Abstract
The carbon (C) pool in forest ecosystems plays a long-term and sustained role in mitigating the impacts of global warming, and the sequestration of C is closely linked to the nitrogen (N) cycle. Accurate estimates C and N storage (SC, SN) of forest can improve our understanding of C and N cycles and help develop sustainable forest management policies in the content of climate change. In this study, the SC and SN of various forest ecosystems dominated respectively by Castanopsis carlesii and Lithocarpus mairei (EB), Pinus yunnanensis (PY), Pinus armandii (PA), Keteleeria evelyniana (KE), and Quercus semecarpifolia (QS) in the central Yunnan Plateau of China, were estimated on the basis of a field inventory to determine the distribution and altitudinal patterns of SC and SN among various forest ecosystems. The results showed that (1) the forest SC ranged from 179.58 ± 20.57 t hm−1 in QS to 365.89 ± 35.03 t hm−1 in EB. Soil, living biomass and litter contributed an average of 64.73%, 31.72% and 2.86% to forest SC, respectively; (2) the forest SN ranged from 4.47 ± 0.94 t ha−1 in PY to 8.91 ± 1.83 t ha−1 in PA. Soil, plants and litter contributed an average of 86.88%, 10.27% and 2.85% to forest SN, respectively; (3) the forest SC and SN decreased apparently with increasing altitude. The result demonstrates that changes in forest types can strongly affect the forest SC and SN. This study provides baseline information for forestland managers regarding forest resource utilization and C management.
Similar content being viewed by others
Introduction
C and N are major constituents of plant and soil organic matter and play a fundamental role in nutrient cycling, plant growth, and ecological functions1,2. Forest SC is the most important part of the global C pool across various terrestrial ecosystems and plays a long-term and continuous role in mitigating the effects of global warming3,4,5. N is a vital and limiting nutrient in forest ecosystems, and C storage is closely linked to the N cycle6. Furthermore, N deposition alters SC and SN 7,8. Consequently, accurate identification of the spatial patterns of forest SC and SN is important for accessing the global C and N pool.
Forest SC is estimated to account for approximately 45% of terrestrial ecosystem SC9,10. In forest ecosystems, C is stored in living biomass, litter and soils11,12,13. Living biomass has a great capacity to sequester atmospheric C and the aboveground living biomass has been considered as a major C pool14,15. Soil is another indispensable component of forest ecosystems and acts as an important C pool in terrestrial ecosystems16,17. The amount of C stored in soil is approximately double the amount in the atmosphere17,18. Consequently, exploring the distribution patterns of SN in forest ecosystems is essential for understanding the C cycle. Many studies have explored the spatial distribution of SC in forest ecosystems at a landscape scale using remote sensing and statistical methods4,12,16,19,20; however, these estimates are not reliable in hilly terrain, because the mountainous and hilly conditions can increase errors of forest vertical structure measured using remote sensors13. Hence, to accurately quantify forest SC at a large scale, it is essential to develop estimates based on ground measurements. Forest inventory data are recognized as one of the most reliable sources of data for global C cycle research4.
The amount of C stored in forest vegetation and soil is considered to be the result of a long-term balance between C absorption and release20,21. The magnitude of SC and SN in forests depends on stand age, species composition, climate variability, geographical circumstances, management strategy and natural disturbances22,23,24. The distribution patterns of SC and SN also differ among spatial landscape patterns, plant species and plant organs25. Mopan Mountain in the central Yunnan Plateau is located in the Yunnan-Guizhou Plateau and the southern margin of the Qinghai-Tibet Plateau. The area belongs to a subtropical mountain climate region26, and vegetation patterns shift vertically due to changes in altitude. The main forest vegetation types are subtropical evergreen broad-leaved forest, subtropical mixed coniferous and broad-leaved forests, coniferous forest and alpine forest. In this region, forests cover more than 72.6% of the land area, and they represent the most important forest resources in the central Yunnan Plateau and in Yunnan Province. The main objectives of this study are to (1) assess the spatial variation in forest biomass based on a field inventory; (2) characterize the spatial variation in C and N density and storage in forest ecosystems; and (3) explore the impact of altitude on biomass and SN and SC in Mopan Mountain. This study will provide baseline information for forestland managers regarding forest resource utilization and C and N management.
