Abstract
Bamboo (Phyllostachys pubescens) expansion into adjacent forests is a widespread phenomenon in subtropical regions, and it has greatly changed the dominance hierarchy from trees to bamboos. This process may be accompanied by changes in productivity, nutrients accumulation and biogeochemical cycles. We compared the net primary production (NPP) and major pools and fluxes of nitrogen (N) in bamboo-dominant forest (BDF) and neighboring secondary evergreen broadleaved forest (EBF) in South China using the space-for-time substitution method. We found that the mean NPP of the BDF was 30.0 t ha−1 yr−1, which was 51.5% greater than that of the EBF (19.8 t ha−1 yr−1). The plant N pool for the BDF was 37.5% larger than that of the EBF, whereas the soil inorganic N pool significantly decreased by 31.2% with conversion of the EBF to BDF. Additionally, the ratio of N return to N uptake was 0.69 in the BDF and 0.88 in the EBF because of the lower litter N return of the BDF compared with that of the EBF. These results indicated that the expansion of P. pubescens significantly increased the NPP and plant N accumulation but reduced the soil N available pool and slowed the N cycling rate, which could lead to soil degradation.
Similar content being viewed by others
Introduction
Bamboos(Poaceae: Bambusoideae) are perennial tree-like grasses widely distributed in tropical, subtropical and temperate forest ecosystems1. These plants frequently expand into their neighboring forest ecosystems by clonal and sexual reproduction and become dominant species in the canopy or understory with human or natural disturbances2,3,4. Here, this phenomenon is referred to as “bamboo expansion”. Bamboo expansion may significantly impact the colonized forests with regards to the species composition5,6, community structure2,7, and forest landscapes8,9, all of which have drawn worldwide attention of ecologists.
Moso bamboo (Phyllostachys pubescens Mazel ex J. Houz.) is the most broadly distributed bamboo species in subtropical China10. Because it grows quickly and has a leptomorph (running) rhizome system, P. pubescens can spread rapidly into disturbed forests as well as undisturbed forests, including areas characterized by shrubs11, coniferous forest12, mixed needle and broadleaved forest13 and pure broadleaved forest14,15. The unbridled expansion of P. pubescens has imposed great ecological consequences on these forests4,13, therefore, it has been considered as one of the greatest contemporary threats to woody forests in China.
Evergreen broadleaved forest (hereafter, EBF), the typical vegetation type in subtropical areas, is recognized as an important global vegetation type that contributes to both biodiversity and environmental protection16. Despite their formerly widespread geographical distribution, remnant EBFs next to bamboo forest unfortunately are now suffering from extensive expansion by bamboo15,17. Until now, a viable strategy has not been proposed for preventing bamboo expansion into EBFs, even in natural reserves and ecological public-welfare forest areas. Therefore, the ecological effects of P. pubescens expansion on EBFs must be assessed.
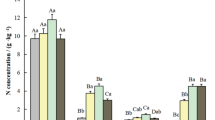
Recent studies have reported the loss of plant diversity13 following P. pubescens expansion, and simplified community structure2 and reduced tree regeneration8 because bamboo are more competitive with regard to capturing light and other resources18,19. Changes in the species composition from trees to bamboos are expected to exert great influences on the net primary productivity (NPP) and biomass of evergreen broadleaved forest, because bamboo are characterized by active rhizomatous clonal growth and high belowground allocation of biomass10. For example, Yang et al.20 found that Phyllostachys edulis expansion affected biomass accumulation of evergreen broad-leaved forest in Dagangshan mountain. Apart from its effects on plant biomass and production, nitrogen dynamics are essentially driven by the development of a bamboo biomass pool. It is likely that bamboo have relatively higher leaf N concentration than those of the dominant species in broadleaved forest14. This shift in tissue quality, along with a shift toward greater belowground biomass, will alter aboveground N pools and soil N pool, as well as litter dynamics. Changes in nitrogen cycling would in turn affect the ecosystem NPP and biomass accumulation21,22. However, to date, little is known about the underlying N biogeochemistry cycling, which is crucial for better understanding of ecosystem functioning after expansion.
Based on the increasing awareness of bamboo expansion, pairs of bamboo–dominated forests (hereafter, BDF) and EBFs in Dagangshan National Forest Ecological Station in Jiangxi Province of China were used as the research objects. We aimed to comprehensively assess the ecological consequences of biomass, production, N pools and fluxes after the expansion of P. pubescens into adjacent EBFs. Our specific objectives are to (1) quantify the size and distribution of biomass and net primary production (NPP) and (2) compare the main ecosystem N pools and N fluxes in BDF and front uninvaded EBF growing on similar soils. Our results can provide experimental data and parameters to evaluate the ecological effects of P. pubescens expansion into native, broad-leaved forest in equivalent or similar climatic areas, which can help us better understand how bamboo expansion affects soil N pool and serve as a scientific basis for restoration of bamboo forest conversion to evergreen broadleaved forests.
