Abstract
The microgravity environment during space flight imposes numerous adverse effects on animal and microbial physiology. It is unclear, however, how microgravity impacts those cellular interactions between mutualistic microbes and their hosts. Here, we used the symbiosis between the host squid Euprymna scolopes and its luminescent bacterium Vibrio fischeri as a model system. We examined the impact of simulated microgravity on the timeline of bacteria-induced development in the host light organ, the site of the symbiosis. To simulate the microgravity environment, host squid and symbiosis-competent bacteria were incubated together in high-aspect ratio rotating wall vessel bioreactors and examined throughout the early stages of the bacteria-induced morphogenesis. The host innate immune response was suppressed under simulated microgravity; however, there was an acceleration of bacteria-induced apoptosis and regression in the host tissues. These results suggest that the space flight environment may alter the cellular interactions between animal hosts and their natural healthy microbiome.
Similar content being viewed by others
Introduction
Space flight, in particular microgravity, presents a unique set of physiological and environmental stresses on life. For animals, including humans, microgravity exposure can lead to changes in muscle atrophy, blood and plasma volume and bone loss1,2. Another critical change in animal physiology during microgravity conditions is the dysregulation of the immune system3. Evidence has shown that both the adaptive and innate immune response is impacted by space flight, thus rendering animals more susceptible to pathogenic infections4. Specifically, cells associated with the innate immune response have shown changes in development, number and function under microgravity exposure4. For example, monocytes collected from astronauts post-flight had a reduced ability to engulf pathogenic Escherichia coli and an inability to generate an oxidative burst5.
In addition to physiological changes in animals, microbes are impacted by microgravity as well. Microbes respond to the changes in the mechanical and physical forces (i.e., low-shear) associated with microgravity by modifying their physiology, gene expression and pathogenicity6. One physiological characteristic that has been known for several decades is the increased growth rate in liquid cultures in microgravity7,8. Although the precise mechanisms that explain these differences have yet to be determined, research has indicated that the lag phase is shortened and the exponential growth phase is lengthened8. Additionally, some pathogenic microbes exhibit an increase in virulence, resistance to environmental stress and increased survival in host macrophages under microgravity conditions, which have been correlate with differential gene expression at both the transcriptional and translational levels6,9,10. Although significant progress has been made understanding microbial responses to microgravity, the vast majority of these studies have focused on pathogenic microbes. The effects of microgravity and the low shear fluid dynamics on mutualistic bacteria and their subsequent associations with animal tissues are virtually unknown.
All animals form associations with beneficial microbes11 and it is becoming increasingly apparent that the co-existence between animals and their microbial consortia is essential for health and normal development12. To examine the impact of simulated microgravity on healthy bacteria-animal interactions, we used the symbiotic association between the Hawaiian squid Euprymna scolopes and the luminescent bacterium Vibrio fischeri as a model system (Fig. 1). For more than two decades the squid/vibrio system has served as a tractable model to examine the influences of bacteria on animal development13,14. Although invertebrates, such as E. scolopes (Fig. 1a), have fewer associations with bacteria and less complex immune systems than humans, research using such models has provided key insight into homologous pathways in human physiology15,16. Furthermore, much of the research on the effects of microgravity on animal physiology has also relied on model systems, such as cell culture or murine models. Results have paralleled many of the physiological responses seen in humans during space flight17.
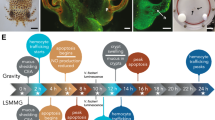
Overview of the bacteria-induced development of the E. scolopes light organ.
(a) Adult E. scolopes. Bar = 1 cm. (b) Newly hatched juvenile squid. Arrow indicates the location of the light organ. Bar = 500 μm. (c) Micrograph of light organ depicting the fields of ciliated epithelial cells that form two appendage-like structures on either side of the light organ. These structures are referred to as the ciliated epithelial appendages (CEA). (d) Symbiotic light organ depicting the regression of the CEA after 96 h post-inoculation with V. fischeri. (e) Rotary cell culture system with eight HARV chambers in the simulated microgravity position. (f) Cartoon depicting the rotation of the HARV chambers under simulated microgravity and gravity conditions.
In the squid/vibrio system the mutualism is initiated when symbiosis-competent strains of V. fischeri colonize a specialized host structure called the light organ (Fig. 1b)13. On either side of the incipient light organ there are two appendage-like structures comprised of ciliated epithelial cells overlying a blood sinus (Fig. 1c). The function of these ciliated epithelial appendages (CEA) is to entrain bacteria from the environment into the vicinity of pores found on the surface of the light organ. If symbiosis-competent strains of V. fischeri are not present in the environment then the light organ does not undergo morphogenesis and the CEA are retained. However, if symbiosis-competent V. fischeri are present then the bacteria enter the light organ through one of the surface pores and travel to an epithelial-lined crypt space whereupon they then begin to divide18. Upon reaching a critical cell density (≈ 12 h) the bacteria begin to luminesce within the light organ through a process called quorum sensing19. The squid then uses the luminescence in an anti-predator behavior called counter-illumination20.
