Abstract
Human anaplastic lymphoma kinase (ALK) has been identified as an oncogene that is mutated or amplified in NBLs. To obtain a better understanding of the molecular events associated with ALK in the pathogenesis of NBL, it is necessary to clarify how ALK gene contributes to NBL progression. In the present study, we found that ALK expression was significantly high in NBL clinical samples with amplified MYCN (n = 126, P < 0.01) and in developing tumors of MYCN-transgenic mice. Indeed, promoter analysis revealed that ALK is a direct transcriptional target of MYCN. Overexpression and knockdown of ALK demonstrated its function in cell proliferation, migration and invasion. Moreover, treatment with an ALK inhibitor, TAE-684, efficiently suppressed such biological effects in MYCN amplified cells and tumor growth of the xenograft in mice. Our present findings explore the fundamental understanding of ALK in order to develop novel therapeutic tools by targeting ALK for aggressive NBL treatment.
Similar content being viewed by others
Introduction
Neuroblastoma (NBL) is an embryonal malignancy derived from precursor cells of the sympathetic nervous system and accounts for 7–10% of childhood cancers and around 15% of cancer deaths in children1. Though some subsets of NBL undergo spontaneous regression without therapy, about 60–70% of high-risk NBL patients are resistant to currently available therapies and have poor prognoses1,2,3. The genetic feature most consistently associated with treatment failure is an amplification of the MYCN proto-oncogene, which is strongly correlated with advanced disease4,5,6,7. Even in otherwise favorable localized disease, MYCN amplification indicates poor outcome, underscoring its biological importance. Indeed, upregulation of MYCN in NBL cells resulted in accelerated proliferation, migration and invasion8,9,10,11. Consistent with these observations, transgenic mice overexpressing MYCN in neural crest-derived tissues displayed frequent development of NBL12, suggesting that upregulated expression of MYCN is causative in the genesis and development of NBL in vivo. However, the role of MYCN expression and its molecular mechanisms to induce an aggressive phenotype are still unclear. Identification of its direct transcriptional target gene(s) may provide a novel insight into understanding the functional contribution of MYCN in malignant phenotypes of aggressive NBL.
The MYC family of proto-oncogenes belongs to the basic helix-loop-helix leucine-zipper class of transcription factors. MYC proteins (MYCN and c-Myc) share several regions of homology and similar cellular functions that target proliferative pathways vital for cancer progression. Members of this family function as heterodimers with MAX and exert transcriptional activity by specifically binding to a consensus E-box motif (CACGTG) located within the promoter regions of a diverse set of target genes13,14,15. Although a handful of MYCN target genes involved in MYCN-driven cell proliferation and apoptosis have been identified, the target genes responsible for MYCN-mediated cell migration and invasion remain elusive.
Anaplastic lymphoma kinase (ALK) has been identified as a gene upregulated in unfavorable NBL, suggesting a possible oncogenic role for this receptor tyrosine kinase, which was previously linked with NBL16,17. Recently, ALK point mutations were described in 3–11% of sporadic NBL and were found to be one of the most important types of mutations in hereditary NBL18,19,20,21,22. More recently, Passoni et al. described NBL patients with high levels of ALK expression without ALK gene mutations. They showed that regardless of mutation status, high ALK levels were strongly correlated with poor prognosis23. This correlation between high ALK levels and unfavorable prognosis was also confirmed by some other investigators24,25,26. Moreover, Di Paolo et al. demonstrated that RNA interference (RNAi)-based knockdown of ALK, regardless of its genetic status, showed reduced proliferation and increased apoptosis in NBL cells and inhibited NBL tumor growth as well as prolonged survival in vivo27.
In the present study, we found that ALK directly mediates MYCN-induced oncogenic properties. The promoter region of ALK gene contains a non-canonical E-box located upstream of the transcription initiation site and MYC proteins bind onto the promoter region and regulate its transcription. Wild-type ALK functions as a modulator of proliferation as well as cell migration and invasion. In addition, those biological activities and tumor growth in xenograft model derived from NBL cell lines with MYCN amplification were inhibited by targeting wild-type ALK with TAE-684, suggesting that highly expressed ALK in MYCN amplified cells could be inhibited by ALK inhibitor in the same manner as mutated or amplified ALK. These findings may be beneficial to the understanding of the molecular mechanism of wild-type ALK function and contribute to the development of a possible therapeutic strategy for ALK-expressing NBLs.
Results
ALK mRNA expression is associated with MYCN amplification and expression in neuroblastomas
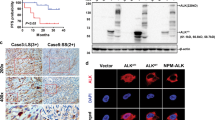
The expression of MYCN and ALK mRNA was measured by quantitative real-time PCR (qRT-PCR) for cDNA samples obtained from NBL clinical tissues. MYCN expression was significantly higher in tumors with MYCN amplification than MYCN-non-amplified tumors (P < 0.001; Figure 1a). In this subset of NBLs with MYCN amplification, mRNA expression of ALK was significantly higher as compared with MYCN-non-amplified tumors (P < 0.01; Figure 1a). High expression of ALK was also observed in NBLs at stages 3, 4 and 4S (Figure 1b), suggesting that ALK might contribute to an aggressiveness and metastasis of NBL.
Endogenous ALK expression is correlated with MYCN expression levels.
(a) ALK expression in NBL clinical samples. The expression levels of MYCN (left) and ALK (right) mRNA in subsets of MYCN-non-amplified and amplified NBL clinical samples. (b) ALK mRNA in different stages of NBL (INSS stages). (c) High expression of MYCN and Alk mRNA in MYCN-transgenic mice. MYCN and Alk expression were investigated by RT-PCR in SMG tissues of 2-week-old wild-type and MYCN-hemizygous mice (left) and in adrenal gland tumor tissues of MYCN-hemizygous mice at 9, 10 and 11 weeks old (right).