Results
Shifts in vegetation biomass
Significant differences were generally not observed in total community biomass between the EBF and BDF (201.2 t ha−1 vs 205.5 t ha−1) (F1, 4 = 0.17, P = 0.579). It was because the decrease of the tree biomass (127.1 t ha−1) was offset by the increase of the bamboo biomass (131.2 t ha−1) from EBF to BDF (Fig. 1A). The biomass spatial allocation clearly varied after bamboo expansion. The aboveground biomass (WA) of the BDF (139.1 t ha−1) was 14.1% lower than that of the EBF (161.9 t ha−1) (F1, 4 = 35.65, P = 0.021), with the WA of the stems, branches and leaves reduced by 11%, 25% and 14%, respectively. Conversely, the belowground biomass in the BDF was 66.4 t ha−1, which was 69.0% greater than that of the EBF (39.3 t ha−1) (F1, 4 = 40.49, P = 0.024). Consequently, the ratio of the aboveground biomass to belowground mass (WA/WB) significantly decreased from 4.1 in the EBF to 2.1 in the BDF (F1, 4 = 22.44, P = 0.042).
Shifts in vegetation NPP
Between 2007 and 2011, the mean annual NPP for EBF and BDF was 19.8 t ha−1 yr−1 and 30.0 t ha−1 yr−1, respectively (Fig. 2), therefore, bamboo expansion led to a NPP increase of 51.5% (F1, 4 = 39.89, P = 0.024). This result was mainly caused by the more than 8.2 t ha−1 yr−1 of fine root production in belowground NPP of BDF than that of EBF. No significant differences were observed in the aboveground NPP between EBF and BDF (15.0 t ha−1 yr−1 versus 15.8 t ha−1 yr−1) (F1, 4 = 1.018, P = 0.906). Thus, the proportion of belowground components in the total NPP increased from 24.2% to 47.3% with the conversion of EBF to BDF.
Plant N content
Almost all organs (except branches) of bamboo presented greater N content compared with the corresponding organs of broadleaved trees (Table 1). The order of N content among old bamboo shoots (>1 yr) organs was leaves > rhizomes > branches > coarse roots > stems, and all trees measured showed similar patterns in different organs. The N content for the organs of young bamboo individuals (≤1 yr) was higher than that of old individuals (>1 yr), particularly for the stems, leaves and rhizomes (Table 1).
Shifts in ecosystem N pools
Plant N pool
The plant N pool of the BDF was 756.0 kg N ha−1 (Fig. 3), which was 37.5% greater than that of EBF (550.0 kg N ha−1) (F1, 4 = 33.58, P = 0.004), and its allocation pattern of N was different in the two forests. The N accumulation in bamboos increased from 0 to 599.9 kg N ha−1, and that in trees decreased by 393.8 kg N ha−1 from the EBF to the BDF (Fig. 3A). The N pool of the aboveground compartments were 426.0 kg N ha−1 and 452.1 kg N ha−1 for the EBF and BDF, respectively (F1, 4 = 2.07, P = 0.109); however, the N pool of the belowground compartment shifted from a value of 124.0 kg N ha−1 for the EBF to 303.9 kg N ha−1 for the BDF (Fig. 3B), which represented an increase of 145.1% (F1, 4 = 122.56, P = 0.008).
Litter N pool
A significant increase in the total litter N pool was observed with the conversion of EBF to BDF (53.0 kg N ha−1 versus 113.0 kg N ha−1) (F1, 4 = 26.84, P = 0.002), with the belowground litter contributing the greatest amount for the BDF (Fig. 4). The litter N pool was 3.5-fold greater in the belowground litter of the BDF relative to that of the EBF (92.4 kg N ha−1 versus 26.4 kg N ha−1) (F1, 4 = 32.33, P = 0.001), whereas the aboveground litter N pool did not show differences between the BDF and EBF (20.8 kg N ha−1 and 26.3 kg N ha−1, respectively) (F1, 4 = 0.25, P = 0.643).
Soil N pool
The soil inorganic N (SIN) of BDF at a soil depth of 0–20 cm was 11.0 kg N ha−1 (Fig. 5), which was 31.2% lower than that of EBF (16.0 kg N ha−1) (F1, 4 = 8.25, P = 0.045), but the soil organic N (SON) pool did not show significant differences between EBF and BDF (4234 kg N ha−1 versus 4019 kg N ha−1) (F1, 4 = 0.85, P = 0.43).
(A) Evergreen broadleaved forest, EBF; and (B) bamboo-dominant forest, BDF. N pools included plant biomass (NB), standing litter (NL), soil organic N(SON) and inorganic N(SIN) within 0–20 cm. N fluxes included annual plant N uptake (+NUP), N return by litter (+NR, the former data in the parenthesis is N return by aboveground litterfall, the latter is by fine root mortality)), net N mineralization rate (+NM), and N output from harvested bamboo (−NOP). Values are the mean of three plots from each stand.