One of the first symbiosis-induced phenotypes in the host squid occurs during the external aggregation of V. fischeri near the light organ pores. V. fischeri cells begin to attach to the cilia of the superficial epithelial appendages and trigger trafficking of macrophage-like hemocytes into the blood sinus21. Hemocytes, which are an integral component of the invertebrate innate immune response, first infiltrate the CEA blood sinus 2 h after V. fischeri exposure22. The trafficking is continuous until the sinus is filled with hemocytes, which occurs approximately 24 h after initial exposure to V. fischeri. The function of the hemocytes in the blood sinus is unclear, but they may play a role in the signaling of other V. fischeri developmental events16.
Approximately 6 h after V. fischeri colonization of the light organ the cells of the CEA appear to show early signs of apoptosis, which includes condensation of the nuclear chromatin23. The number of condensed (i.e., pycnotic) nuclei peaks approximately 14 h after exposure to V. fischeri24. The bacterial trigger of the apoptosis event is the microbe-associated molecular pattern (MAMP) molecule lipopolysaccharide (LPS)14. LPSs are endotoxins found on the cell membrane of gram-negative bacteria and have been shown to trigger low levels of early apoptotic cell death in the CEA14. Although purified LPS can initiate apoptosis, it cannot, however, induce full light organ morphogenesis. A second MAMP, tracheal cytotoxin, which is a derivative of peptidoglycan, works synergistically with LPS to induce the gradual regression of the CEA over a 4–5 d period, thus completing morphogenesis (Fig. 1d)22.
Together the binary nature of the symbiosis (i.e., one host, one symbiont) coupled with the rapid induction of measurable bacteria-induced phenotypes render the squid/vibrio symbiosis an ideal model for microgravity studies. To simulate the microgravity environment of space flight several ground-based devices have been developed including the high-aspect-ratio rotating wall vessel bioreactors (HARVs; Fig. 1e). The HARVs were designed by the NASA Biotechnological Group (JSC, Houston, TX)25,26 to mimic the low shear, low turbulent space environment for cell growth and maintain the organisms in a state of constant fluid suspension (Fig. 1f). The hydrodynamic forces within the HARV offset the effects of gravity within the chamber such that the squid or bacteria within the HARV chamber “falls” at a constant terminal velocity. Although the addition of the squid (2–3 mm) to the HARVs does likely have a small impact on the low-shear environment it is unlikely to have a significant impact on the symbiosis. Previous modeling experiments with various sized beads have shown that the maximum shear forces (5.2–7.8 dynes per cm2) generated by a 2–3 mm object remain low and do not exceed the natural fluid shear levels that microbes typically encounter when associating with animal tissues27,28. Additionally, studies using three-dimensional (3-D) aggregates of various human tissues, which are also similar in size to the juvenile squid, continue to grow and differentiate in the low-shear environment of rotating wall bioreactors6,29,30. These 3-D aggregates have also been shown to retain key morphological and physiological characteristics in the low shear environment of the rotating wall bioreactors31. Together, these previous studies suggest the change in the shear environment by the presence of the squid is likely confined to a small area around the animal and is similar to levels naturally seen by bacteria that typically associate with animal epithelia27,28,30,31. The use of low-shear simulated microgravity (LSSMG) has been widely used to mimic the space flight environment and examine the subsequent changes in bacterial morphology, physiology and gene expression9,32,33. LSSMG experimental results have also been shown to correlate to those obtained from actual space flight6,34.
In this study we examined the impact of LSSMG, from here on referred to as simulated microgravity, on the developmental time line of V. fischeri-induced morphogenesis of the host squid light organ. Both the host and symbiont were simultaneously exposed to simulated microgravity conditions and the onset of the symbiosis, as well as key developmental phenotypes, such as hemocyte trafficking, apoptosis and CEA regression, were monitored. By understanding how healthy animal-microbe interactions are initiated and maintained under simulated microgravity conditions, we can begin to determine the sensitivity of these mutualistic associations to perturbations in the space environment.
Results
Rapid growth rate of Vibrio fischeri cultures and normal onset of luminescence in simulated microgravity
The growth rates of V. fischeri ES114 (V. fischeri) cells under simulated microgravity and normal gravity conditions were compared. Results indicated that V. fischeri cultures grown in the HARV chambers under simulated microgravity conditions had a higher cell density beginning 8 h post-inoculation compared to gravity controls (Fig. 2). This trend continued throughout the exponential growth phase of V. fischeri until 18 h when the simulated microgravity-treated cells reached stationary phase. The gravity controls required an additional 2–4 h to reach stationary phase as compared to the simulated microgravity-treated cultures. However, by the time that both treatments reached stationary phase there was no statistical difference in the cell density of the cultures.