Both MYCN and c-Myc regulates ALK expression
In a MYCN transgenic mice model, the expression of the human MYCN oncogene was targeted to neural crest cells with the use of a tyrosine hydroxylase promoter12. This promoter is active in migrating cells of the neural crest early in the development of sympathetic ganglia and the adrenal medulla from which NBLs often arise28. Expression of both human MYCN and endogenous Alk mRNA was induced in superior mesenteric ganglion (SMG) tissues of 2-week-old MYCN-hemizygous mice (Figure 1c), which continued in adrenal tumor tissues until the mice were at least 11 weeks old. Consistent with the transgenic mice data, overexpression of MYCN or c-Myc in NBL (wild-type ALK cell line; NBL-S and NLF and mutated ALK cell line; SH-SY5Y) and non-NBL (U2OS and HeLa) cells induced ALK expression in dose- and time-dependent manners at the mRNA (Figures 2a–c and Supplementary Figure S1A) and protein (Supplementary Figures S1B and C) levels. Next, we performed siRNA-mediated knockdown of MYCN experiment in SK-N-AS cells as described previously29 and found that MYCN knockdown decreased expression of ALK (Figure 2d). To confirm a possible relationship between MYCN and ALK, we employed MYCN-inducible neuroblastoma cells (Tet21/N) derived from a parental neuroblastoma cell line, SHEP8. The Tet21/N cells constitutively expressed MYCN in the absence of tetracycline (Tc), whereas the addition of Tc to the culture decreased MYCN expression levels. At the indicated time points after Tc depletion, total RNA was prepared and subjected to RT–PCR. As shown in Figure 2e, Tc deprivation led to an induction of MYCN in association with a significant increase in the expression levels of ALK. In contrast, addition of Tc significantly reduced the expression levels of MYCN with a concomitant decrease in ALK expression levels (Figure 2e), suggesting that ALK might be a direct transcriptional target of MYCN.
MYC proteins regulate the expression of ALK.
(a) NBL-S cells were transfected with different amounts of MYCN or c-Myc expression vector. (b) SH-SY5Y (F1174L ALK mutation) cells were transfected with MYCN expression vector as (a). (c) U2OS cells were transfected with MYCN or c-Myc expression vector and the expression of ALK mRNA was examined in a time- (left) or dose- (middle and right) dependent manner. At the indicated time points (for time-dependent) or at 24 h (for dose-dependent) after transfection, the expression of ALK, MYCN or c-MYC was checked by RT-PCR. (d) siRNA-mediated knockdown of MYCN downregulated ALK expression. SK-N-AS cells were transfected with control siRNA or siRNA targeting MYCN (siMYCN-1 and -2). Seventy-two hours after transfection, total RNA was prepared and processed for RT-PCR. (e) Induction of ALK in MYCN-inducible SHEP Tet21/N cells (F1174L ALK mutation). RT-PCR of ALK and MYCN expression was performed after the removal (left) or addition (right) of tetracycline (Tc, 100 ng/ml) at the indicated time intervals.
ALK is a direct transcriptional target of MYCN and c-Myc
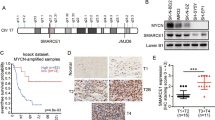
To identify a possible promoter region of ALK gene, we generated luciferase reporter constructs containing −2056 bp to +30 bp fragments of ALK gene. We performed promoter study using NBL (mutated ALK cell line; SH-SY5Y and wild-type ALK cell line; SK-N-AS) and non-NBL (U2OS and HeLa) cells, which showed co-related regulation of ALK gene with MYCN or c-MYC expression (Figures 2b–d and Supplementary Figure S1A). An increase in luciferase activity was observed in cells transfected with pGL4.17 ALK (−2056 bp) compared with empty pGL4.17-basic vector (Figure 3a and Supplementary Figure S2A). The luciferase activity with pGL4.17 ALK (−2056 bp) was enhanced by co-expression of increasing amounts of MYCN or c-Myc expression vector (Figure 3b and Supplementary Figure S2B).
Transcription of ALK is directly regulated by MYC proteins.
(a) SH-SY5Y cells were transfected with ALK luciferase reporter construct (−2056 bp) or empty vector and subjected to luciferase reporter assays. (b) Overexpression of both MYCN and c-Myc enhanced the basal promoter activity of ALK. SH-SY5Y cells were co-transfected with ALK (−2056 bp) and increasing amounts of MYCN or c-Myc expression vector. Luciferase assays were then performed to measure the promoter activity. (c) Both MYCN and c-Myc were recruited onto the ALK promoter region. Schematic drawing of the 5′-upstream region of human ALK indicates the positions of putative E-boxes (E1 and E2). Primer sets (F1 R1 and F2 R2) used for ChIP assays are indicated by arrows (top). HeLa cells were transiently transfected with MYCN expression plasmid. Forty-eight hours after transfection, ChIP assays were performed using anti-MYCN antibody (middle). To detect the recruitment of endogenous MYCN (bottom left) and c-Myc (bottom right), ChIP assays were carried out in the indicated cell lines using anti-MYCN or anti-c-Myc antibodies. (d) E1 and E2 are important for the transcriptional activation of ALK. Site-specific deletions were introduced into the parental core promoter (−350 bp) of the luciferase reporter construct at the indicated E-boxes (left panel). SK-N-AS cells were simultaneously transfected with parental or deletion mutants of luciferase reporter constructs together with MYCN or c-Myc expression vector. The graph shows the relative luciferase activity driven by the expression of MYC proteins.
Next, we performed ChIP assays with anti-MYCN and anti-c-Myc antibodies to determine whether they could directly bind to the ALK promoter. As shown in Figure 3c, MYCN was recruited onto the ALK promoter region. Endogenous MYCN was recruited onto the same region in TNB1 cells (MYCN amplification and high expression of ALK, Supplementary Figure S7A) but not in RISA cells (MYCN-non-amplified and low ALK expression). Moreover, in non-NBL cell lines, the recruitment of endogenous c-Myc was more obvious in A875 cells (high ALK expression, Supplementary Figure S7B) than HeLa cells (low ALK expression) (Figure 3c). Since the region to which MYC proteins were recruited contains two possible E-boxes (E-box1 and E-box2), E-box deletions were introduced in the luciferase reporter construct (−350 bp). Deletion of the E-box1 and/or E-box2 resulted in a significant reduction of ALK gene promoter activity in SK-N-AS (Figure 3d) and HeLa cells (Supplementary Figure S2C).