Shifts in ecosystem N fluxes
The annual N uptake by plants in the BDF (162.0 kg N ha−1 yr−1) was slightly higher than that in the EBF (150.0 kg N ha−1 yr−1) (F1, 4 = 4.19, P = 0.109); however, the total litter N return (aboveground + belowground) to soil was significantly lower in the BDF than the EBF (113.0 kg N ha−1 yr−1 versus 132 kg N ha−1 yr−1) (F1, 4 = 11.24, P = 0.041), thus representing a decrease of 14.4% (Fig. 5). The N cycling rate calculated as dividing the N return by N uptake was 0.69 in the BDF and 0.88 in the EBF, representing a 21.6% decrease for the BDF (F = 7.13, P = 0.056). Additionally, the N output from the BDF because of the harvesting of dead or old bamboos was approximately 22.0 kg N ha−1 yr−1; however, this output was neglected (Fig. 5).
Discussion
Expansion of bamboo changed vegetation biomass allocation pattern
Forest biomass is one of the best indicators of ecosystem function23. We found that the expansion of P. pubescens had few effects on the total vegetation biomass (Fig. 1). The averaged values of EBF biomass obtained in the present study was 201.2 t ha−1, a result that was higher than the mean value of EBFs in subtropical China (163.7 t ha−1)23 but close to the EBF in east China (225.3 t ha−1) reported by Yang et al.24. The total biomass of the BDF was 205.5 t ha−1, which was in the range of 132.8–309.2 t ha−1 previously reported for equivalent vegetation in various regions of China and Japan14,25,26. The biomass showed limited changes because the increase in bamboo biomass (131.2 t ha−1) was nearly sufficient to compensate for the loss of tree biomass (127.1 t ha−1) (Fig. 1A); although the vegetation density and BA increased approximately 5-fold and 1-fold after the P. pubescens expansion, respectively (Table 2), the biomass of hollow culm of individual bamboo was less than the biomass of individual tree in the same size. Our findings were consistent with those reported by Fukushima et al.14, who noted that an obvious trend of biomass did not occur in conjunction with temperate secondary broadleaved forest conversion into bamboo forest.
The biomass spatial distribution pattern was modified by the bamboo expansion. The ratio of the aboveground biomass to the belowground biomass (WA/WB) significantly decreased from 4.1 in the EBF to 2.1 in the BDF (Fig. 1B). This ratio is well within the range of 3–7 for broadleaved forest stands23 and 0.1–0.3 for grassland sites in China27. The stand structure of the BDF was between that of forest and grassland sites, and this structure is also suited for other bamboos, such as Phyllostachys bambusoides stands in Japan28 or Guadua weberbaueri-dominant open rain forests in Brazil29. A large amount of biomass allocated belowground in BDF. This is attributed to bamboos’ inherent extensive rhizome and root system10,25, which reflects a strategy for maintaining the advantageous regeneration of P. pubescens that could contribute to its rapid expansion25.
Expansion of bamboo increased vegetation NPP
We found that the expansion of P. pubescens significantly increased the vegetation NPP. The NPP in the EBF estimated in the present study was 19.8 t ha−1 yr−1 (Fig. 2), which was close to the mean NPP value (14.5 t ha−1 yr−1) for subtropical EBFs23. The NPP of the BDF averaged 30.0 t ha−1 yr−1, which is equivalent to the 28.9 t ha−1 yr−1 of pure P. pubescens forest observed in the Wuyishan Biosphere Reserve of Southeast China26. This result was consistent with the findings of Isagi et al.25, who reported that P. pubescens forests had greater gross production compared with temperate broadleaved and/or coniferous forest stands in Japan. The greater total NPP after bamboo expansion was related to the greater belowground NPP, which was caused by the >8.2 t ha−1 yr−1 of fine root production in the BDF relative to that in the EBF (Fig. 2). Because fine roots are short lived and have a faster return19, their presence can explain the limited differences in biomass but greater NPP in the BDF compared with the EBF.
The NPP in forest ecosystems is often closely related to the forest type30, soil conditions31, and intrinsic plant properties21. Generally, higher NPP is found when soil N availability is higher (i.e., larger SIN pool and/or greater soil N mineralization rate)31,32,33. In this case, both the SIN pool and soil N mineralization rate were reduced after bamboo expansion (Fig. 5). Therefore, the difference in NPP between the two types of forest was likely independent of the soil N availability but related to the intrinsic advantages of the species themselves. Nitrogen use efficiency (NUE) of plant is important for governing the pattern of nutrient processes in many ecosystems34,35, and higher productivity could be promoted by higher NUE21,35. According to ecosystem-level NUE, which is calculated as the NPP per unit N returned in litter34, we found that the BDF has greater NUE (265.5) than the EBF (150.0). In addition, the leaf N concentration of P. pubescens exceeded the corresponding values of woody trees (Table 1) partly as a result of high leaf N reabsorption rate of bamboos15, indicating that P. pubescens has a higher photosynthetic ability36. Importantly, even P. pubescens in poor sites can obtain nutrients from rich sites by clonal integration to meet their N demand37. Therefore, more organic production can be generated in BDF with the increase of bamboo predominant. Regrettably, belowground components contribute most to the total NPP, leading to some aboveground functional shrinkage.