The initial colonization of the host light organ by V. fischeri was examined under simulated microgravity conditions by placing both partners in the rotating HARV chambers. Host squid were exposed to levels of V. fischeri commonly found in the shallow sand flats of their natural environment (1 × 105 cells per ml of seawater) and showed no delay in the onset of symbiosis in simulated microgravity conditions. The effectiveness of V. fischeri colonization was monitored by measuring the luminescence of the host squid. The results indicated there were no differences in the onset or intensity of light production between animals maintained under simulated microgravity or gravity conditions (data not shown).
Delay in the trafficking of hemocytes in simulated microgravity conditions
Upon colonization, one of the earliest symbiosis-induced events is the infiltration of macrophage-like hemocytes (i.e., invertebrate blood cells) into the blood sinus that underlies the CEA (Fig. 3a–c). Within two hours after exposure to symbiosis-competent V. fischeri there was a significant increase in the number of hemocytes in normal gravity controls compared to simulated microgravity conditions (Fig. 3d). This increase in the trafficking of hemocytes peaked approximately 12 h post inoculation and remained high throughout the light organ morphogenesis process. In simulated microgravity conditions, however, the number of hemocytes observed in the sinus remained at background levels until 12 h post inoculation, which was more than 10 h after hemocytes were observed in gravity controls. The total number of hemocytes present in the sinus did not reach levels observed in gravity controls throughout the duration of the experiment (Fig. 3d).
The trafficking of hemocytes into the blood sinus of the ciliated epithelial appendages (CEA) upon exposure to V. fischeri.
(a) Micrograph of one half of symbiotic light organ at 2 h showing the first appearance of hemocytes (arrow) in the CEA blood sinus (s). (b) By 6 h there is an increase in the abundance of hemocytes in the sinus. (c) The increase in hemocyte trafficking continues over time and peaks approximately 24 h post inoculation. (d) Quantification of the number of hemocytes in both aposymbiotic (apo) and symbiotic (sym) light organs over time. Animals were incubated under both gravity (+) and simulated microgravity (−) conditions. Asterisks indicate statistically significant differences between the gravity and simulated microgravity treatments with P ≤ 0.03. Error bars reflect the standard error of the mean.
Simulated microgravity accelerated bacteria-induced cell death in host light organ
Examination of bacteria-induced cell death in the squid light organ under simulated microgravity conditions revealed a similar spatial distribution of pycnotic nuclei compared to normal gravity conditions (Fig. 4a–h), however, the timing of the cell death event differed between treatments (Fig. 4i). In symbiotic light organs of both simulated microgravity and gravity treatments, pycnotic nuclei first occurred along the ridge of ciliated epithelial cells at the base of the light organ and then appeared throughout the appendage-like structures of the light organ. In normal gravity conditions, these first signs of bacteria-induced apoptosis along the ridge occurred approximately 6–8 h after inoculation with symbiosis-competent V. fischeri. By 12 h pycnotic nuclei appeared throughout the CEA and peaked 14–16 h after inoculation with V. fischeri. In simulated microgravity-treated light organs, however, the peak of pycnotic nuclei occurred 8 h post-inoculation, approximately 6–8 h earlier than in normal gravity treatments. The number of pycnotic nuclei within the light organ under simulated microgravity conditions was statistically higher (P ≤ 0.03) than gravity controls until 16 h post inoculation, which was the peak of cell death in normal gravity controls.
Induction of apoptotic cell death by V. fischeri under simulated microgravity and gravity conditions.
Only one half of acridine orange-stained light organ shown. (a–d). Juvenile squid incubated in HARV chambers under normal gravity conditions. (a) Micrographs of aposymbiotic light organ revealing the pronounced ciliated epithelial appendages (CEA). No pycnotic nuclei are present in aposymbiotic animals under normal gravity conditions at 24 h. (b) Micrograph of symbiotic light organs exposed to normal gravity depicting the pronounced pattern of cell death along the CEA by 24 h. (c) Aposymbiotic animals under normal gravity continue to show no signs of cell death in the CEA at 48 h. (d) At 48 h post-inoculation, symbiotic animals show full cell death pattern as well as initial regression of the CEA as the appendage lengths are shortened. (e–h). Juvenile squid incubated in HARV chambers under simulated microgravity conditions. (e) Aposymbiotic light organ under simulated microgravity showing few pycnotic nuclei at 24 h. (f) Micrograph of symbiotic light organ under simulated microgravity showing a full pattern of bacteria-induced cell death at 24 h. (g) Aposymbiotic squid incubated under simulated microgravity conditions show a cell death pattern at 48 h in the absence of symbiosis-competent bacteria. (h) Symbiotic light organ incubated under simulated microgravity conditions for 48 h show an accelerated level of CEA regression. Bar = 150 μm. (i) The numbers of pycnotic nuclei were quantified over 24 h in aposymbiotic (apo) and symbiotic (sym) animals under both normal gravity (+) and simulated microgravity (−) conditions. Asterisks indicate statistically significant differences between the gravity and simulated microgravity treatments with P ≤ 0.03. Error bars reflect the standard error of the mean.