Materials and methods
Study area
This study was conducted in Mopan Mountain National Forest Park (23°46′18″N–23°54′34″N, 101°16′06″E–101°16′12″E) in the central Yunnan Plateau of Yunnan province, southwestern China. The total area of the forest is about 7348.5 ha with an altitude from 1260 to 2614 m a.s.l.
The area belongs to a subtropical mountain climate region. The temperature ranges from – 2.2 to 33 °C with a mean annual temperature 15 °C, and the annual rainfall is approximately 1050 mm. Precipitation shows strong seasonal variation with approximately 85% occurring in the rainy season from May to October and the left 15% occurring in the dry season from November to the next April26.
The study sites were occupied by subtropical evergreen broad-leaved forest, coniferous forest and alpine forest dominated respectively by C. carlesii and Lithocarpus mairei (EB), Pinus yunnanensis (PY), Pinus armandii (PA), Keteleeria evelyniana (mixed with the Quercus species, KE) and Quercus semecarpifolia (QS). The characteristics of these forests are listed in Table 1.
Study design and sampling
Study design
To test the variation in SC and SN among various forests, 16 sample plots in each forest were chosen for analysis. These 16 sample plots contained one 100 m × 100 m and fifteen 30 m × 30 m tree plots, and each tree plot had three shrub and three herb plots. The sizes of the shrub and herb plots were 5 m × 5 m and 1 m × 1 m, respectively17. The sample plots were distributed across the altitude range as follows: for QS, from 2467 to 2611 m; for PY, from 2012 to 2151 m; for PA, from 2035 to 2381 m; for KE, from 1865 to 2265 m; and for EB, from 1450 to 2436 m.
Plant census and sampling
In each tree plot, census of plant individuals which diameter at breast height was more than 1 cm was performed. In addition, in each shrub and herb plot, the species name and abundance were recorded2,17,27. All plant individuals in each plot were collected with different parts for C and N testing, i.e., trees with roots, trunks, leaves, branches and bark; shrubs with roots, stems and leaves; and herbs with above- and belowground part.
Litter sampling
Triplicate plots with a size of 1 m × 1 m were established in the tree plots for ground litter sampling28. For each of these samples, horizons L, F and H were separated and carefully placed in plastic bags for determining of the dry weight and C and N contents. The L horizon was composed of fresh or slightly discoloured material that was not weak or friable; the F horizon was composed of medium to strongly fragmented material with many mycelia and thin roots; and the H horizon consisted of humified amorphous material.
Mineral soil sampling
Mineral soil samples were collected from each tree plot, with three replicates. Most of the slope gradients of these soil profiles were less than 15°. After removal of the forest floor mass, soil samples were collected from three layers: 0–20 cm, 20–40 cm and 40–60 cm, and the corresponding soil bulk density (BD) of each layer was measured using the cutting-ring method29. The soil samples were placed in sacks and air dried for soil C and N testing.
Laboratory analysis
Shrub, herb and ground litter samples were dried to a constant weight at 105 °C and then weighed for biomass estimation. Plant and soil total N concentrations were determined by a continuous flow analytical system (Analytical AA3, SEAL, Germany) with sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) digestion30. The total C concentration was determined by an elemental analyser (Vario TOC cube, Elementar, Germany)31.
The estimation of biomass, SC, and SN
The estimation of forest vegetation biomass
Tree biomass (roots, trunks, leaves, branches and bark biomass) was estimated using allometric equations based on long-term practical measurements of forest vegetation in southwestern China32,33,34,35,36,37,38. The shrub and herb biomass was directly expressed as their dry weights. For each forest plot, the total biomass was the sum of the biomass of each vegetation type in the plot.
The estimation of plant, litter and soil SC and SN
The SC (t ha−1) and SN (t ha−1) of trees, shrubs, and herbs were obtained by multiplying the forest vegetation biomass (t ha−1) by the corresponding C and N content coefficient17,29.
The litter SC and SN were the sum of the SC and SN of horizons L, F and H. The litter SC and SN storage was calculated by the following formula17:
where SC (t ha−1) and SN (t ha−1) are the respective litter C and N storage; \(TC_{i}\) and \(TN_{i}\) are the C and N (g kg−1) contents of horizons L, F and H, respectively; and \(LB_{i}\) is the litter biomass (dry litter weight) of horizons L, F and H.