Expansion of bamboo slowed ecosystem N cycling
N cycling in the ecosystem is primarily composed of three N pools (plant, litter and soil) and three N flux processes (N uptake, N return and N mineralization)38. The presence of P. pubescens interrupts N cycling in colonized ecosystems. The plant N pool and litter N pool increased remarkably under bamboo expansion. The values of the plant N pool were 550. kg ha−1 in the EBF and 756.0 kg ha−1 in the BDF (Fig. 3). The shift in the plant N pool was similar to that found in many studies of shrub invasion into grassland in which the accrual of plant N was promoted35,39,40. However, the shift was not consistent with the findings reported by Fukushima et al.14, who found that the plant N stock was not influenced by the expansion of P. pubescens into temperate broadleaved forest. The differentiation between two studies may be due to the invaded ecosystems between two studies were different in species composition, stem density, site conditions, and the interference degree by bamboos. The large plant N accumulation in the BDF in this study was caused by the higher N content in bamboo tissue than in trees (Table 1) because the biomass exhibited no changes between the two forests. The total litter N pool increased by 60.0 kg N ha−1 with the conversion of EBF to BDF (Fig. 4), mainly resulting from more than 66 kg N ha−1 belowground litter N accumulation in BDF. The belowground litter N accumulation generally depends on the N input through root mortality and N output into soil by decomposition38. Because the N input by the fine root mortality in BDF(52 kg ha−1 yr−1) was higher than that in EBF (13 kg ha−1 yr−1, Fig. 5) and fine root decomposition rates varied little between BDF and EBF41, more N was accumulated below ground in the BDF than EBF.
In contrast to the N pools for plant and litter, we found that the SIN pool significantly decreased from 16.0 kg N ha−1 to 11 kg N ha−1 after EBF conversion to BDF (Fig. 5), which indicated a depletion of the available N in the soil pool but only a slight decrease on the SON pool despite some SON was output through bamboo harvesting. A similar observation was found in areas colonized by dwarf bamboo (Sasa kurilensis) invasion, which caused a lower soil N available N in Betula ermanii forests in northern Japan3.
The size of the SIN pool was mainly determined by litter N release, soil N mineralization rate and plant N uptake38,42. The expansion of P. pubescens decreased the N return by litter and the soil N mineralization but increased the annual N uptake by plants. The annual N return by litter (aboveground + belowground) decreased, but the standing litter N accumulation increased with the conversion of EBF to BDF (Fig. 5), which suggested a slower N release rate (N return/N storage) from the litter to soil. In addition, the amount of soil N mineralization decreased from 145 kg N ha−1 yr−1 in the EBF to 106.0 kg N ha−1 yr−1 in BDF in the topsoil at 20 cm15 (Fig. 5), which represented a 26.9% reduction. The lower litter N release and soil N mineralization rate could be attributed to the changes of microorganism abundance, community structure and activities17,43,44. Studies found that the abundance of fungi were decreased after bamboo expansion into adjacent forests44, similar decreased pattern was found in the soil cellulose and xylanase activities43. The annual N uptake by the BDF (162.0 kg N ha−1 yr−1) was greater than that by the EBF (150.0 kg N ha−1 yr−1), indicating that the demand greatly exceeded the annual soil N supply by mineralization in the BDF. Thus, the lower litter N release rate, the lower N mineralization rate and the higher N uptake led to a smaller SIN pool after bamboo expansion.
Increase N accumulation in plant and litter intercepted the N flux from the aboveground to belowground, thereby slowing the N cycling in colonized ecosystem by bamboo expansion. Reduced N return and release, slower soil mineralization rate and increased N uptake by vegetation led to a small SIN pool when bamboo spread into the former broadleaved forests. A decrease in soil N availability and increase in N uptake resulted from bamboo–soil feedback loops, which could limit original tree growth, seed germination and future seedling establishment, thereby threatening plant biodiversity. However, the same factors would have fewer effects on bamboo growth and reproduction because of bamboo’s nutrient integration by extensive rhizomes37 and high N resorption rate15. These findings represent consequences of bamboo expansion and act as a mechanism for P. pubescens expansion, which implies that NPP and N cycling should be considered when remnant forests are conserved to prevent bamboo expansion and bamboo forests are restored to EBFs.