In addition to temporal differences observed in symbiotic light organs, simulated microgravity-induced phenotypes were also detected in aposymbiotic light organs. Aposymbiotic animals showed significantly higher levels of pycnotic nuclei at 24 h compared to aposymbiotic gravity controls (P ≤ 0.03; Fig. 4a, e, i). By 48 h, the pattern of pycnotic nuclei resembled the typical, spatial pattern of cell death associated with the bacteria-triggered event, although the total number of pycnotic nuclei never reached levels observed in symbiotic animals (Fig. 4c, g, i). Animals maintained aposymbiotically in the HARV bioreactors under normal gravity conditions showed few, if any, pycnotic nuclei in the light organs even after 96 h post hatching (data not shown).
Simulated microgravity increased host sensitivity to lipopolysaccharide-induced cell death
Experimental addition of exogenous lipopolysaccharides (LPS) to aposymbiotic squid under simulated microgravity conditions resulted in an increase in the number of pycnotic nuclei present in the ciliated epithelium of the light organ compared to normal gravity (Fig. 5). A range of LPS concentrations (0.01 ng, 0.1 ng, 1 ng, 10 ng per ml of seawater) were tested under simulated microgravity and gravity conditions. By 24 h of exposure there was an approximate 2-fold increase in the number of pycnotic nuclei present within the LPS-treated light organs in simulated microgravity conditions compared to normal gravity (Fig. 5). The onset of the LPS-induced apoptosis also appeared dose-dependent. At concentrations lower than 0.01 ng/ml the onset of apoptosis occurred at 6 h and peaked 24 h after exposure (data not shown). At LPS concentrations of 0.1 ng/ml and higher there was a significant increase in the number of pycnotic nuclei by 14 h post-exposure (P ≤ 0.05; Fig. 5). In both simulated microgravity and gravity treatments the LPS-induced pycnotic nuclei were statistically lower than in symbiotic control animals exposed to intact V. fischeri at all time points after 6 h post-exposure (P ≤ 0.001).
Onset of apoptotic cell death in response to exogenous lipopolysaccharide (LPS) under normal gravity and simulated microgravity conditions.
Pycnotic nuclei were quantified at 2 (black), 6 (white), 14 (dark gray) and 24 (light gray) h post-exposure. Results of exposure to 1 and 10 ng of LPS per ml of seawater were not statistically different (P ≤ 0.75) from 0.1 ng and thus are not shown. Symbiotic animals (sym) were examined in parallel as a positive control. Error bars reflect the standard error of the mean.
Regression of the ciliated epithelial appendages accelerated under simulated microgravity conditions
In addition to the apoptotic cell death event, the light organ undergoes extensive morphogenesis that results in the regression of the CEA. Typically, the complete loss of the CEA in normal gravity-treated symbiotic animals occurs over a 96–120 h period (Fig. 1d)24,35. A comparison of symbiotic animals incubated in the HARV chambers during simulated microgravity and gravity conditions revealed a difference in the rate of regression of the CEA (Fig. 6). For the first 12 h after inoculation with symbiosis competent V. fischeri, there was no difference between simulated microgravity and gravity treatments. By 14 h, however, there was a significant decrease in the length of the anterior CEA in those symbiotic animals exposed to simulated microgravity (P ≤ 0.04). This trend continued until 48 h when the normal gravity treated animals showed comparable regression of the CEA and were not significantly different from simulated microgravity treated animals (P ≤ 0.18). For the remainder of the light organ morphogenesis (i.e., 48–96 h) there was no significant difference between simulated microgravity and gravity treatments (data not shown).
Discussion
This study examined the impact of simulated reduced gravity, or modeled microgravity, on the symbiotic association between the squid Euprymna scolopes and its luminescent bacterial partner Vibrio fischeri. The findings of this work offer insight into the effects that simulated microgravity may have on the interactions between mutualistic bacteria and their animal hosts. Specifically, the results of this study show that: 1) the trafficking of the host innate immune cells were significantly delayed and attenuated under simulated microgravity; 2) the onset of bacteria-induced apoptosis was accelerated in simulated microgravity conditions and the number of apoptotic cells increased; 3) the host light organ exhibited an increased sensitivity to the V. fischeri surface molecule lipopolysaccharide (LPS) under simulated microgravity conditions; and 4) initial regression of the symbiotic host light organ ciliated epithelial appendages (CEA) was accelerated in simulated microgravity conditions.