ALK shows oncogenic potential in NBL cells
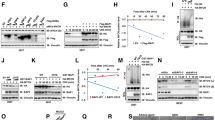
Consistent with previous reports23,30, our results also showed that ectopic expression of wild-type or mutated (F1174L) ALK induced the phosphorylation of both ALK and an ALK-associated signaling molecule, AKT (Supplementary Figure S4A). The siRNA-mediated knockdown of endogenous ALK resulted in reduced phosphorylation of AKT in NBL cells harboring a wild-type allele (Supplementary Figure S4B). Next, we investigated cell growth study using NBL cells (SK-N-DZ, SK-N-AS and NBL-S), which showed activation of the downstream signaling molecule AKT by ALK overexpression (Supplementary Figure S4A). We also performed siRNA mediated knockdown of ALK experiment in MYCN amplified NLF cells. Figure 4a and 4b shows that overexpression of ALK enhanced cell proliferation, whereas siRNA-mediated knockdown of ALK was correlated with an inhibition of proliferation of NBL cells. Colony formation assays also revealed that the number and size of ALK-expressing viable clones were higher than those of vector control cells (Figures 4c and 4d).
ALK promotes cell proliferation.
(a) SK-N-DZ cells were transfected with the expression plasmid for ALK or empty plasmid and subjected to WST-8 assays at the indicated times after transfection. (b) NLF cells were transfected with control siRNA or siRNA against ALK. Twenty-four hours after transfection, cells were seeded in 96-well cell culture plates. At the indicated time points, the numbers of viable cells were measured by WST-8 assays. (c) and (d) Colony formation assays. SK-N-AS (c) and NBL-S (d) cells were transfected with pcDNA3-ALK or empty plasmid. Forty-eight hours after transfection, cells were transferred to fresh medium containing G418 (400 μg/ml for SK-N-AS and 500 μg/ml for NBL-S cells). Images were taken after crystal violet staining. Numbers and sizes of colonies were counted. All experiments were performed in triplicate.
To examine whether ALK contributes to metastatic activity of NBL cells, we performed wound healing assays in MYCN-non-amplified NBL (mutated ALK cell line; SH-SY5Y and wild-type ALK cell line; SK-N-AS) and low ALK expressing non-NBL (HeLa) cells and found that ALK expression enhanced cell migration (Supplementary Figure S5). Consistent with these results, Boyden chamber migration and invasion assays also showed a significant increase in the number of migrated and invaded cells arising from ALK-expressing cells compared with the vector control cells (Figure 5a and Supplementary Figure S6A). This study we performed using MYCN-non-amplified cell line SK-N-AS, as shown downstream signaling molecule AKT was activated upon ALK overexpression (Supplementary Figure S4A) and low ALK expressing non-NBL cell line HeLa. Migration and invasion of NBL cells were significantly suppressed by knockdown of ALK expression (Figure 5b and Supplementary Figure S6B). For knockdown of ALK experiment, we used MYCN-non-amplified NBL-S cells, which have a high expression of MYCN protein and invasive potency as described previously10 and phosphorylation of the downstream molecule AKT was reduced with ALK knockdown (Supplementary Figure S4B). We also performed knockdown of ALK study in MYCN amplified cell line SK-N-DZ. Both cell lines have a wild-type ALK allele.
ALK contributes to cell migration and invasion.
(a) Overexpression of ALK enhanced NBL cell migration and invasion. SK-N-AS cells were transfected with pcDNA3-ALK or empty plasmid and ALK ectopic expression (220 kDa) was determined by immunoblotting (left, full-length blots are presented in Supplementary Figure S9). Migration assays (middle) and invasion assays (right) were performed in Boyden chambers. (b) siRNA-mediated knockdown of ALK suppressed NBL cell migration and invasion. NBL-S cells were transfected with control siRNA or siRNA targeting ALK. Knockdown of ALK mRNA expression in NBL-S cells was confirmed by qRT-PCR (left). Migration assays (middle) and invasion assays (right) were performed as (a). All experiments were performed in triplicate.
ALK mediates MYCN-induced oncogenesis
We next tested the effect of growth inhibitory stimulus such as retinoic acid (RA) on the proliferative and colony-forming ability of cells expressing wild-type or F1174L-mutant ALK. RA is a well known MYCN suppressor and the addition of RA to proliferating NBL cells halts their division and leads to either differentiation or apoptosis31,32,33. We analyzed RA treatment experiment using MYCN amplified NBL cells (GOTO), which have a wild-type ALK allele and showed sensitivity to RA as previously described33. Figures 6a and b showed that after treatment with RA, the proliferation as well as the number and size of colony of NBL cells were significantly suppressed. As expected, MYCN expression was decreased after RA treatment and ALK expression was also downregulated (Figure 6c). However, both wild-type and F1174L mutated ALK-overexpressing cells had a higher number of live clones compared with the control cells following treatment with RA, suggesting that the prior ALK expression partially prevented the effect of RA on cell proliferation.
MYCN induces oncogenesis through regulating ALK expression.
(a) and (b) Effect of RA on the proliferative and colony forming abilities of NBL cells overexpressing ALK. Cell proliferation assays (a) and colony formation assays (b) to examine the proliferation of GOTO cells with wild-type or F1174L mutated ALK showed a significant difference in cell proliferation following treatment with RA (10 μM). (c) GOTO cells were transfected with pcDNA3-ALK (wild-type or mutated) or empty plasmid. Twenty-four hours after transfection, cells were treated with or without RA. Forty-eight hours after RA treatment, the expression of ALK and MYCN mRNA was verified by RT-PCR. (d) Cell migration assay. NBL-S cells were transfected with siRNA against ALK, followed by the transfection of MYCN expression vector or empty vector 24 h after siRNA transfection. Seventy-two hours after the first transfection, cell migration was examined (upper panel) and the expression levels of ALK and MYCN were determined (lower panel) by RT-PCR. All experiments were performed in triplicate.
We also examined the effect of MYCN-induced cell migration in the presence or absence of ALK expression. This study we performed using NBL-S cells, which showed upregulation of endogenous ALK with MYCN overexpression (Figure 2a and Supplementary Figure S1B). Consistent with previous reports10,11, MYCN expression enhanced cell migration. As expected, the migration was suppressed by siRNA-mediated knockdown of ALK in NBL cells (Figure 6d), suggesting that MYCN function in cell migration is at least partly regulated by ALK expression.