In summary, P. pubescens expansion can spatially modify biomass allocation by reducing aboveground and tree biomass although with a limited influence on the total vegetation biomass, and increase NPP (especially belowground) through higher NUE. In addition, bamboo expansion may hinder N cycling in the colonized ecosystems, as evidenced by higher N uptake, higher plant N accumulation, lower N return and lower soil N mineralization rate, which may lead to potential soil degradation. These findings have great implications for assessing the ecological consequences and understanding the mechanisms of P. pubescens expansion, and they also provide fruitful insights into cases of other bamboo expansions. However, the effects of P. pubescens expansions and proliferation into EBFs could vary over time as well as on a site-by-site basis. Therefore, the long-term dynamics of these expanding ecosystems should be investigated further to accurately monitor the changes in NPP and N cycling that accompany P. pubescens expansion and proliferation in a variety of forest systems.
Methods
Site description
This study was conducted at Dagangshan National Forest Ecological Station in Jiangxi Province, South China (27°30′–27°50′N, 114°30′–114°45′E). The region has a middle subtropical monsoon climate, an annual mean temperature of 15.8–17.7 °C, and extreme temperatures in January of −8.3 °C and July of 39.9 °C. The annual mean precipitation is 1591 mm, which mainly occurs from March to August. This region has a subgroup of mountain red and yellow soil with well-weathered coarse granite of the Presinian system45. The climax vegetation is EBF; however, P. pubescens is also a suitable species and is spreading into adjacent EBFs20.
To understand the impacts of bamboo expansion on ecosystem properties, a space-for-time substitution was used as the evaluation method46. In August 2011, three pairs of stands straddling BDF and unexpanded EBF were continuously selected. EBF was selected to represent a reference condition, and neighboring BDF was recognized as the experimental unit. A 20 m × 20 m plot was established in each stand with the same slope and aspect. The plots were considered to have similar historical community structures and soil conditions. Therefore, differences in the current NPP and N cycling were assumed to have resulted from the bamboo expansion. Details of this site’s soil physical and chemical properties have been previously described by Song et al.15.
The EBF was located in the front of the BDF, and it was approximately 50 years old and dominated by the evergreen broadleaved tree Castanopsis fargesii, accompanied by Castanopsis sclerophylla, Symplocos laurina, Schima superba, and Symplocos sumuntia. The trees’ density, height and basal areas were 1031 stems ha−1, 16.9 m and 22.3 m2 ha−1, respectively, of which C. fargesii contributed 58.0% of the total breast area (BA) (Table 2).
The BDF was formed when P. pubescens expanded into secondary EBF approximately 30 years ago. The BDF was dominated by P. pubescens, accompanied with some resident broadleaved trees, including Symplocos laurina, C. fargesii and C. sclerophylla. The stand density was 5379 stems ha−1, with a ratio of P. pubescens over broadleaved trees of 9:1 (by individual). The diameter at breast height (DBH) of the bamboo and trees was 9.8 cm and 16.2 cm, respectively, and the corresponding mean height was 12.9 cm and 11.7 cm, respectively. The BA was 46.2 m2 ha−1, with P. pubescens accounting for 81.0% (Table 2). Fertilization was not performed at the bamboo sites, and the old (>10 a) and dead bamboos were occasionally harvested for firewood or housing.
Biomass and NPP estimation
Biomass
The diameter at breast height (DBH) and height (H) were recorded for all trees and bamboos with DBH >5 cm in all 6 plots in August, 2011. The ages of bamboos were also measured in each bamboo sites. The biomass of the stems, leaves, branches, and coarse roots (including stumps) of the species in each subplot was estimated from the DBH and H using the allometric equation W = a × (DBH2 × H)^b (Supplementary Table S1), which was established with equivalent vegetation from the study site of Yang et al.20. To estimate the bamboo biomass of the belowground rhizome, 5 vertical blocks of 100 cm length, 100 cm width and 90 cm depth were dug in each bamboo plot. All of the bamboo rhizomes in the block were picked out, and separated into current-year and older-year components. These components were then dried at 80 °C for 72 h to determine the dry mass and N content. The biomass of the fine root (<2 mm) of BDF and EBF was obtained from Liu et al.19 in the same study site. The aboveground biomass (WA), belowground biomass (WB) and total biomass (WT) were calculated using the following formulas:



where WS, WB and WL represent the biomass of the stems, branches and leaves, respectively and WC, WF and MR represent the biomass of the coarse roots, fine roots and rhizomes, respectively.
NPP
The annual NPP, including the aboveground NPP (ANPP) and belowground NPP (BNPP) of vegetation were calculated using the following equations47:



where ∆BA is the annual aboveground increment of wood biomass, including the stems and branches; ∆BB is the annual belowground increment of wood biomass, including the coarse roots and rhizomes; L is the annual aboveground litterfall production observed monthly from 2011 to 2012, obtained from Song et al.15; and F is the annual growth of fine root measured between 2010 and 2011, obtained from Liu et al.19.
