the glass transition domain, between \({T}_{g}^{l}\) and \({T}_{g}^{u}\), the activation energies were computed at each temperature to reveal any changes in the local dynamics. For this, the average of the two slopes associated with a frequency in the Arrhenius diagram is carried out. Three activation energies were thus considered: \({E}_{a}^{at}\) stemming from the consideration of all backbone transitions (Fig. 3), \({E}_{a}^{ct}\) coming from the cooperative transitions, and \({E}_{a}^{it}\) extracted from isolated transitions which perturb only neighboring atomic positions. Their behavior with respect to temperature are displayed in Fig. 6 for both polymers.
General behavior of the different activation energies with respect to temperature (Fig. 6) are comparable for both polymers. The slight difference in the curve shape observed can origin from the presence of a bulky side-group in PS. Discussion on such effect request further development since cooperativity exists between the lateral group rotation and the backbone motion11. Values of \({E}_{a}^{at}\) associated with all the transitions are primarily investigated. The actual values stemming from the linear fit in the Arrhenius diagram (Fig. 3) correspond to the average of \({E}_{a}^{at}\) in the graph of Fig. 6 between Tg and \({T}_{g}^{u}\). At this latter temperature, \({E}_{a}^{ct}\) reaches a maximum value. A decrease after this temperature is observed. The reason for this behavior lies in the procedure to count transitions (cf Simulation methods). To be recognized as a transition, the new dihedral angle must not change during 1.5 ps. At high temperatures, especially at temperature higher than \({T}_{g}^{u}\), fluctuations increase leading to uncertainties in the definition of a transition. This simulation artifact is no further discussed as the focus is on describing the glass transition domain. At low temperatures, below \({T}_{g}^{l}\), behavior of \({E}_{a}^{at}\) and \({E}_{a}^{it}\) are merged. Only isolated transitions subsist, in agreement with previous conclusions from Fig. 5. This finding also agrees with published data: transitions can occur without any involvement of a rotation in neighboring bonds31,34. An interesting statement is that for both polymers, values of \({E}_{a}^{it}\) below \({T}_{g}^{l}\) are equivalent, and equal to 1.5 kcal/mol. However, it is difficult to be certain about the unicity of this value since below \({T}_{g}^{l}\), the number of transitions is limited. Further studies on other polymers are needed. Nevertheless, we argue that it corresponds to the minimum potential energy required to generate one transition between rotameric states along a carbon backbone chain. Such an energy is not sufficient to prompt a transition in another bond (Fig. 2), and thus to engender cooperativity, but it is enough to involve changes in the position of neighboring atoms (Figure in the Additional Information).
The requested energy to generate a rotameric transition in another bond is \({E}_{a}^{ct}\) at \({T}_{g}^{l}\) where (i) curves of \({E}_{a}^{at}\) and \({E}_{a}^{it}\) split (Fig. 6) and (ii) percentage of CT begins to increase (Fig. 5). At \({T}_{g}^{l}\), the value of \({E}_{a}^{ct}\) is 2.5 kcal/mol and in order of 5 kcal/mol for PE and PS, respectively. For PE, this latter value corresponds exactly to the barrier height between gauche and trans states (Fig. 2). Based on previous correlation (Fig. 4), we can thus claim that 5 kcal/mol is the potential energy height for gauche to trans transition in PS along the backbone chain with a phenyl side-group. At \({T}_{g}^{u}\), \({E}_{a}^{ct}\) reaches its maximum value. These values are 4.5 kcal/mol and 8.6 kcal/mol for PE and PS, respectively. For PE, this value corresponds to the total energy barrier height (Fig. 2). From all the information gained from the study of the activation energies, a simple atomistic picture of the glass transition can be retrieved. It is displayed in Fig. 7. The values of the energy are associated with those stemming from PE but can be transferred to PS and other polymers.
In summary, the chemical pictures of the two limit temperatures, and the resulting domains of temperatures (Fig. 7) are as follows:
-
\(T < {T}_{g}^{l}\): Transitions between rotameric states mainly lead to changes in neighboring atoms position.
-
\({T}_{g}^{l}\): Temperature at which there is enough potential energy for transitions between rotameric states to generate rotation in another dihedral angle. This energy corresponds to the barrier height to go from gauche to trans states.
-
\({T}_{g}^{l} < T < {T}_{g}^{u}\): Domain of cooperativity between two bonds, i.e. changes in rotameric states are achieved between two bonds along the polymer chain.
-
\({T}_{g}^{u}\): Temperature corresponding to the maximum of the potential energy barrier.