Gravity has been one of the few environmental constants throughout the evolutionary history of Earth and all life forms have evolved mechanisms to respond and cope with this force36. Although the extent of gravitational loading can differ significantly between terrestrial and aquatic organisms36 most, if not all, organisms derive directional cues from gravity and respond through either positive or negative geotaxis37. The use of the aquatic organism E. scolopes as a host for simulated microgravity studies on animal-microbe interactions provided several experimental advantages. For example, in its natural aquatic habitat E. scolopes experiences reduced gravitational loading on the vascular system, supporting tissues and proprio-perception (in terms of body weight), thus potentially lowering the stress response to simulated microgravity conditions. This adaptation coupled with its small size (2–3 mm at hatching), a rapid reproduction rate and embryonic development period (≈ 21 days) renders E. scolopes an ideal host organism for simulated microgravity studies. Another beneficial feature of the squid/vibrio system was that although there were several distinct permutations with the normal developmental process in simulated microgravity conditions, symbiosis-competent V. fischeri were able to successfully colonize the host tissues and induce development. Typically background levels of V. fischeri in the natural sandy sediments approximate 105 cells per ml of seawater43. Previous studies, however, have shown that as few as 1–10 cells of V. fischeri are all that is required to initiate the symbiosis37 suggesting that the natural populations of V. fischeri far exceed the numbers required for effective colonization and subsequent light organ morphogenesis. In this study we inoculated host animals with levels of symbionts they naturally see in their environment and did not see differences in the onset of luminescence, a critical indicator of colonization, under simulated microgravity conditions. However, future work is required to assess whether there are differences in the minimal number of symbionts required to colonize the host under simulated microgravity conditions.
One physiological difference was the overall growth rate of V. fischeri in simulated microgravity. V. fischeri grew slightly faster under simulated microgravity conditions compared to the gravity controls, which corresponded to several other previous studies38,39. However, by 18 h the overall cell density of V. fischeri cultured in simulated microgravity conditions was not significantly higher than gravity conditions, which may reflect the motile nature of V. fischeri. V. fischeri is motile until it colonizes the host light organ, whereupon it loses its flagella40. Motility has been shown to impact microbial growth in microgravity primarily by disrupting the quiescent fluid and nutrient environment of the suspension, thus changing the physiology and growth rates of the microbes39,41. The lack of a significant higher cell density at the end of the growth curve correlates to other simulated microgravity studies with motile pathogenic bacteria, including strains of Salmonella enterica serovar Typhimurium, E. coli and Pseudomonas aeruginosa39,41,42.
Upon V. fischeri colonization of the host squid light organ there was a delay in the bacteria-induced trafficking of the host innate immune cells, known as hemocytes, under simulated microgravity conditions. The innate immune system in animals has been shown to be a critical mediator for the interactions and communication between beneficial microbes and their hosts. Additionally, the cellular mechanisms associated with the innate immune response appear to be well conserved in animals43,44. In normal gravity conditions the squid hemocytes began to migrate into the blood sinus of the CEA within 2 h. These results correlate with a previous study on hemocyte trafficking during the early stages of the squid/vibrio symbiosis45. This previous study showed that the microbe-associated molecular pattern (MAMP) molecule peptidoglycan, which is shed by V. fischeri into the aggregating mixture of cells, triggered the production of mucus by the host squid45. Bacteria then accumulate in the mucus and then migrate with the material into the light organ pores, thus initiating colonization18. Previous studies demonstrated that peptidoglycan release and the subsequent bacterial migration into the pores facilitate the trafficking of the hemocytes45. In simulated microgravity the light organ colonization by V. fischeri appeared to occur normally within a few hours of exposure, as determined by luminescence readings. However, there was a significant decrease in the numbers of hemocytes within the blood sinus even after 10 h post-inoculation with V. fischeri. These results suggest that there was a suppression of the overall response time of hemocyte migration and innate immune response. This delay in response time could be the result of a decrease in the production of host-derived mucus that facilitates bacterial aggregation or in the extent of bacterial signaling via peptidoglycan shedding.
Despite the initial delay in hemocyte trafficking, by 12 h post-inoculation hemocyte numbers within the blood sinus were significantly higher, although they were persistently lower in simulated microgravity conditions compared to normal gravity controls. This boost in hemocyte abundance may be triggered by light organ venting, which occurs approximately 12 h after bacterial exposure45,46. Each morning the host squid contracts muscles in the light organ and expels 95% of the bacterial population into the surrounding seawater46. The venting process then potentially exposes the epithelium of the crypt, pores and CEA to additional V. fischeri peptidoglycan, thus potentially triggering a more intense hemocyte response. The lower levels of hemocytes within the blood sinus during simulated microgravity treatments may reflect a reduced capacity of these cells to respond to the bacterial stimulus. Space flight has been shown to suppress the innate immune response of animals, including humans, through decreases in the proliferation of blood cells and cytokine production3,47. Although further experiments are required to assess the specific relationship between host mucus production, MAMP production and hemocyte trafficking under simulated microgravity conditions, the results suggest that the innate immune response, which plays a critical role in coordinating signaling between beneficial microbes and their hosts, is negatively impacted during simulated microgravity conditions.