The ∆BA and ∆BB of the trees were based on the DBH increment of each individual and calculated from a tree ring analysis. Based on the above biomass measurements, the species C. fargesii, C. sclerophylla, S. laurina and S. superba were selected from the plots using the average standard tree method (3 trees for each species) and cut down to analyze the age and annual ring width using the LINTAB™ 5 ring analyzer (Frank Rinn, Germany) of accuracy 0.01 mm. For each tree ring disc at DBH, an average incremental growth (in diameter) for the previous 5 years (2007–2011) was determined according to four directions. The mean annual incremental increase in DBH was then subtracted from the current diameter to estimate the average previous year’s diameter. To estimate the annual incremental increase of unmeasured species, we calculated the weighted mean increment based on the BA of each species in each plot. Previous year’s height was calculated by the relationship between DBH and H established by Yang et al.20 (Supplementary Table S1). Both the current DBH and H, the average previous year’s DBH and H were entered into the allometric equations to calculate biomass. The difference in biomass between the current and the previous year was the tree woody growth increment.
The annual production of ∆BA of living bamboo was calculated as the weight of new bamboo shoots. For the number of the new bamboo shoots has a regular biennial cycle, but was constant in each cycle48. Thus, we calculated the total aboveground biomass of living bamboos within recent 3 biennial cycles (2006–2011, 6 years). The ∆BA of living bamboo was calculated as dividing the total aboveground biomass by 6 according to Li et al.48. ∆BB of living bamboo is calculated from the ratio of aboveground woody biomass to the belowground biomass of bamboos. The average annual amount of harvested bamboo was calculated by the annual amount of the bamboo culm production.
N pool and flux estimation
The stem, branch, leaf and coarse root of the main tree species were sampled to measure the N concentration in each stand. We selected three adult trees for each species in each plot. Four subsamples were selected randomly from each adult tree and mixed them together as one sample, thus there was a total of 9 samples for each species. A previous study showed that the N concentration of P. pubescens leaves varies significantly by culm age49. So, the aboveground compartments of P. pubescens were separately sampled on current-year shoots and those older than 1 year. All of the samples were transported to the laboratory, oven dried at 70 °C, and then ground and sieved through a 0.25-mm mesh to measure the N concentration. The fine root samples were obtained by the study published by Liu et al.19. The total N concentration was measured by the micro-Kjeldahl method, in which 0.25 g sample was digested in 5 ml concentrated H2SO4 with a catalyst mixture (CuSO4, K2SO4 and selenium powder) and then distilled50.
N pools were calculated using the following formulas:




where the values of the standing litter storage, including aboveground compartments, were obtained from Song et al.15 and those for the belowground compartment (rhizome, coarse and fine root debris) were obtained from Liu et al.41. The SON content, SIN (NH4+-N+ NO3−-N) content and soil bulk density from the 0–20 soil layers were observed monthly between 2011 and 2012 from Song et al.15.
Main N fluxes were estimated using the following equations47:




All of these estimates of standing storage for biomass and N are expressed as an oven-dry weight per hectare. N fluxes are expressed on an annual basis as the weight per hectare.
Statistical analysis
An ANOVA was used to detect significant differences in the biomass, NPP, N storage, N uptake, N return and N cycling rate between the BDF and EBF. Differences in the N content of plant organs (including stems, branches, leaves, coarse roots, and rhizomes) between the bamboo and broadleaved tree species were detected by the ANOVA and a protected least significant difference test (LSD). P < 0.05 was the criterion for significant differences for all tests. Statistical analyses were performed using SPSS 17.0 for Windows.
Additional Information
How to cite this article: Song, Q.-n. et al. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci. Rep. 7, 40383; doi: 10.1038/srep40383 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Soderstrom, T. R. & Calderon, C. E. A commentary on the bamboos (Poaceae: Bambusoideae). Biotropica, 161–172 (1979).
Okutomi, K., Shinoda, S. & Fukuda, H. Causal analysis of the invasion of broad-leaved forest by bamboo in Japan. J Veg Sci 7, 723–728 (1996).
Tripathi, S. K. et al. The effects of understorey dwarf bamboo (Sasa kurilensis) removal on soil fertility in a Betula ermanii forest of northern Japan. Ecol Res 21, 315–320 (2006).
Yang, Q. P. et al. Ecological studies on bamboo expansion: process, consequence and mechanism. J Plant Ecol 39, 110–124 (2015).
Nakashizuka, T. Regeneration of beech (Fagus crenata) after the simultaneous death of undergrowing dwarf bamboo (Sasa kurilensis). Ecol Res 3, 21–35 (1988).
González, M. E., Veblen, T. T., Donoso, C. & Valeria, L. Tree regeneration responses in a lowland Nothofagus-dominated forest after bamboo dieback in South-Central Chile. Plant Ecol 161, 59–73 (2002).