The soil SC and SN were calculated as the sum of the SC and SN of the 0–20 cm, 20–40 cm and 40–60 cm soil layers. The soil SC and SN were calculated using the following formula21,39:
where SC and SN are soil total C storage (t ha−1) and N storage (t ha−1), respectively, \(BD_{i}\) is the soil BD (g cm-3), \(TC_{i}\) and \(TN_{i}\) are the soil total C and N contents (g kg−1), respectively, and \(D_{i}\) is the soil layer thickness (cm).
Statistical analysis
Statistical analyses were carried out using the software Statistical Package for the Social Sciences 19 (SPSS 19) and Microsoft Office Excel (version 2013). One-way ANOVA was used to test whether the variations in SC and SN were significantly different among the plant, litter and soil and forest type components. Duncan’s shortest range test was used to examine the difference among different forest types at P < 0.05. The relationships between altitude and biomass, SC and SN were examined by linear regression.
Results
Biomass in forest ecosystems
The biomass of the forest ecosystems in the central Yunnan Plateau ranged from 142.36 ± 18.36 to 271.77 ± 34.71 t ha−1. The biomass of the forest ecosystems was significantly different among the various forests (Table 2). Plant biomass made a significant contribution to ecosystem biomass and accounted for a much higher proportion (more than 90%) than forest litter. Tree biomass was significantly higher than that of shrubs and herbs in PY, PA, KE and EB and accounted for 99.64%, 94.46%, 95.33% and 95.88% of the total plant biomass, respectively. The tree and shrub biomass in QS accounted for a nearly equal proportion of plant biomass at 46.72% and 51.01%, respectively. The biomass of each component of plants and litter is presented in Fig. 1.
C and N concentrations
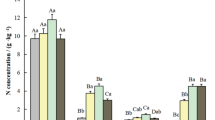
The C and N concentrations in plants, shrubs and herbs varied significantly among forests and their components (Fig. 2 A-B). Generally, the N concentration was classified into three levels by the lines in the figure. The N concentration was highest in the leaves of trees and shrubs and the aboveground parts of herbs, and it ranged from 6.64 ± 2.01 to 21.99 ± 6.66 g·kg−1. The N concentration in tree branches, shrub stems and the L, F and H litter horizons ranged from 3.86 ± 0.90 to 8.78 ± 1.73 g·kg−1, and these values were higher than those in the roots and trunks of trees, shrub roots and soil, which had N concentrations lower than 4.89 ± 1.31 g·kg−1. Significant differences were not observed in the C concentrations in the plant and litter components among different forests, which ranged from 323.21 ± 63.58 to 503.00 ± 97.56 g·kg−1, and the mean C concentration of the forest vegetation and litter was 425.80 ± 100.34 g·kg−1. However, the soil C concentrations were significantly lower than those in the plants and litter, i.e., less than 81.08 ± 13.62 g kg−1, with a mean of 29.74 ± 12.20 g·kg−1.
SC and SN
The ecosystem SC was calculated as the sum of the plant SC, litter SC and soil SC. The ecosystem SC was significantly different among the forests (Table 3 and Fig. 3 A). The ecosystem SC ranged from a high of 365.89 ± 35.03 t hm−1 in EB to a low of 179.58 ± 20.57 t hm−1 in QS. The levels of ecosystem SC in PY, PA and KE were 258.38 ± 24.92, 203.01 ± 19.79 and 326.89 ± 31.71 t hm−1, respectively. The soil SC contributed 62.40–67.06% to the ecosystem SC in various forests and was higher than the contributions of plants and litter. Plant SC accounted for 29.50–34.91%, and litter SC accounted for only 1.35–5.15% of ecosystem SC.
The plant SC of different forests varied significantly from 62.70 ± 11.33 t hm−1 in QS to 120.35 ± 13.01 t·hm−1 in EB, although the difference between QS and PA was not significant. Tree SC contributed more than 94% to the plant SC in PY, PA, KE and EB; however, the tree SC of QS contributed only 47.11% to the plant SC. The shrub SC of QS accounted for a high proportion of 50.78% of the plant SC, whereas the shrub and herb SC in the other four forests contributed less than 1% to the plant SC. The litter SC varied from a high concentration of 13.20 ± 2.12 t hm−1 in PY to a low concentration of 4.82 ± 0.77 t·hm−1 in QS. Generally, the SC of different layers among the forests decreased in the order of H > F > L, while the litter SC in EB decreased in the order of H > L > F. The highest soil SC was in EB at 240.59 ± 32.90 t hm−1. The soil SC in KE was 219.21 ± 29.98 t hm−1, which was significantly lower than that in EB but significantly higher than that in PY and PA, which were 164.42 ± 22.90 t hm−1 and 129.20 ± 17.67 t hm−1, respectively. The lowest soil SC was in QS at 112.06 ± 15.32 t hm−1. In KE, the SC at 20–40 cm was higher than that at 0–20 cm and the soil SC decreased with increasing soil depth.