Whereas the host innate immune response appeared to be suppressed, several other bacteria-induced events, such as the onset of apoptosis and CEA regression, were accelerated under simulated microgravity conditions. There was a significant increase in the number of cells within the superficial epithelium that exhibited signs of apoptosis in simulated microgravity treatments compared to gravity controls. Previous studies have shown that simulated microgravity can cause an increase in cellular apoptosis in a wide range of cell types, including osteoblasts, reproductive and endothelial cells48,49. A common mechanism associated with the increase in simulated microgravity-induced apoptosis in these cell types was the activation and upregulation of the NF-κB pathway48,49. The NF-κB protein complex has been shown to play a critical role in regulating the host innate immune response50. The NF-κB pathway, which has been identified in E. scolopes51, is often activated through the presence of MAMPs such as peptidoglycan and LPS, both of which are released by V. fischeri. Although the precise mechanism by which simulated microgravity triggers the upregulation of the NF-κB pathway is unknown, once activated the NF-κB complex has been shown to interact with p53, a family of proteins known to mediate and regulate the apoptosis cascade. Members of the p53 protein family have been identified in E. scolopes and immunocytochemistry has localized expression of p53 to the nuclei CEA cells in both aposymbiotic and symbiotic animals52. The observed increase in the number of apoptotic cells within the CEA during simulated microgravity treatments might be the result of an activated NF-κB and p53 pathway. The activation of these two pathways might also explain why low levels of cell death were observed in 24 and 48 h aposymbiotic animals when no symbiosis-competent bacteria were present. Aposymbiotic animals might be poised for programmed cell death and the simulated microgravity-induced activation of the NF-κB/p53 pathway might initiate these early developmental mechanisms in the absence of the bacteria.
The increase in the number of apoptotic cells could also be the result of an increased sensitivity to the MAMP molecule LPS. LPS molecules have been shown to induce apoptosis of the CEA and work synergistically with peptidoglycan derivatives to induce full morphogenesis of the light organ14,22. A significant increase in cell death was caused by the exogenous addition of LPS to aposymbiotic squid under simulated microgravity as compared to gravity controls. LPS is a major trigger of the host innate immune response in both pathogenic infections as well as in the development of symbioses53,54. Typically, LPS molecules bind to a soluble protein such as LPS-binding protein (LBP), which as a complex binds to Toll-like receptors on the cell surface. Upon activation of Toll, the host innate immune response is activated through pathways such as the NF-κB pathway55. Space flight has been shown to increase the responsiveness of host cells to LPS through the enhanced expression of Toll-like receptor 4 and LBP in human monocytes56. Proteins with homology to LBP and Toll-like receptors have been identified in transcriptome libraries of the host E. scolopes51; however, whether the expression of these proteins is altered in simulated microgravity conditions remains to be determined. Although simulated microgravity enhanced the LPS-induced apoptosis event in the host light organs, the response was still significantly lower than the level of apoptosis associated with intact V. fischeri. These results reinforce the concept that LPS is only one of several developmental cues needed for complete light organ remodeling and development.
In addition to the increase in apoptotic cell death in animals exposed to simulated microgravity, there was also a significant increase in the rate of CEA regression during the first 24 h of light organ morphogenesis. Previously, it had been hypothesized that the hemocytes might serve as mediators for the regression process45. However, the delay in hemocyte trafficking into the CEA blood sinus suggests that hemocyte migration and regression events might be uncoupled. The increase in CEA regression in simulated microgravity conditions could be due in part to the loss of the basement membrane underlying the ciliated epithelium and/or a restructuring of the CEA's cytoskeleton. The cells of the CEA form a single layer of epithelial cells, which is lined by a basement membrane23. Basement membranes anchor and provide structural support for the epithelial cell layers. They are comprised of various extracellular matrix (ECM) molecules such as collagens and integrins57 and have been shown to be critical to prevent apoptotic cell death in the overlying epithelial cells58. In simulated microgravity, previous studies have shown that the expression of several ECM molecules is differentially regulated. For example, in mesenchymal stem cells expression of genes associated with collagen I are down-regulated59 and the signaling between collagen and integrin molecules is disrupted60. Additionally, several cytoskeletal molecules such as α- and F-actin are also down-regulated in microgravity and the cytoskeleton can undergo extensive remodeling61. Based on these previous studies, it is possible that simulated microgravity might have hastened the collapse of the basement membrane in symbiotic animals through a disruption of signaling between the ECM and epithelial cytoskeleton. The loss of the basement membrane and structural scaffolding might have accelerated the initial CEA regression in the light organ.