Lima, R. A. F., Rother, D. C., Muler, A. E., Lepsch, I. F. & Rodrigues, R. R. Bamboo overabundance alters forest structure and dynamics in the Atlantic Forest hotspot. Biol Conserv 147, 32–39 (2012).
Isagi, Y. & Torii, A. Range expansion and its mechanisms in a naturalized bamboo species, Phyllostachys pubescens, in Japan. J Sustain For 6, 1–2, 127–141 (1998).
Kudo, G., Amagai, Y., Hoshino, B. & Kaneko, M. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: pattern of expansion and impact on species diversity. Ecol Evol 1, 85–96 (2011).
Yi, T. P., Shi, J. Y., Ma, L. S., Wang, H. T. & Yang, L. Bamboo altas of China (Science Press, Beijing, China, 2008).
Suzuki, S. & Nakagoshi, N. Expansion of bamboo forests caused by reduced bamboo-shoot harvest under different natural and artificial conditions. Ecol Res 23, 641–647 (2008).
Lin, Y. T. et al. Changes in the soil bacterial communities in a Cedar plantation invaded by moso bamboo. Microb Ecol 67, 421–429 (2014).
Bai, S. B. et al. Plant species diversity and its dynamics in forests invaded by Moso bamboo (Phyllostachys edulis). Biodivers Sci 21, 1–8 (2013).
Fukushima, K., Usui, N., Ogawa, R. & Tokuchi, N. Impacts of moso bamboo (Phyllostachys pubescens) invasion on dry matter and carbon and nitrogen stocks in a broad-leaved secondary forest located in Kyoto, western Japan. Plant Spec Biol 30, 81–95 (2015).
Song, Q. N. et al. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 404, 113–124 (2016).
Song, Y. C. & Chen, X. Degradation mechanism and ecological restoration of evergreen broad-leaved forest ecosystem in east China (Science Press, Beijing, China, 2007).
Xu, Q. F. et al. Bamboo invasion of native broadleaf forest modified soil microbial communities and diversity. Biol Invasions 17, 433–444 (2015).
Liu, S., Zhou, G. M. & Bai, S. B. Light intensity changes on Cunninghamia lanceolata in mixed stands with different concentrations of Phyllostachys pubescens . J Zhejiang A & F Univ 28, 550–554 (2011).
Liu, J. et al. Strategy of fine root expansion of Phyllostachys pubescens population into evergreen broad-leaved forest. J Plant Ecol 37, 230–238 (2013).
Yang, Q. P. et al. Effects of Phyllostachys edulis expansion on carbon storage of evergreen broad-leaved forest in Dagangshan mountain, Jiangxi. Acta Agri Univ Jiangxiensis 33, 529–536 (2011).
Norris, M. D., Blair, J. M. & Johnson, L. C. Altered ecosystem nitrogen dynamics as a consequence of land cover change in Tallgrass Prairie. Am Nat 158, 432–445 (2007).
Hughes, R. F. et al. Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Glob Change Biol 12, 1733–1747 (2006).
Fang, J. Y., Liu, G. H. & Xun, S. N. Biomass and net production of forest vegetation in China. Acta Ecol Sin 16, 497–508 (1996).
Yang, T. H., Song, K., Da, L. J., Li, X. P. & Wu, J. P. The biomass and aboveground net primary productivity of Schima superba-Castanopsis carlesii forests in east China. Sci China Life Sci 53, 811–821 (2010).
Isagi, Y., Kawahara, T., Kamo, K. & Ito, H. Net production and carbon cycling in a bamboo Phyllostachys pubescens stand. Plant Ecol 130, 41–52 (1997).
Li, Z. J., Lin, P., He, J. Y., Yang, Z. W. & Lin, Y. M. Silicon’s organic pool and biological cycle in moso bamboo community of Wuyishan Biosphere Reserve. J Zhejiang Univ Sci B 7, 849–857 (2006).
Piao, S. L., Fang, J. Y., He, J. S. & Xiao, Y. Spatial distribution of grassland biomass in China. J Plant Ecol 28, 491–498 (2004).
Isagi, Y., Kawahara, T. & Kamo, K. Biomass and net production in a bamboo Phyllostachys bambusoides stand. Ecol Res 8, 123–133 (1993).
Torezan, J. M. D. & Silveira, M. The biomass of bamboo (Guadua weberbaueri Pilger) in open forest of the Southwestern Amazon. Ecotropica 6, 71–76 (2000).
Yan, J. H., Wang, Y. P., Zhou, G. Y. & Zhang, D. Q. Estimates of soil respiration and net primary production of three forests at different succession stages in South China. Glob Change Biol 12, 810–821 (2006).
Reich, P. B., Grigal, D. F., Aber, J. D. & Gower, S. T. Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78, 335–347 (1997).
Pastor, J., Aber, J. D., McClaugherty, C. A. & Melillo, J. M. Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65, 256–268 (1984).
Nadelhoffer, K. J., Aber, J. D. & Melillo, J. M. Fine roots, net primary production, and soil nitrogen availability: a new hypothesis. Ecology 66, 1377–1390 (1985).