The SN of the forest ecosystems varied significantly among the forests, although significant differences were not found between PY and PA (Table 3 and Fig. 3 B). The Ecosystem SN ranged from 8.91 ± 1.83 t·ha−1 in EB to 4.47 ± 0.94 t·ha−1 in PA, and the SN in KE, QS and PY was 7.13 ± 1.52 t·ha−1, 6.36 ± 1.19 t ha−1 and 5.14 ± 1.10 t ha−1, respectively. Soil was the most important contributor to total SN in the forest ecosystems and accounted for an average of 86.88% of Eco SN. Plant contributions to Eco SN ranged from a high concentration of 15.83% in QS to a low concentration of 6.63% in KE, and litter only contributed an average of 2.85%.
The plant SN differed significantly among the forests with different species and ranged from a high concentration of 1.11 ± 0.33 t·ha−1 in EB to a low concentration of 0.39 ± 0.17 t·ha−1 in PA. The tree SN of PY, PA, KE and EB accounted for more than 85% of the living biomass SN, and the shrubs and herbs contributed less than 15%. However, the shrubs in QS stored more N than trees and the SN of shrubs and trees contributed 24.42% and 70.36% to the living biomass SN, respectively. The estimated mean SN of forest litter was 0.17 ± 0.01 t·ha−1, and the H layer stored approximately half of the litter N. The soil is a large N pool in forest ecosystems, and in this study, the soil SN accounted for 86.88% on average of Eco SN. More SN was stored in the topsoil (0–20 cm), with a contribution of 53.69%, 45.71%, 45.87%, 39.27% and 42.85% to the total soil (0–60 cm) SN in QS, PY, PA, KE and EB, respectively.
Correlation analysis of biomass, SC and SN in forest ecosystems and altitude
The generalized linear model illustrates the effects of altitude on biomass, SC and SN of forest ecosystems, which decreased with increasing altitude (Fig. 4A-C). Whether calculated within the same forest or across all forests, significant correlations were found between the altitude and biomass at the P < 0.005 level (Table 4). The SC of QS, PA, KE and EB was also significantly (P < 0.005) correlated with altitude, but for PY, the correlation between SC and altitude was significant at the P < 0.05 level. In all forests, SC decreased significantly (P < 0.001) with increasing altitude. A significant correlation (P < 0.005) was observed between SN and altitude in QS, PA, KE and EB, and a less significant correlation was observed between SN and altitude (P < 0.01) in PY. However, for all forests, the variation in SN was not significant (P = 0.400) with respect to altitude.
Discussion
Different forest ecosystems have different C sequestration capacities. The total SC values of the forest ecosystems of KE and EB in Mopan Mountain in the central Yunnan Plateau are apparently higher than the average values of forest ecosystems (258.83 t C∙ha−1) across China27,40, whereas the total SC values of the forest ecosystems of QS PA and PY are lower. The results of the present research show that changes in forest types can strongly affect SC and SN values. Generally, broad-leaved species can store more C and N than conifers30,41. Although the alpine forest (QS) had the lowest SC, its SN was higher than that in PY and PA. The SC and SN of forests in Mopan Mountain in the central Yunnan Plateau averaged 266.75 ± 26.40 t ha−1 and 6.40 ± 1.32 t ha−1, respectively. With respect to SC, the living biomass, litter layer and soil accounted for 31.72, 3.55% and 64.73% of the total C storage, respectively. The corresponding SN accounted for 10.27%, 2.85% and 86.88% of the total N storage, respectively. The current and previous results indicate that the soil is the most important component for SC and SN in forest ecosystems42,43.