ALK inhibitors suppressed NBL cell growth, migration and invasion and inhibited tumor growth in xenograft model
Finally, we analyzed the effect of ALK inhibition on NBL cell proliferation, migration and invasion. Cell proliferation of NBL cells with MYCN amplification (SK-N-DZ, NLF and GOTO) were effectively inhibited by TAE-684 compared to MYCN-non-amplified cells (SK-N-AS and RISA) (Figure 7a). The IC50 values of SK-N-DZ, NLF, GOTO, RISA and SK-N-AS cells were 75.6 nM, 95.5 nM, 132.7 nM, 349.0 nM and 894.7 nM, respectively. Consistence with these results, efficient suppression of cell migration and invasion were observed in MYCN amplified NBL cells (SK-N-DZ and NLF) after TAE-684 treatment, whereas no significant suppression was detected in MYCN-non-amplified SK-N-AS cells (Figure 7b). Similar results in cell growth and migration assays using NBL cells with MYCN amplification were obtained by two selective ALK inhibitors, crizotinib and CH5424802 (Supplementary Figure S8). In addition, TAE-684 treatment significantly suppressed tumor growth of xenograft generated from MYCN amplified NBL cells (SK-N-DZ and NLF) (Figure 7c).
Effects of ALK inhibitor TAE-684 on NBL cells and xenograft tumors.
(a) TAE-684 reduced the proliferation of NBL cells. MYCN-non-amplified (SK-N-AS and RISA) or amplified (GOTO, NLF and SK-N-DZ) NBL cells were cultured with varying concentrations of TAE-684 for 72 h and cell proliferation was measured. The values are mean ± SD of triplicate experiments. (b) TAE-684 suppressed cell migration and invasion of NBL cells with MYCN amplification. NBL cells with MYCN amplification (SK-N-DZ and NLF) or without amplification (SK-N-AS) were treated with 50 nM or 200 nM of TAE-684 or DMSO as control and cell migration (upper panel) or invasion (lower panel) assays were performed. The values are mean ± SD of triplicate experiments. (c) TAE-684 suppressed tumor growth in mice. SK-N-DZ (left panel) and NLF (right panel) cells were subcutaneously injected into mice. When palpable tumors appeared, mice were orally treated with TAE-684 or carrier solution (vehicle) and tumor sizes were measured. Tumor sizes are displayed as mean ± SD at the indicated time interval of TAE-684 treatment (upper panel). Pictures of subcutaneous tumors for each group are shown (lower panel). n = 6 for each group of SK-N-DZ cells and n = 5 for each group of NLF cells.
Discussion
MYCN amplification occurs in approximately 25% of primary NBLs and is one of the most reliable prognostic factors identified to date1,5,6,7. It is significantly associated with advanced disease stages, rapid tumor progression and poor prognosis. However, the molecular mechanisms how MYCN induces aggressive NBL have not yet been fully elucidated. Our present findings clearly provided the evidence that MYCN-mediated ALK induction promotes cell proliferation, migration and invasion.
To our knowledge, this is the first report showing a direct role of MYCN in the transcriptional regulation of ALK in NBL. Consistent with the evidence that ALK expression was significantly correlated with MYCN amplification in primary NBLs, overexpression of MYCN induced promoter activity of the ALK gene, leading to a high level of ALK expression in NBL cells. The induction of ALK expression was also observed in non-NBL cells, suggesting that the transcription of ALK gene is generally regulated by MYCN. Moreover, in agreement with the previous results showing that c-Myc recognizes and binds to the E-box in the same manner as MYCN14,15,34, ALK expression was also transcriptionally regulated by c-Myc in non-NBL cells. Taking this into account, ALK is a direct target gene of MYC proteins. Our data are also informative to explain why ALK expression is high in MYCN transgenic mice. High levels of ALK expression were observed in 2-week-old MYCN-hemizygous mice, indicating that a spontaneously arising NBL expresses ALK in the early stage of tumor development driven by MYCN. Recently, PHOX2b has been reported as a transcription factor targeting ALK35. While the researchers identified transcriptional activity in the promoter construct of ALK gene from −672 to +384 bp, we observed comparable basal promoter activity in the −350 to +30 bp promoter region that contains no PHOX2b binding motif. According to our results, this minimum region of the ALK promoter possesses MYC-binding sites and transcriptional activity of ALK gene.
More recently, Schonheer et al. showed that both wild-type and gain-of-function ALK mutants were able to stimulate transcription at the MYCN promoter through the activation of a downstream molecule, ERK and initiate mRNA transcription of MYCN in both neuronal and NBL cells36. Furthermore, Berry et al. demonstrated that the F1174L mutation of ALK enhanced MYCN protein stabilization and found that endogenous Mycn mRNA was upregulated in the tumors of MYCN/ALKF1174L transgenic mice37. Taken together, these data suggest a positive feedback loop for MYCN which, in turn, directly regulates ALK expression to potentiate the oncogenic activity of MYCN, leading to rapid malignant transformation.
The theory that ALK overexpression contributes to oncogenic activity is supported by the data obtained from patients with NBL. As described previously, high expression of ALK mRNA25,26 and/or protein23,24 was significantly correlated with poor outcome of NBL. Consistent with these earlier reports, our present data clearly showed that aggressive and metastatic NBLs (stages 3, 4 and 4s) exhibited a significantly higher expression level of ALK mRNA compared with localized and favorable NBLs (stages 1 and 2), suggesting an oncogenic relevance of ALK in NBL. Previously obtained evidence has indicated an important role of ALK in both familial and sporadic NBL pathogenesis18,19,20,21,22. However, the contribution of wild-type ALK to NBL development was not well understood. Intensive studies have been mainly performed examining a correlation between activating mutations in the tyrosine kinase (TK) domain of ALK and poor clinical outcome in NBLs. However, mutations in the TK domain are observed in limited cases. According to De Brouwer and colleagues, only 6.9% missense mutations and 1.7% focal amplifications in ALK gene were detected among 709 NBL patients25. They have also revealed that there were no significant survival differences observed in tumors with or without ALK mutations or amplifications25, suggesting that wild-type ALK might have an important role in NBL pathogenesis. Consistent with Passoni et al. and Di Paolo et al., our present findings indicate that wild-type ALK can exert oncogenic activity in NBL cells, in addition to its mutated isoforms.