Vitousek, P. Nutrient cycling and nutrient use efficiency. Am Nat 119, 553–572 (1982).
Mckinley, D. C. & Blair, J. M. Woody plant encroachment by Juniperus virginiana in a mesic native grassland promotes rapid carbon and nitrogen accrual. Ecosystems 11, 454–468 (2008).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: global convergence in plant functioning. PNAS 94, 13730–13734 (1997).
Li, R., Werger, M. J. A., Kroon, H. d., During, H. J. & Zhong, Z. C. Interactions between shoot age structure, nutrient availability and physiological integration in the giant bamboo Phyllostachys pubescens . Plant biol, 437–446 (2000).
Castro‐Díez, P., Godoy, O., Alonso, A., Gallardo, A. & Saldaña, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta‐analysis. Ecol Lett 17, 1–12 (2014).
Norris, M. D., Blair, J. M., Johnson, L. C. & McKane, R. B. Assessing changes in biomass, productivity, and C and N stores following Juniperus virginiana forest expansion into tallgrass prairie. Can J For Res 31, 1940–1946 (2001).
Lett, M. S., Knapp, A. K., Briggs, J. M. & Blair, J. M. Influence of shrub encroachment on aboveground net primary productivity and carbon and nitrogen pools in a mesic grassland. Can J Bot 82, 1363–1370 (2004).
Liu, J. et al. Contribution of fine root to soil nutrient heterogeneity at two sides of the bamboo and broad-leaved forest interface. J Plant Ecol 37, 739–749 (2013).
Knops, J. M. H. & Laungani, R. Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. PNAS 106, 12400–12405 (2009).
Chang, E. H. & Chiu, C. Y. Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Applied Soil Ecology 91, 1–7 (2015).
Li, Y. C. et al. Effects of Phyllostachys edulis invasion of native broadleaf forest on soil fungal community. Chinese Journal of Applied Ecology 27, 585–592 (2016).
Wang, B., Li, H. J. & Guo, Q. S. Study of forest biodiversity in Dagang Mountain, Jiangxi province (China Forestry Publishing House, Beijing, China, 2005).
Yan, E. R., Wang, X. H., Huang, J. J., Li, G. Y. & Zhou, W. Decline of soil nitrogen mineralization and nitrification during forest conversion of evergreen broad-leaved forest to plantations in the subtropical area of Eastern China. Biogeochemistry 89, 239–251 (2008).
Tateno, R. & Takeda, H. Nitrogen uptake and nitrogen use efficiency above and below ground along a topographic gradient of soil nitrogen availability. Oecologia 163, 793–804 (2010).
Li, R., Werger, M. J. A., During, H. J. & Zhong, Z. C. Biennial variation in production of new shoots in groves of the giant bamboo Phyllostachys pubescens in Sichuan, China. Plant Ecology 135, 103–112 (1998).
Li, R., Werger, M. J. A., During, H. J. & Zhong, Z. C. Carbon and nutrient dynamics in relation to growth rhythm in the giant bamboo Phyllostachys pubescens . Plant Soil 201, 113–123 (1998).
Nelson, D. W. & Sommers, L. E. Total carbon, OC, and organic matter. In: Page, A. L., Miller, R. H., Keeney, D. R. (eds) Methods of soil analysis, Part 2 (Agronomy Society of America and Soil Science Society of America, Madison, WI, 1982).
Acknowledgements
We are grateful to the Dagangshan National Forest Ecological Station for providing the study site. We also thank to Dingkun-Yu and Ming Ouyang for their help with field and laboratory work and two anonymous reviewers for helpful comments on this manuscript. This study was supported by the National Natural Science Foundation of China (31260120, 41371328 and 31460077).
Author information
Authors and Affiliations
Contributions
H.L. and Q.P.Y. designed the experiments. Q.N.S. and J.L. carried out the experiments and analyzed the data. Q.N.S., H.L. and Q.P.Y. contributed to interpreting the results and writing the paper. J.Y. and G.Y.Y. helped to revise the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Song, Qn., Lu, H., Liu, J. et al. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci Rep 7, 40383 (2017). https://doi.org/10.1038/srep40383
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40383
This article is cited by
-
Contrasting nitrate leaching from an abandoned Moso bamboo forest and a Japanese cedar plantation: role of vegetation in mitigating nitrate leaching
Plant and Soil (2023)
-
Accessing the nursing behaviour of Moso bamboo (Phyllostachys edilus) on carbohydrates dynamics and photosystems
Scientific Reports (2020)
-
Tall-statured grasses: a useful functional group for invasion science
Biological Invasions (2019)
-
Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China
Annals of Forest Science (2018)
-
Moso bamboo (Phyllostachys pubescens) forests as a significant carbon sink? A case study based on 4‐year measurements in central Taiwan
Ecological Research (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.