-
\({T}_{g}^{u} < T\): Domain of more complex cooperativity.
Conclusion
Due to very rapid cooling rate, molecular dynamics simulation leads to a spread in the glass transition domain bordered by a lower (\({T}_{g}^{l}\)) and an upper (\({T}_{g}^{u}\)) temperatures. The actual enlargement has been seen as an overcranking effect as the coefficient of thermal expansion and the heat capacity vary progressively11. However, it challenges the very definition of Tg from atomistic simulation, as it corresponds to an intersection between two linear fits in the dilatometry simulation, not to a change in physical properties. To address this important issue, local dynamics of two polymers, polyethylene (PE) and polystyrene (PS), was investigated thanks to the computation of the activation energy, Ea.
By confronting computed activation energies with previously published values of Ea stemming from PVDF derivatives, we clearly demonstrated that Ea is directly related to Tg. This value corresponds to a single barrier height in dihedral potential energy diagram. To better grasp the meaning of Ea extracted from the consideration of the backbone transitions, two different kind of transitions were considered: isolated and cooperative transitions. For the former ones, it was shown that they are mainly exhibited at low temperatures in agreement with published studies. It is worth noting that both polymers exhibit the same Ea, 1.5 kcal/mol. We argue that this value corresponds to the minimum potential energy required to generate one transition between rotameric states along a carbon backbone chain. However, the unique value for PE and PS cannot be generalized at this point. Further studies are needed. At \({T}_{g}^{l}\), alternative transitions at another bond come into play. The associated Ea is 2.5 kcal/mol which corresponds to the potential energy for a gauche state to go in a trans state, in the dihedral potential energy diagram. The maximum value is reached at \({T}_{g}^{u}\) where Ea is 4.5 kcal/mol for PE which is the maximum energy in the dihedral potential energy carbon-carbon. For PS, these values are 5 and 8.6 kcal/mol respectively. We thus clearly answer the question raised by Boyd in effect that does Ea behavior appropriate to a single barrier height or whether higher values might arise due to cooperative effect. Accordingly, a simple atomistic picture of the glass transition was shown. This atomic representation should be applied to other systems for which particular behaviour at the glass transition has been reported such as thin films38.
Methods
More details of the whole molecular simulation procedure to get Tg can be found in previous articles39,40,41. Selection of the initial configurations and their relaxation process are crucial. A cell with periodic boundary conditions is constituted by a chain of 250 monomers long for PE, and 125 monomers long for PS. The generation of the chains imbedded in the cell was done through the Self-Avoiding Walk procedure of Theodorou-Suter42 and Meirovitch43 scanning methods, implemented in the Amorphous_Cell© code, in the Materials Studio environment. 50 configurations were thus first obtained. A first selection was made by considering their radius of gyration not to stray too far from the average value. Second, 8 configurations with the lowest energy are finally selected. A heating-cooling process was then employed to eliminate any endemic stress. Molecular dynamics (MD) in the NPT (i.e., constant number of particles, pressure, and temperature) ensemble was used. The integration of Newton’s equation of motion was performed using the velocity Verlet integration algorithm with a 1 fs integration time step44. The Nosé-Hoover thermostat and Parrinello-Rafman barostat algorithms were used to maintain constant temperatures and pressures, respectively45,46. The pcff force field was chosen. The non-bonded interactions have been computed using the Ewald summation, to take into account long-range effects3. All the MD simulations have been carried out using the LAMMPS code47. The heating-cooling process consists in a fast heating process (50 K/200 ps) followed by a lower cooling rate (20 K/ns). It has been shown that to get reproducible values of Tg, the initial configuration must be in mechanical equilibrium, a “quasi-static” equilibrium state where the stress in the cell balances the internal pressure48. A uniform hydrostatic compression is imposed to the system until the internal energy reaches a minimum. During the cooling down process, 20 K/ns, the specific volume is reported with respect to the temperature, for each ensuing configuration. This simulated dilatometry leads to the value of Tg. MD of 10 ns are then run at each temperature, and a configuration is saved at each 500 fs.
Details concerning the calculation of Ea can be found in a previous publication. The important initial step is to account for a transition. The method we used can be summarized into 4 key points. The procedure corresponds to a slightly modified Wu’s procedure18. (1) Fluctuations in the dihedral angle around the rotameric states are smoothed by a sliding average. (2) In order for a transition to be identified, the difference between the two involved dihedral angles must be greater than 40 deg. (3) The time interval that is requested for a new state along a trajectory to lose memory of its previous state was set at 3 ps. 4) The torsion angle of the new rotameric state must exist for more than 1.5 ps to avoid counting abrupt changes of states.