In the current study, overexpression of either wild-type or mutated ALK partially restored the decreased cell proliferation caused by RA treatment, implicating that RA-mediated reduction of MYCN expression resulted in decreased cell proliferation partially through the downregulation of ALK expression. The contribution of native ALK to migration and invasion implies a role for ALK in tumor progression and metastasis of NBLs. MYCN-induced cell migration was also inhibited by siRNA-mediated knockdown of ALK, suggesting that ALK is one of the key target genes of MYCN to conduct NBL cell migration. Moreover, treatment with TAE-684 effectively inhibited cell proliferation, migration and invasion of NBL cells with MYCN amplification compared to MYCN-non-amplified cells. Xenograft tumors derived from MYCN amplified NBL cells were significantly suppressed with TAE-684 treatment, indicating that ALK had a pivotal role in the development of NBL with MYCN amplification. Taken together, these data provide an important and direct mechanism by which MYCN is able to sensitize cells and tumors to aggressive characteristics through the regulation of ALK expression.
In conclusion, this study provides several lines of evidence that ALK is a direct transcriptional target of MYC proteins and a key molecule for MYC proteins to exert influence towards oncogenesis. Thus, our present findings might help to explain a novel molecular mechanism for the development and progression of aggressive NBL with or without MYCN amplification and suggest that a therapy targeting ALK should be considered in combination with more conventional agents to treat NBLs with high expression of ALK.
Methods
Patient population
One hundred and twenty-six patients with NBL were diagnosed clinically and histologically, using surgically removed tumor specimens according to the International Neuroblastoma Pathological Classification (INPC). According to the International NBL Staging System (INSS)38, 22 patients were diagnosed as stage 1, 12 were stage 2, 23 were stage 3, 63 were stage 4 and 6 were stage 4S. MYCN and ALK amplification were determined using fluorescence in situ hybridization (FISH). This study was approved by the Ethics Committee of the Faculty of Biology and Medicine at the Chiba Cancer Center and appropriate informed consent was obtained from all patients.
Transgenic mice samples collection and RT-PCR
Tyrosine hydroxylase (TH)-MYCN mice were maintained through hemizygotic matings, as previously described39. Superior mesenteric ganglion (SMG) tissues were obtained from 2-week-old wild-type or MYCN-hemizygous mice (n = 3 mice per group). Adrenal gland tumor tissues were collected from 9-, 10- or 11-week-old MYCN-hemizygous mice (n = 3 mice per group). All animals were handled in accordance with institutional guidelines for safe and ethical treatment of mice and this study was approved by the Animal Care and Use Committee of Nagoya University Graduate School of Medicine. Total RNA extraction was performed using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. cDNA was generated from total RNA using SuperScript III reverse transcriptase and random primers following the manufacturer's recommendations (Invitrogen, Carlsbad, CA, USA). The resultant cDNAs were subjected to PCR-based amplification using the following primer sets and annealing temperatures (Ta): human MYCN, 5′-CGACCACAAGGCCCTCAGTA-3′ (sense) and 5′-CAGCCTTGGTGTTGGAGGAG-3′ (antisense), Ta, 56°C; mice Alk, 5′-GACAGGATGGCTCCACCACA-3′ (sense) and 5′-CGGAAGCAGAGCGCACACAA-3′ (antisense), Ta, 56°C; mice Gapdh, 5′-GGTGGTGAAGCAGGCATCTG-3′ (sense) and 5′-GGAGGCCATGTAGGCCATGA-3′ (antisense), Ta, 57°C.
Cell culture
Human-derived NBL cell lines harboring wild-type ALK, including SK-N-AS, SK-N-DZ, NBL-S, NLF, RISA, NB69, SK-N-BE, NMB, NBTU1, NB9, KP-N-NS, SMS-KAN, GOTO, IMR32, GANB, CHP-134 and mutated ALK, including SH-SY5Y (F1174L), TNB1 (R1275Q) and SHEP Tet-21/N (F1174L), OAN (D1091N), RTBM1 (F1174L), TGW (R1275Q), SMS-SAN (F1174L), NGP (D1529E), LHN (R1275Q), LAN5 (R1275Q), KCN (R1275Q) and ALK amplified NB1 (Amp), were cultivated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and penicillin (100 IU/ml)/streptomycin (100 μg/ml). Non-NBL cell lines including HeLa, U2OS, A-875, TTC-11, ASPS-KY, RMS-Mk, NOS-1, SAOS-2, OST, G-361, G32TG, A549, H1299, HACAT, HEK-293T, HEK-293, COLO 320, MCF-7 and MDA-MB-453, were maintained in Dulbecco's modified Eagle's medium with the same supplements. Cells were grown at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. NBL cell lines were obtained from the CHOP cell line bank (Philadelphia, PA, USA). SHEP Tet 21/N cell line was kindly provided by Dr. M. Schwab (German Cancer Research Center, Heidelberg, Germany) and RISA cell line was established at Chiba Cancer Center Research Institute, Chiba, Japan. Non-NBL cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). For transient transfection, cells were transfected with the indicated expression of plasmids using Lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's recommendations.
Plasmid constructs
ALK promoter construct and different deletion constructs of ALK promoter were generated using an In-Fusion HD Cloning Kit (Clontech Laboratories, Mountain View, CA, USA) and inserted into the luciferase pGL4.17- basic plasmid (Promega, Madison, WI, USA). The protein coding region of c-Myc was inserted into the pcDNA3 plasmid (Invitrogen). pcDNA3-ALK wild-type and pcDNA3-ALK (F1174L ALK mutation) mutated expression plasmids were kindly provided by Dr. J. Takita (The University of Tokyo, Tokyo, Japan). pUHD-MYCN expression plasmid was kindly provided by Dr. M. Schwab (German Cancer Research Center, Heidelberg, Germany). All constructs were verified by DNA sequencing.