The living biomass of forests is one of the major C and N pools. Quantification of stored C in the living biomass of a forest is necessary for future management 44. The estimated mean living biomass SC in this study was 84.12 t·ha−1, which is much higher than the average values of vegetation C storage in Chinese forest ecosystems (57.07 t ha−1)45,46. This finding is mainly because of the high tree density and low anthropogenic disturbance at the location of Mopan Mountain National Forest Park. The tree growth rate and biomass allocation to different tree parts and varying rates of C sequestration in ecosystem components can affect the rate of C sequestration and longevity of C storage2,41. The present study showed that the SC in plants ranged from 62.70 ± 11.33 t·ha−1 in QS to 120.35 ± 13.01 t·ha−1 in EB, which accounted for 31.72% of the total C storage. Among all forests, QS had the lowest SC in living biomass, which was caused by its lower biomass and lower C concentration in living biomass. However, the higher biomass in EB resulted in higher SC of living biomass compared with the other groups in Mopan Mountain in the central Yunnan Plateau. The SN in living biomass varied from a high of 1.11 ± 0.33 t·ha−1 in EB to a low of 0.39 ± 0.17 t·ha−1 in PA, with a mean contribution of 10.27% to total SN.Tree SC and SN accounted for a large proportion of living biomass SC and SN in PY, PA, KE and EB, whereas shrubs contributed more C and N than trees to living biomass SC and SN in QS. The SC and SN of vegetation are mainly determined by the biomass of live vegetation components and C and N contents. Consequently, the interspecific differences in tree biomass caused by inherent variation in growth rates47,48,49 were the main reasons for the variations in SC and SN allocation among forests. Furthermore, the effect of forest species on the growth and diversity of understorey plant biomass2,30,50 also resulted in the variation in SC and SN allocation in forest vegetation.
Forest litter and its decomposition rate are key factors in nutrient cycling in forest ecosystems51, and the current litter SC in the world’s forests is estimated at 43 ± 3 Pg·C (5% of total forest C)52. In the present study, the estimated mean litter SC and SN in the forests were 8.93 ± 1.44 t ha−1 and 0.17 ± 0.01 t ha−1, which accounted for 3.55% and 2.85% of the total SC and SN, respectively. The mean litter SC in this study is slightly higher than the mean litter SC in China (8.21 t·ha−1)49. The study also found that conifer litter stored more C and N than broadleaf litter, and a similar result was found in previous studies41,53. The above results occurred mainly because conifer litter is more difficult to decompose than broadleaf litter, resulting in a higher rate of litter accumulation on the forest floor.
The estimated mean soil SC and SN of different forests in this study were 173.70 ± 23.75 t·ha−1 and 5.56 ± 1.08 t·ha−1, which accounted for 64.73% and 86.88%, respectively, of the total SC and SN. The results showed that soil is the largest C pool in forest ecosystems, similar to a previous study conducted in China2,30,42. The mean reported value of soil SC was 193.55 t ha−1 in Chinese forest ecosystems 45,46, and the soil SN was 6.27 t ha−1 in subtropical forests of China54. The SC and SN of KE and EB was higher and that of the other forests in this study was lower than the mean soil SN in China and soil SN in subtropical forests in China. The C stored in soil is significantly influenced by the C inputs (e.g., litter decomposition) and soil organic matter decomposition55. Therefore, SC is determined by the balance between the input or output patterns and controlled mainly by tree species under similar environmental conditions17,41. There were significant differences in the soil SC and SN at depths of 0–20 cm, 20–40 cm and 40–60 cm among the forests. The topsoil (0–20 cm) in the forests stored 43.38% of the C and 45.48% of the N from 0 to 60 cm. The soil C and N were mainly stored in the topsoil42,43,56, which is probably because of the variation in the soil bulk density and concentrations of C and N in soil layers, which are two important determining factors of SC and SN at fixed soil depths 17,57. Although the soil bulk density decreased with increasing soil depth, the topsoil contained more C and N.
The forest ecosystem biomass (158.47 ± 27.37 to 283.68 ± 37.14 t·ha−1, with an average of 208.81 ± 29.60 t ha−1), SC (179.58 ± 20.57 to 365.89 ± 35.03 t·ha−1, with an average of 266.75 ± 26.40 t ha−1) and SN (4.47 ± 0.94 to 8.91 ± 1.83 t ha−1 with an average of 6.40 ± 1.32 t ha−1) in the five forests in Mopan Mountain decreased with increasing altitude, although the SC of PY and the SN of all forest ecosystems in this study were not highly significantly correlated with altitude. Previous reports indicated that the soil SC in forest ecosystems increases with altitude58,59 and the living biomass and total SC of forest ecosystems decreased significantly with increasing latitude in different regions60,61 because increasing altitude changed the climate factors (i.e., temperature and precipitation) and resulted in the shifting of vegetation types and a decline in net primary production and litterfall58,62,63. The vegetation patterns in the study area shifted vertically due to changes in altitude. With increasing altitude, the forest vegetation types in this area shifted from subtropical evergreen broad-leaved forest, subtropical mixed coniferous and broad-leaved forest, and coniferous forest to alpine forest, and the living biomass of the forests declined significantly. Therefore, the total SC and SN of forest ecosystems exhibited decreasing trends with increasing altitude.