Together, the results of this study suggest that the cellular interactions between animal hosts and their natural healthy microbiome are altered in microgravity-analogue conditions. As most microbes that associate with animals are commensal or mutualistic in nature, it is important to assess the impact that space flight, in particular microgravity, has on these beneficial interactions. Furthermore, as much of the microbial machinery and genetic pathways required to interact with host cells is shared by both beneficial and pathogenic microbes, it becomes increasingly important to understand how the host innate immune response recognizes and differentially responds to these different modes of biological interactions in microgravity. The symbiosis between the E. scolopes and V. fischeri represents an ideal model to examine the effects of microgravity on animal-microbe interactions as both partners can be simultaneously examined in simulated microgravity conditions. By using less complex models that have rapidly inducible phenotypes it is possible to more clearly assess the specific impact that microgravity has on bacteria-induced host development pathways and innate immune responses.
Methods
Ethics statement
University of Florida Institutional Animal Care and Use Committee (IACUC) was contacted prior to the study, however, as they do not consider cephalopods regulated animals IACUC approval was not required. No permits were required for the described field sites as Euprymna scolopes is not an endangered or protected species. Animals were anesthetized prior to examination in a 1:1 solution of 0.37 M MgCl2 and filtered sterilized seawater.
General procedures
Adult E. scolopes were collected from the shallow sand flats of O'ahu, Hawai'i and transported to the Space Life Science Lab at the Kennedy Space Center, FL where they were maintained in individual aquaria. Egg clutches, laid by the female E. scolopes, were incubated separately from the adults for their full development cycle (≈ 21 days) at 23°C on a 12 h light/dark cycle. Upon hatching, juvenile squid (2–3 mm in size) were repeatedly rinsed in filter-sterilized seawater (FSW) to prevent premature inoculation with V. fischeri and maintained in borosilicate scintillation vials. Juvenile squid were rendered symbiotic by placing them in FSW containing 1 × 105 cells per ml of V. fischeri ES114, a strain previously isolated from the light organ of the adult squid62. The onset of symbiosis was determined by measuring bacterial luminescence with a photometer (GloMax 20/20 Luminometer, Promega Corp., Madison, WI). For control purposes, a subset of animals were maintained aposymbiotically (i.e., without symbiosis-competent V. fischeri present) for all experimental treatments. All reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Statistical comparisons used a two-tailed Student's t-test with an n ≥ 8 squid.
Simulated microgravity treatments
To simulate a low shear microgravity environment two separate rotary culture systems each with four 10-ml volume high aspect ratio vessels (HARVs; Synthecon, Houston, TX) were used at 13 rpm. The HARVs were either rotated around a horizontal axis to simulate microgravity or a vertical axis to serve as a normal gravity (1 × g) control (Figure 1F). Aeration of the FSW in the HARVs was maintained through a semipermeable membrane on the back of each individual bioreactor. At least three technical replicates were completed for all described experiments.
For V. fischeri growth curves, cells were grown in seawater tryptone broth (SWT) that contained 5 g tryptone, 3 g yeast extract, 3 ml of glycerol, 700 ml of FSW and 300 ml of distilled water. A starting culture of 1 × 106 cells per ml of SWT was incubated in each of the HARV bioreactors at 23°C. Every 2 h 100-μl aliquots were removed in triplicate from each reactor and monitored spectrophotometrically (DU800, Beckman Coulter, Fullerton, CA) at A600 nm.
To initiate animal-microbe interactions under simulated microgravity conditions, each HARV vessel contained 10 ml FSW with an inoculum of 1 × 105 cells per ml of V. fischeri. Juvenile squid were added to each HARV chambers through ports on the surface of the vessel. For each treatment a subset of vessels was maintained without symbiosis-competent V. fischeri (i.e., aposymbiotic) as a control. All host-microbe HARV experiments were incubated at 23°C on a 12 h light/dark cycle.
Monitoring of hemocyte trafficking
To observe hemocytes being trafficked into the light organ CEA, juvenile squid were stained with fluorescently labeled DNase I, which targets the large g-actin stores contained within hemocytes63. Specifically, juvenile animals were fixed in 4% paraformaldehyde in marine phosphate-buffered saline (mPBS; 50 mM sodium phosphate buffer with 0.45 M sodium chloride, pH 7.4) for 18 h at 4°C. The light organs were then dissected out of the squid and permeabilized with a solution of mPBS containing 1% Triton-X overnight at 4°C. The light organs were then incubated with 0.64 μM of Alexa Fluor® 488 conjugated DNase I and 0.19 μM tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin (Invitrogen, Carlsbad, CA) for 48 h at 4°C in the dark. After incubation, the light organs were washed four times with mPBS for 15 min each rinse and counterstained for 20 min with a 2 mM solution of TOTO-3 (Invitrogen, Carlsbad, CA). The light organs were rinsed twice in mPBS for 15 min then mounted on a depression glass slide using Vectashield medium to reduce photobleaching (Vector Labs, Burlingame, CA). The stained light organs were then analyzed with a PerkinElmer Ultra View ERS-3E confocal microscope (PerkinElmer, Waltham, MA).