RNA isolation and RT-PCR
Total RNA was prepared from the indicated cell lines using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's recommendations. cDNA was generated from total RNA using SuperScript II reverse transcriptase and random primers following the manufacturer's conditions (Invitrogen). The resultant cDNAs were subjected to PCR-based amplification using the following primer sets and annealing temperatures (Ta): human MYCN, 5′- CTTCGGTCCAGCTTTCTCAC-3′ (sense) and 5′- GTCCGAGCGTGTTCAATTTT-3′ (antisense), Ta, 58°C; human GAPDH, 5′-ACCTGACCTGCCGTCTAGAA-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense), Ta, 58°C; human ALK, 5′-AGGACCCGGATGTAATCAAC-3′ (sense) and 5′- CTTGTGCAACTCCGAAGGAG-3′ (antisense), Ta, 58°C; human c-Myc, 5′- CTCGACTACGACTCGGTGCA-3′ (sense) and 5′ TGGTGGGCGGTGTCTCCTCA-3′ (antisense), Ta, 60°C. To control for the integrity and uniformity of the sample preparation, GAPDH mRNA was amplified. All PCR amplifications were carried out with a GeneAmp PCR 9700 (Applied Biosystems, Foster City, CA, USA), using rTaq DNA polymerase (Takara, Shiga, Japan). PCR products were separated by 2.0% agarose gel electrophoresis and stained with ethidium bromide.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from clinical samples and NBL as well as non-NBL cell lines using TRIzol reagent (Invitrogen) according to the manufacturer's instructions and reverse transcription was performed with SuperScript II reverse transcriptase (Invitrogen). qRT-PCR was carried out using an ABI Prism 7700 sequence detection system (Applied Biosystems), according to the manufacturer's protocol. TaqMan probe for ALK (Assay ID: Hs00608292_m1) and β-actin control reagent kit were purchased from Applied Biosystems. MYCN mRNA expression was measured by the SYBR green real-time PCR system using the following primer set: 5′-GGACACCCTGAGCGATTCAGA-3′ (sense) 5′-AGGAGGAACGCCGCTTCT-3′ (antisense). The mRNA levels of each gene were standardized by β-actin.
siRNA-mediated knockdown
Cells were transiently transfected with 10 nM siRNA targeting MYCN, siRNA-1: 5′-GAACCCAGACCUCGAGUUUUU-3′ (sense); 5′-PAAACUCGAGGUCUGGGUUCUU-3′ (antisense) and siRNA-2: 5′-UCACGGAGAUGCUGCUUGAUU-3′ (sense), 5′-PUCAAGCAGCAUCUCCGUGAUU-3′ (antisense) or a control non-targeting siRNA (Thermo Fisher Scientific, Waltham, MA, USA) using Lipofectamine RNAiMAX transfection reagent (Invitrogen) according to the manufacturer's instructions. Seventy-two hours after transfection, total RNA was prepared and subjected to RT-PCR. To knockdown endogenous ALK expression, cells were transfected with 100 nM of control siRNA or with siRNA against ALK target sequences: 5′-CCUGUAUACCGGAUAAUGA-3′, 5′-GUUGGGGUCAUAGAUGUUU-3′, 5′-UGAUUAUUUUACAUGGAAU-3′, 5′-GGAGGUGGCUGGAAUGAUA-3′ (Thermo Fisher Scientific) according to the manufacturer's recommendations. Seventy-two hours after transfection, cell lysates were prepared and analyzed for ALK and GAPDH expression levels by qRT-PCR or the expression levels of ALK, pALK, AKT and pAKT by immunoblotting analysis.
Site-specific deletion
The luciferase reporter constructs (−350 bp)-Δ E1 and (−350 bp)-ΔE 1/2 deletion constructs were generated with QuikChange Site-Directed Mutagenesis System (Invitrogen) on the basis of the parental construct (−350 bp), according to the manufacturer's instructions. The following primer sets were used: (−350 bp)–ΔE1, 5′-GCGCGGCTCAGCCAGCTGGCGGGCGCCCAG-3′ (sense) and 5′-CATCTGCCTGGGCGCCCGCCAGCTGGCTGAG-3′ (antisense); (−350 bp)–ΔE2, 5′-GCAGCAGCGCGGAGTTGGAGCCCCGCCCC-3′ (sense) and 5′-CCCGGAGGGGGCGGGGCTCCAACTCCGCG-3′ (antisense). The deletions were verified by DNA sequencing.
Luciferase reporter assays
Cells (5 × 104) were seeded in 12-well cell culture plates (Becton Dickinson, NJ, USA) and allowed to adhere overnight. Cells were then co-transfected with expression plasmid, luciferase reporter construct and pRL-TK Renilla luciferase cDNA. Total amounts of plasmid DNA per transfection were kept constant with empty plasmid pcDNA3 and/or pGL4.17. Forty-eight hours after transfection, cells were lysed and both firefly and Renilla luciferase activities were measured with the Dual-Luciferase Reporter Assay system (Promega), according to the manufacturer's instructions. The firefly luminescence signal was normalized based on the Renilla luminescence signal.
ChIP assays
Chromatin immunoprecipitation (ChIP) assays were performed using a chromatin immunoprecipitation assay kit according to the protocol provided by Millipore (Bedford, MA, USA). In brief, TNB1 (high ALK expression), RISA (low ALK expression), A-875 (high ALK expression) and HeLa cells (low ALK expression) were cross-linked with formaldehyde and cross-linked chromatin was sonicated followed by immunoprecipitation with anti-IgG (Cell Signaling), polyclonal anti-MYCN and/or monoclonal anti-c-Myc (anti-MYC-Tag (9B11)) antibody. DNA of the immunoprecipitates and control input DNA were purified and then analyzed by standard PCR using the following primer sets: F1 (−350), 5′-GCTCGCTAGCCTCGAAGTTCTCACATTTGCTCC-3′ (sense); R1 (+30), 5′- TCTTGATATCCTCGAGTACCAGCTGCTACC-3′ (antisense) and F2(-1813), 5′-GGAGAGGGGTATTATTAGAGAACG-3′ (sense); R2 (−1433), 5′- GGGCAAAGAATTATCTCACCC- 3′ (antisense).
Cell proliferation assays
Cells (1 × 103) were seeded in 96-well cell culture plates (Becton Dickinson, NJ, USA) and allowed to adhere overnight. At the indicated time points, cell proliferation was measured using a cell counting kit-8 (Dojindo, Kumamoto, Japan) according to manufacturer's instructions.