References
Sharrow, S. H. & Ismail, S. Carbon and nitrogen storage in agroforests, tree plantations, and pastures in western Oregon, USA. Agrofor. Syst. 60(2), 123–130 (2004).
Yang, L. L. et al. Carbon and nitrogen storage and distribution in four forest ecosystems in Liupan Mountains, Northwestern China. Acta. Ecol. Sin. 35(15), 5215–5227 (2015).
Watson, R. T. et al. Land use, land-use change, and forestry. In: Published for the Intergovernmental Panel on Climate Change. Cambridge University Press, pp. 308 (2000).
Zhao, M. M. et al. Estimation of China’s forest stand biomass carbon sequestration based on the continuous biomass expansion factor model and seven forest inventories from 1977 to 2013. For. Ecol. Manag. 448, 528–534 (2019).
Dale, V. H. et al. Climate change and forest disturbances. Bioscience 51, 723–734 (2001).
Gunderson, P. Carbon—Nitrogen Interactions in Forest Ecosystems—Final Report. Danish Centre for Forest, Landscape and Planning, Denmark (2006).
Hook, P. B. & Burke, I. C. Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate. Ecology 81, 2686–2703 (2000).
Vourlitis, G. L., Zorba, G., Pasquini, S. C. & Mustard, R. Carbon and nitrogen storage in soil and litter of southern Californian semi-arid shrublands. J. Arid Environ. 70, 164–173 (2007).
Bonan, G. B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008).
Liu, G. H., Fu, B. & Fang, J. Y. Carbon dynamics of Chinese forests and its contribution to global carbon balance. Acta. Ecol. Sin. 20(5), 733–740 (2000).
IPCC. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (2007).
Phillips, J. et al. Live aboveground carbon stocks in natural forests of Colombia. For. Ecol. Manag. 374, 119–128 (2016).
Gibbs, H. K., Brown, B., Niles, J. O. & Foley, J. A. Monitoring and estimating tropical forest carbon stocks: making REDD a reality. Environ. Res. Lett. 2(4), 1–13 (2007).
Aragão, L. et al. Above- and below-ground net primary productivity across ten Amazonian forests on contrasting soils. Biogeosciences 6, 2759–2778 (2009).
Malhi, Y. et al. Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Glob. Chang. Biol. 15, 1255–1274 (2009).
Post, W. M. & Kwon, K. C. Soil carbon sequestration and land use change: processes and potential. Glob. Chang. Biol. 6, 317–327 (2000).
Ma, J. et al. Ecosystem carbon storage distribution between plant and soil in different forest types in Northeastern China. Ecol. Eng. 81, 353–362 (2015).
Davidson, E. A., Trumbore, S. E. & Amundson, R. Biogeochemistry—soil warming and organic carbon content. Nature 408, 789–790 (2000).
Chaturvedi, R. K. & Raghubanshi, A. S. Aboveground biomass estimation of small diameter woody species of tropical dry forest. New For. 44, 509–519 (2013).
Wen, D. & He, N. P. Forest carbon storage along the north-south transect of eastern china: spatial patterns, allocation, and influencing factors. Ecol. Indic. 61, 960–967 (2016).
Fan, S. et al. A large terrestrial carbon sink in North America implied by atmospheric andoceanic carbon dioxide data and models. Science 282, 442–446 (1998).
Gough, C. M., Vogel, C. S., Schmid, H. P. & Curtis, P. S. Controls on annual forest carbon storage: lessons from the past and predictions for the future. Bioscience 58, 609–622 (2008).
Van Deusen, P. Carbon sequestration potential of forest land: Management for products and bioenergy versus preservation. Biomass Bioenerg. 34, 1687–1694 (2010).
Bradford, J. B., Jensen, N. R., Domke, G. M. & D’Amato, A. W. Potential increases in natural disturbance rates could offset forest management impacts on ecosystem carbon stocks. For. Ecol. Manag. 308, 178–187 (2013).
Park, A. Carbon storage and stand conversion in a pine-dominated boreal forest landscape. For. Ecol. Manag. 340, 70–81 (2015).