References
Donth, E.-J. The Glass Transition. 48, (Springer Berlin Heidelberg, 2001).
Ngai, K. L. Why the glass transition problem remains unsolved? J. Non. Cryst. Solids 353, 709–718 (2007).
Allen, P. & Tildesley, D. J. Computer simulation of liquids-2nd Edition. (Oxford University Press, 2017).
Rigby, D. & Roe, R.-J. Molecular dynamics simulation of polymer liquid and glass. II. Short range order and orientation correlation. J Chem Phys 89, 5280 (1988).
Binder, K., Baschnagel, J. & Paul, W. Glass transition of polymer melts: test of theoretical concepts by computer simulation. Prog. Polym. Sci. 28, 115–172 (2003).
Wu, C. Re-examining the procedure for simulating polymer Tg using molecular dynamics. J. Mol. Model. 23, 270 (2017).
Gee, R. H. & Boyd, R. H. The role of the torsional potential in relaxation dynamics: a molecular dynamics study of polyethylene. Comput. Theor. Polym. Sci. 8, 93–98 (1998).
Soldera, A. & Metatla, N. Glass transition of polymers: Atomistic simulation versus experiments. Phys. Rev. E 74, 61803 (2006).
Sperling, L. H. Introduction to Physical Polymer. (John Wiley & Sons, Inc., 2006).
Wunderlich, B. Thermal Analysis of Polymeric Materials. (Springer, 2005).
Godey, F., Fleury, A., Ghoufi, A. & Soldera, A. The extent of the glass transition from molecular simulation revealing an overcrank effect. J. Comput. Chem. https://doi.org/10.1002/jcc.25069 (2017).
Takeuchi, H. & Roe, R.-J. Molecular dynamics simulation of local chain motion in bulk amorphous polymers. II. Dynamics at glass transition. J. Chem. Phys. 94, 7458 (1991).
Anousheh, N., Godey, F. & Soldera, A. Unveiling the impact of regioisomerism defects in the glass transition temperature of PVDF by the mean of the activation energy. J. Polym. Sci. Part A Polym. Chem. 55, 419–426 (2017).
Angell, A. Liquid landscape. Nature 393, 521 (1998).
Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature 410, 259–67 (2001).
Parisi, G. & Sciortino, F. Structural glasses: Flying to the bottom. Nat. Mater. 12, 94–5 (2013).
Hotston, S. D., Adolf, D. B. & Karatasos, K. An investigation into the local segmental dynamics of polyethylene: An isothermal/isobaric molecular dynamics study. J. Chem. Phys. 115, 2359 (2001).
Wu, R., Zhang, X., Ji, Q., Kong, B. & Yang, X. Conformational transition behavior of amorphous polyethylene across the glass transition temperature. J. Phys. Chem. B 113, 9077–9083 (2009).
Liang, T., Yang, Y., Guo, D. & Yang, X. Conformational transition behavior around glass transition temperature. J. Chem. Phys. 112, 2016–2020 (2000).
Boyd, R. H. & Smith, G. D. Polymer Dynamics and Relaxation. (Cambridge University Press, 2007).
Godey, F., Bensaid, M. O. & Soldera, A. Extent of the glass transition in polymers envisioned by computation of mechanical properties. Polymer 164, 33–38 (2019).
Harmandaris, V. A., Adhikari, N. P., Van Der Vegt, N. F. A. & Kremer, K. Hierarchical modeling of polystyrene: From atomistic to coarse-grained simulations. Macromolecules 39, 6708–6719 (2006).
Kanaya, T., Kaji, K. & Inoue, K. Local Motions of Cis-1,4-Polybutadiene in the Melt - a Quasi-Elastic Neutron-Scattering Study. Macromolecules 24, 1826–1832 (1991).
Adolf, D. B. & Ediger, M. D. Brownian dynamics simulations of local polymer dynamics. Macromolecules 24, 5834–5842 (1991).
Wu, R., Kong, B. & Yang, X. Conformational transition characterization of glass transition behavior of polymers. Polymer 50, 3396–3402 (2009).
Boyd, R. H., Gee, R. H., Han, J. & Jin, Y. Conformational dynamics in bulk polyethylene: A molecular dynamics simulation study. J. Chem. Phys. 101, 788 (1994).
Lačević, N. et al. Spatially heterogeneous dynamics investigated via a time-dependent four-point density correlation function. J. Chem. Phys. 119, 7372–7387 (2003).