Colony formation assays
Cells were seeded at a final density of 2 × 104 cells per well in 6-well cell culture plates (Becton Dickinson, NJ, USA) and allowed to attach overnight. Cells were then transfected with the empty or pcDNA3-ALK expression plasmid. Forty-eight hours after transfection, cells were transferred to the fresh medium containing G418. After 14 days, viable colonies were washed in PBS, fixed with 4% paraformaldehyde (PFA) and stained with crystal violet solution.
Retinoic acid treatment
To study the effect of retinoic acid (RA, Sigma, St. Louis, MO, USA) on the proliferation or colony formation of GOTO cells expressing wild-type or mutated ALK, transfected cells were grown in normal growth medium and dimethylsulfoxide (DMSO) or 10 μM RA (with 50 μg/ml of G418 for colony formation assays) was added to the medium the following day. The culture medium was changed every 48 h to replenish the RA and G418. Cell viability and colony formation assays were performed as described above.
Cell migration and invasion assays
Cell motility was measured using 8-μm pore size FluoroBlok Transwell chambers (BD Biosciences, San Diego, CA, USA). Cells were collected by a brief treatment with trypsin/EDTA solution, washed once with serum-containing medium, centrifuged, resuspended in medium containing 0.1% bovine serum albumin and then placed in the inserts at a concentration of 2.5 × 104 cells in 250 μl (migration assays) or 5 × 104 cells in 250 μl (invasion assays). In the lower compartments of the chambers, 750 μl of medium containing 10% FBS was added as a chemoattractant. Invasion assays were carried out by the same procedure except that the filters of the Transwell chambers were coated with 50 μg Matrigel (BD Biosciences). After incubation at 37°C for 16 h (migration assays) or 24 h (invasion assays), cells that migrated through the pores to the lower chamber were stained with Giemsa solution or 1 μM Calcein AM (Invitrogen). Stained cells were analyzed by either counting under the microscope or by detecting the fluorescence intensity using microplate reader (Perkin-Elmer, Waltham, MA, USA).
Immunoblot analysis
Immunoblotting was performed as previously described40 using the following antibodies: monoclonal anti-ALK was from Beckman Coulter (Marseille, France), polyclonal anti-phosphorylated Tyr-1604 ALK, polyclonal anti-AKT, polyclonal anti-phosphorylated AKT Ser-473, polyclonal anti-MYCN and monoclonal anti-MYC-Tag (9B11) were from Cell Signaling (Danvers, MA, USA), polyclonal anti-Actin was from Sigma-Aldrich (St. Louis, MO, USA). All antibodies were used at a dilution of 1:1000 in immunoblot analysis.
Drug treatment
ALK inhibitors, TAE-68441,42 and CH542480243 were purchased from Active Biochemicals (Hong Kong, China) and crizotinib36,37,44 was purchased from Selleck (Houston, TX, USA). For cell proliferation assay; NBL cells (3 × 103) were seeded in 96-well cell culture plates and treated with varying concentrations of TAE-684, crizotinib or CH5424802 for 72 h and cell proliferation was measured as described above. The IC50 (half maximal inhibitory concentration) values of NBL cells were calculated by nonlinear regression (variable slope) using the GraphPad Prism 6 software (La Jolla, CA, USA). Cell migration and invasion assay; cell migration and invasion assays were performed as described above with or without the treatment of 50 nM or 200 nM of TAE-684, crizotinib or CH5424802.
Xenograft study; NBL cells with MYCN amplification (SK-N-DZ and NLF) were subcutaneously injected (5 × 106 cells/inoculate in RPMI 1640 together with an equal volume of Matrigel, Becton Dickinson) in the right flank of C.B-17-SCID female mice at 6 weeks old. After implantation, tumor sizes were measured using the following formula: [(width)2 × length]/2. Treatment of TAE-684 was initiated at the time when tumor size exceeds 85 mm3. TAE-684 was resuspended in 10% N-methyl-2-pyrrolidinone (Wako, Osaka, Japan)- 90% PEG (polyethylene glycol, molecular weight 300) (Wako) solution. Mice were administered TAE-684 (10 mg/kg) by oral gavage once daily. All mice were housed in a pathogen-free conditions strictly following the Chiba Cancer Center Research Institute guidelines and these studies were approved by the Institutional Animal Care and Use Committee of Chiba Cancer Center Research Institute.
Data analysis
Results were expressed as the mean ± SD. Student's t tests and two-independent samples t tests were used to compare the differences in the means of different groups. P values of <0.05 were considered statistically significant. P values of <0.05, <0.01 and <0.001 were denoted by a single asterisk, double asterisks and triple asterisks, respectively. n.s. indicates not significant at the 0.05 probability level.
References
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216 (2003).
Nakagawara, A. et al. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N. Engl. J. Med. 328, 847–854 (1993).
Ogawa, S., Takita, J., Sanada, M. & Hayashi, Y. Oncogenic mutations of ALK in neuroblastoma. Cancer Sci. 102, 302–308 (2011).
Brodeur, G. M., Seeger, R. C., Schwab, M., Varmus, H. E. & Bishop, J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–4 (1984).
Seeger, R. C. et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 13, 1111–6 (1985).
Kohl, N. E., Gee, C. E. & Alt, F. W. Activated expression of the N-myc gene in human neuroblastomas and related tumors. Science 226, 1335–7 (1984).
Riley, R. D. et al. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin. Cancer Res. 10, 4–12 (2004).
Lutz, W. et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 13, 803–12 (1996).
Bernards, R., Dessain, S. K. & Weinberg, R. A. N-myc amplification causes down-modulation of MHC class I antigen expression in neuroblastoma. Cell 47, 667–74 (1986).
Goodman, L. A. et al. Modulation of N-myc expression alters the invasiveness of neuroblastoma. Clin. Exp. Met. 15, 130–139 (1997).
Tanaka, N. & Fukuzawa, M. MYCN downregulates integrin alpha1 to promote invasion of human neuroblastoma cells. Int. J. Oncol. 33, 815–2 (2008).
Weiss, W. A., Aldape, K., Mohapatra, G., Feuerstein, B. G. & Bishop, J. M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16, 2985–95 (1997).
Alex, R., Sozeri, O., Meyer, S. & Dildrop, R. Determination of the DNA sequence recognized by the bHLH-zip domain of the N-Myc protein. Nucleic Acids Res. 20, 2257–63 (1992).