Wang, S. J., Zhao, J. X. & Chen, Q. B. Controlling factors of soil CO2 efflux in Pinusyunnanensis across different stand ages. PLoS ONE 10(5), e0127274. https://doi.org/10.1371/journal.pone.0127274 (2015).
Liu, J. et al. Distinct soil bacterial communities in response to the cropping system in a Mollisol of northeast China. Appl. Soil Ecol. 119, 407–416 (2017).
Kavvadias, V. A. et al. Litterfall, litter accumulation and litter decomposition rates in four forest ecosystems in northern Greece. For. Ecol. Manag. 144, 113–127 (2001).
Dai, W. et al. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of Southeastern China. For. Ecol. Manag. 409, 288–297 (2018).
Liu, S. et al. Carbon and nitrogen storage and distribution in different forest ecosystems in the subalpine of western Sichuan. Acta. Ecol. Sin. 37(4), 1074–1083 (2017).
Kern, J., Giani, L., Teixeira, W., Lanza, G. & Glaser, B. What can we learn from ancient fertile anthropic soil (Amazonian Dark Earths, shell mounds, Plaggen soil) for soil carbon sequestration?. CATENA 172, 104–112 (2019).
Zhang, Z. H., Wang, L. C., Luo, J. X. & Zheng, D. R. Study on tree biomass models of Pinus Yunnanensis Faranch in Northwest Yunnan Province. J. Shandong For. Sci. Technol. 4, 4–6 (2011) ((in Chinese)).
Chen, C. Biomass and production of the Arbor-Layers in Pinus armandii forests. J. Northwestern Coll. For. 1, 1–18 (1984) ((in Chinese)).
Liu, S. R., Su, Y. M., Cai, X. H. & Ma, Q. Y. Aboveground biomass of quercus aquifolioides shrub community and its responses to altitudinal gradients in balangshan mountain, Shichuan province. Sci. Silvae. Sin. 42, 1–7 (2006) ((in Chinese)).
Li, J. L., Liang, S. C. & Chen, S. Z. A preliminary study on the biomass models of keteleeria davidiana var chien-peii colony in qingyan town of Guizhou province. J. Guizhou Normal Univ. 15, 7–12 (1997) ((in Chinese)).
Yang, L. L. et al. Carbon and nitrogen storage and distribution in four forest ecosystems in Liupan Mountains, northwestern China. Acta. Ecol. Sin. 35, 5215–5227 (2015) ((in Chinese)).
Xie, S. C., Liu, W. Y., Li, S. C. & Yang, G. P. Preliminary studies on the biomass of middle-mountain moist evergreen broadleaved forests in Ailao Mountain, Yunnan. Acta Phytoecol. Sin. 20, 167–176 (1996) ((in Chinese)).
Shen, Y., Tian, D. L., Yan, W. D. & Xiao, Y. Biomass and its distribution of natural secondary quercus fabri + sassafras tsumu+ cunninghamia lanceolata community in Yuanling county, Hunan province. J. Cent. South Univ. For. Technol. 31, 44–51 (2011) ((in Chinese)).
Guo, L. B. & Gifford, R. M. Soil carbon stocks and land use change: a meta analysis. Global Change Biol. 8, 345–360 (2002).
Zhou, Y. R., Yu, Z. L. & Zhao, S. D. Carbon storage and budget of major Chinese forest types. Acta. Phytoecol. Sin. 24, 518–522 (2000) ((in Chinese)).
Eslamdoust, J. & Sohrabi, H. Carbon storage in biomass, litter, and soil of different native and introduced fast-growing tree plantations in the South Caspian Sea. J. For. Res. 29, 449–457 (2018).
He, Y. J. et al. Carbon storage capacity of monoculture and mixed-species plantations in subtropical China. For. Ecol. Manag. 295, 193–198 (2013).
Ren, H. et al. Spatial and temporal patterns of carbon storage from 1992 to 2002 in forest ecosystems in Guangdong, Southern China. Plant Soil 363, 123–138 (2013).
Ali, F., Khan, N., Ahmad, A. & Khan, A. A. Structure and biomass carbon of Olea ferruginea forests in the foot hills of Malakand division, Hindukush range mountains of Pakistan. Acta. Ecol. Sin. 39, 261–266 (2019).
Ren, Y. et al. Potential for forest vegetation carbon storage in Fujian Province, China, determined from forest inventories. Plant Soil 345, 125–140 (2011).