Pant, P. V. K., Han, J., Smith, G. D. & Boyd, R. H. A molecular dynamics simulation of polyethylene. J. Chem. Phys. 99, 597 (1993).
Baysal, C., Atilgan, A. R., Erman, B. & Bahar, İ. Molecular Dynamics Analysis of Coupling between Librational Motions and Isomeric Jumps in Chain Molecules. Macromolecules 29, 2510–2514 (1996).
Moro, G. J. The coupling between librational motions and conformational transitions in chain molecules. II. The rotor chain represented by the master equation for site distributions. J. Chem. Phys. 97, 5749 (1992).
Moe, N. E. & Ediger, M. D. Computer simulations of polyisoprene local dynamics in vacuum, solution, and the melt: Are conformational transitions always important? Macromolecules 29, 5484–5492 (1996).
Helfand, E., Wasserman, Z. R. & Weber, T. A. Brownian dynamics study of polymer conformational transitions. J. Chem. Phys. 70, 2016–2017 (1979).
Helfand, E., Wasserman, Z. & Weber, T. Brownian Dynamics Study of Polymer Conformational Transitions. Macromolecules 13, 526–533 (1980).
Starkweather, H. W. Noncooperative Relaxations. Macromolecules 21, 1798–1802 (1988).
Bahar, I., Erman, B. & Monnerie, L. Kinematics of Polymer Chains with Freely Rotating Bonds in a Restrictive Environment. 1. Theory. Macromolecules 25, 6309–6314 (1992).
Haliloglu, T., Bahar, I., Erman, B. & Mattice, W. L. Relative Contributions of Coupled Rotations and Small-Amplitude Torsions to Conformational Relaxation in Polymers. 9297, 8942–8947 (1996).
de Gennes, P. G. Scaling concepts in polymer physics. (Cornell University Press, 1979).
El Ouakili, A., Vignaud, G., Balnois, E., Bardeau, J. F. & Grohens, Y. Multiple glass transition temperatures of polymer thin films as probed by multi-wavelength ellipsometry. Eur. Phys. J. Appl. Phys. 519, 2031–2036 (2011).
Soldera, A. Atomistic simulations of vinyl polymers. Mol. Simul. 38, 762–771 (2012).
Metatla, N. & Soldera, A. Computation of densities, bulk moduli and glass transition temperatures of vinylic polymers from atomistic simulation. Mol. Simul. 32, 1187–1193 (2006).
Metatla, N. & Soldera, A. The Vogel-Fulcher-Tamman equation investigated by atomistic simulation with regard to the Adam-Gibbs model. Macromolecules 40, 9680–9685 (2007).
Theodorou, D. N. & Suter, U. W. Detailed molecular structure of a vinyl Polymer glass. Macromolecules 18, 1467–1478 (1985).
Meirovitch, H. Computer simulation of self-avoiding walks: Testing the scanning method. J Chem Phys 79, 502 (1983).
Haile, J. M. Molecular Dynamics Simulation. (John Wiley & Sons, 1992).
Victor, R. Berendsen and Nose-Hoover thermostats Temperature in MD MD at constant Temperature - NVT ensemble 1–4 (2007).
Parrinello, M. & Rahman, A. Strain fluctuations and elastic constants. J. Chem. Phys. 76, 2662 (1982).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Metatla, N. & Soldera, A. Effect of the molar volume on the elastic properties of vinylic polymers: A static molecular modeling approach. Macromol. Theory Simulations 20, 266–274 (2011).
Acknowledgements
The computational resources were provided by Calcul Québec and Compute Canada, through the financial support of the Canadian Foundation Innovation (CFI). This work was supported by the Université de Sherbrooke, the Fonds Québécois de la Recherche sur la Nature et les Technologies (FRQNT), and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Contributions
A.S. designed the research and wrote the manuscript. F.G. performed the simulations. A.F. computed the MSD. All the authors revised and proof read the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Godey, F., Fleury, A. & Soldera, A. Local dynamics within the glass transition domain. Sci Rep 9, 9638 (2019). https://doi.org/10.1038/s41598-019-45933-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45933-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.




 ) for PE (a), and PS (b).
) for PE (a), and PS (b).

 ) and isolated transitions (
) and isolated transitions ( ) with respect to temperature for PE (a) and PS (b). Vertical lines representing Tg,
) with respect to temperature for PE (a) and PS (b). Vertical lines representing Tg, 
 ), and isolated transitions,
), and isolated transitions,  ), for PE (a), and PS (b).
), for PE (a), and PS (b).