Blackwood, E. M. & Eisenman, R. N. Regulation of Myc: Max complex formation and its potential role in cell proliferation. Tohoku J. Exp. Med. 168, 195–202 (1992).
Torres, R., Schreiber-Agus, N., Morgenbesser, S. D. & DePinho, R. A. Myc and Max: a putative transcriptional complex in search of a cellular target. Curr. Opin. Cell Biol. 4, 468–74 (1992).
De Preter, K. et al. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 7, R84 (2006).
Osajima-Hakomori, Y. et al. Biological role of anaplastic lymphoma kinase in neuroblastoma. Am. J. Pathol. 167, 213–22 (2005).
Chen, Y. et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455, 971–974 (2008).
George, R. E. et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455, 975–978 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–5 (2008).
Caren, H., Abel, F., Kogner, P. & Martinsson, T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem. J. 416, 153–159 (2008).
Passoni, L. et al. Mutation- independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 69, 7338–7346 (2009).
Duijkers, F. A. et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am. J. Pathol. 180, 1223–3 (2012).
De Brouwer, S. et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin. Cancer Res. 16, 4353–62 (2010).
Schulte, J. H. et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin. Cancer Res. 17, 5082–92 (2011).
Di Paolo, D. et al. Selective therapeutic targeting of the anaplastic lymphoma kinase with liposomal siRNA induces apoptosis and inhibits angiogenesis in neuroblastoma. Mol. Ther. 19, 2201–12 (2011).
Banerjee, S. A., Hoppe, P., Brilliant, M. & Chikaraishi, D. M. 5′ Flanking sequences of the rat tyrosine hydroxylase gene target accurate tissue-specific, developmental and trans synaptic expression in transgenic mice. J. Neurosci. 12, 4460–4467 (1992).
Akter, J. et al. Expression of NLRR3 orphan receptor gene is negatively regulated by MYCN and Miz-1 and its downregulation is associated with unfavorable outcome in neuroblastoma. Clin. Cancer Res. 17, 6681–92 (2011).
Moog-Lutz, C. et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J. Bio. Chem. 280, 26039–26048 (2005).
Thiele, C. J., Reynolds, C. P. & Israel, M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature 313, 404–406 (1985).
Ichimiya, S. et al. Downregulation of hASH1 is associated with the retinoic acid-induced differentiation of human neuroblastoma cell lines. Med. Pediatr. Oncol. 36, 132–4 (2001).
Futami, H. & Sakai, R. All-trans retinoic acid downregulates ALK in neuroblastoma cell lines and induces apoptosis in neuroblastoma cell lines with activated ALK. Cancer Let. 297, 220–225 (2010).
Grandori, C. & Eisenman, R. N. Myc target genes. Trends Biochem. Sci. 22, 177–181 (1997).
Bachetti, T. et al. PHOX2B-mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma. PLoS One 5, e13108 (2010).
Schonheer, C. et al. Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 31, 5193–5200 (2012).
Berry, T. et al. The ALKF1174L mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 22, 117–130 (2012).
Brodeur, G. M. et al. Revisions of the international criteria for neuroblastoma. Diagnosis, staging and response to treatment. J. Clin. Oncol. 11, 1466–77 (1993).
Huang, P. et al. The neuronal differentiation factor NeuroD1 downregulates the neuronal repellent factor Slit2 expression and promotes cell motility and tumor formation of neuroblastoma. Cancer Res. 71, 2938–48 (2011).
Hossain, S. et al. NLRR1 enhances EGF-mediated MYCN induction in neuroblastoma and accelerates tumor growth in vivo. Cancer Res. 72, 4587–96 (2012).
Galkin, A. V. et al. Identification of NVP-TAE684, a potent, selective and efficacious inhibitor of NPM-ALK. Proc. Natl. Acad. Sci. USA 104, 270–275 (2007).
Heukamp, L. C. et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Sci. Trans. Med. 4, 141ra91 (2012).
Sakamoto, H. et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 19, 679–690 (2011).
Christensen, J. G. et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 6, 3314–22 (2007).
Acknowledgements
We are grateful to Dr. N. Koshikawa and Mr. M. A. Rahman (Chiba Cancer Center Research Institute, Japan) for their excellent technical advice and to Dr. A. Nakazawa (National Center for Child Health and Development, Japan) for useful discussions. We also thank Drs. M. Atwa, H. G. A. El_Azeem and E. El_Gezawy (Assiut University, Egypt) for their important suggestions. This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour and Welfare for the Third Term Comprehensive Control Research for Cancer (A. Nakagawara), a Grant-in-Aid for JSPS KAKENHI Grant Number 24249061 and 21390317 (A. Nakagawara), Takeda Science Foundation (A. Nakagawara) and grant-in-aids from the National Cancer Center Research and Development Fund, Japan (K. Kadomatsu and A. Nakagawara).
Author information
Authors and Affiliations
Contributions
M.K.H. and As.N. designed, performed and analyzed cellular and animal experiments, clinical data and wrote the manuscript. A.T. performed and analyzed cellular and animal experiments and wrote the manuscript. S.K. and K.K. performed and analyzed animal experiments and wrote the manuscript. M.O. performed and analyzed clinical data. Y.S., S.H., J.A., A.O. and Y.N. assisted in the experiments and figures. Ak.N. designed and supervised the experiments, analyzed data, wrote and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Hasan, M., Nafady, A., Takatori, A. et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci Rep 3, 3450 (2013). https://doi.org/10.1038/srep03450
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03450
This article is cited by
-
ATM depletion induces proteasomal degradation of FANCD2 and sensitizes neuroblastoma cells to PARP inhibitors
BMC Cancer (2023)
-
Inhibition of PI3 kinase isoform p110α suppresses neuroblastoma growth and induces the reduction of Anaplastic Lymphoma Kinase
Cell & Bioscience (2022)
-
ALK expression, prognostic significance, and its association with MYCN expression in MYCN non-amplified neuroblastoma
World Journal of Pediatrics (2022)
-
Wnt5a induces ROR1 to recruit cortactin to promote breast-cancer migration and metastasis
npj Breast Cancer (2019)
-
ALK positively regulates MYCN activity through repression of HBP1 expression
Oncogene (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.