Fu, W. J. et al. Spatial variation of biomass carbon density in a subtropical region of Southeastern China. Forests 6, 1966–1981 (2015).
Fonseca, W., Alice, F. E. & Rey-Benayas, J. M. Carbon accumulation in aboveground and belowground biomass and soil of different age native forest plantations in the humid tropical lowlands of Costa Rica. New For. 43, 197–211 (2012).
Nelson, A., Saunders, M., Wagner, R. & Weiskittel, A. Early stand production of hybrid poplar and white spruce in mixed and monospecific plantations in eastern Maine. New For. 43, 519–534 (2012).
Gao, Y., Cheng, J., Ma, Z., Zhao, Y. & Su, J. Carbon storage in biomass, litter, and soil of different plantations in a semiarid temperate region of northwest China. Ann. For. Sci. 71, 427–435 (2014).
Fortier, J., Gagnon, D., Truax, B. & Lambert, F. Biomass and volume yield after 6 years in multiclonal hybrid poplar riparian buffer strips. Biomass Bioenerg. 34, 1028–1040 (2010).
González-Rodríguez, H. et al. Litterfall deposition and leaf litter nutrient return in different locations at Northeastern Mexico. Plant Ecol. 212, 1747–1757 (2011).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science https://doi.org/10.1126/science.1201609 (2011).
Bradford, J. B., Birdsey, R. A., Joyce, L. A. & Ryan, M. G. Tree age, disturbance history and carbon stocks and fluxes in subalpine rocky mountain forests. Global Change Biol. 14, 2882–2897 (2008).
Zhang, C. N., Yan, X. D. & Yang, J. H. Estimation of nitrogen reserves in forest soils of China. J. Southwest Agric. Univ. 26, 572-575+579 (2004) ((in Chinese)).
Lee, K. L., Ong, K. H., King, P. J. H., Chubo, J. K. & Su, D. S. A. Stand productivity, carbon content, and soil nutrients in different stand ages of Acacia mangium in Sarawak, Malaysia. Turk. J. Agric. For. 39, 154–161 (2015).
Cao, B., Domke, G. M., Russell, M. B. & Walters, B. F. Spatial modeling of litter and soil carbon stocks on forest land in the conterminous United States. Sci. Total Environ. 654, 94–106 (2019).
Deng, L., Wang, K. B., Chen, M. L., Shangguan, Z. P. & Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. CATENA 110, 1–7 (2013).
Zhu, B. et al. Altitudinal changes in carbon storage of temperate forests on Mt Changbai, Northeast China. J. Plant Res. 123, 439–452 (2010).
Xie, X. L., Sun, B., Zhou, H. Z. & Li, A. B. Soil organic carbon storage in China. Pedosphere 14, 491–500 (2004).
Leuschner, C., Moser, G., Bertsch, C., Röderstein, M. & Hertel, D. Large altitudinal increase in tree root/shoot ratio in tropical mountain forests of Ecuador. Basic Appl. Ecol. 8, 219–230 (2007).
Singh, S. P., Adhikari, B. S. & Zobel, D. B. Biomass, productivity, leaf longevity, and forest structure in the central Himalaya. Ecol. Monog. 64, 401–421 (1994).
Kirschbaum, M. U. F. Will changes in soil organic carbon act as a positive or negative feedback on global warming?. Biogeochemistry 27, 753–760 (2000).
Raich, J. W., Russel, A. E., Kitayama, K., Parton, W. J. & Vitousek, P. M. Temperature influences carbon accumulation in moist tropical forests. Ecology 87, 76–87 (2006).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Granted Nos. 32060345 and 31660146), Applied Basic Research Programs of Yunnan Province (2018FG001-050), Ph.D. Program Foundation of Southwest Forest University (111705) and the Yunnan Provincial Key Laboratory of Soil Erosion and Control.
Author information
Authors and Affiliations
Contributions
J.L. and Q.C. contributed to the conception of the study. J.L., Q.C., Z.L., B.X., J.Z., X.X., B.Z., and D.S. performed the investigation and experimental test. J.L. performed data analysis and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Chen, Q., Li, Z. et al. Distribution and altitudinal patterns of carbon and nitrogen storage in various forest ecosystems in the central Yunnan Plateau, China. Sci Rep 11, 6269 (2021). https://doi.org/10.1038/s41598-021-85710-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85710-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.