Detection of apoptosis and ciliated epithelial appendage (CEA) regression
To observe and quantify apoptotic cell death within the ciliated epithelial surface of the light organ, juvenile squid were anesthetized and stained in a 1:1 solution of 0.37 M MgCl2 and FSW containing 5 ng per ml of acridine orange for 5 min. Acridine orange is a fluorescent molecule that intercalates into the condensed (i.e., pycnotic) chromatin of dying cells and has been used regularly to monitor the onset of cell death23,24. After staining, the mantle and funnel were removed to improve visualization of the light organ. The stained E. scolopes were examined with epifluorescence microscopy using a Zeiss Axioplan Microscope (Carl Zeiss, Jena, Germany). The number of pycnotic nuclei within one of the ciliated epithelial surfaces that cover each half of the light were counted and statistically compared between treatments using a parametric Student t-test as previously described14.
In addition to apoptotic cell death, the extent of regression of the CEA was monitored over time using microscopy. Micrographs of light organs were taken using a Zeiss Axioplan Microscope (Carl Zeiss, Jena, Germany) and the length of the anterior appendage of the ciliated epithelial surface was measured using the Zeiss Micro Imaging software (Carl Zeiss, Jena, Germany). The lengths of the light organ anterior appendages were then statistically compared between treatments using pairwise comparisons and descriptive statistics. The anterior appendage lengths of each treatment were then normalized to those of juvenile squid observed at the start of each experiment (t = 0 h) and reported as a percentage of regression compared to t = 0 h.
Lipopolysaccharide (LPS) preparation and treatments
Commercially available LPS derived from Escherichia coli was prepared in filtered distilled water at stock concentrations of 100 μg per ml. The LPS stock solutions were then vortexed for 2 min to generate homogeneous suspensions. The LPS solution was then diluted to 0.01, 0.1, 1 and 10 ng per ml of FSW as these concentrations have been previously shown to be effective in the induction of apoptosis14. Juvenile squid were exposed to the LPS solutions in the HARV chambers under simulated microgravity and simulated gravity conditions.
References
Smith, S. M. & Heer, M. Calcium and bone metabolism during space flight. Nutrition 18, 849–852 (2002).
Vernikos, J. Human physiology in space. BioEssays 18, 1029–1037 (1996).
Crucian, B. E., Stowe, R. P., Pierson, D. L. & Sams, C. F. Immune system dysregulation following short- vs long-duration space flight. Aviat. Space Environ. Med. 79, 835–843 (2008).
Guéguinou, N. et al. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth's orbit? J. Leukoc. Biol. 86, 1027–1038.
Rykova, M. P., Antropova, E. N., Larina, I. M. & Morukov, B. V. Humoral and cellular immunity in cosmonauts after ISS missions. Acta Astronaut. 63, 697–705 (2008).
Nickerson, C. A., Ott, C. M., Wilson, J. W., Ramamurthy, R. & Pierson, D. L. Microbial responses to microgravity and other low-shear environments. Microbiol. Mol. Biol. Rev. 68, 345–361 (2004).
Ciferri, O., Tiboni, O., Di Pasquale, G., Orlandoni, A. M. & Marchesi, M. L. Effects of microgravity on genetic recombination in Escherichia coli. Naturwissenschaften 73, 418–421 (1986).
Klaus, D., Simske, S., Todd, P. & Stodieck, L. Investigation of space flight effects on Escherichia coli and a proposed model of underlying physical mechanisms. Microbiology 143 ( Pt 2), 449–455 (1997).
Nickerson, C. A. et al. Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 68, 3147–3152 (2000).
Wilson, J. W. et al. Low-Shear modeled microgravity alters the Salmonella enterica serovar typhimurium stress response in an RpoS-independent manner. Appl. Environ. Microbiol. 68, 5408–5416 (2002).
Ruby, E., Henderson, B. & McFall-Ngai, M. We get by with a little help from our (little) friends. Science 303, 1305–1307 (2004).
Xu, J. & Gordon, J. I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 100, 10452–10459 (2003).
McFall-Ngai, M. J. & Ruby, E. G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 254, 1491–1494 (1991).
Foster, J. S., Apicella, M. A. & McFall-Ngai, M. J. Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev. Biol. 226, 242–254 (2000).
McFall-Ngai, M. J. Unseen forces: the influence of bacteria on animal development. Dev. Biol. 242, 1–14 (2002).
McFall-Ngai, M., Nyholm, S. V. & Castillo, M. G. The role of the immune system in the initiation and persistence of the Euprymna scolopes--Vibrio fischeri symbiosis. Semin. Immunol. 22, 48–53 (2010).
Sonnenfeld, G. The immune system in space, including Earth-based benefits of space-based research. Curr. Pharmaceut. Biotechnol. 6, 343–349 (2005).
Nyholm, S. V., Stabb, E. V., Ruby, E. G. & McFall-Ngai, M. J. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 97, 10231–10235 (2000).
Lupp, C. & Ruby, E. G. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 187, 3620–3629 (2005).
Stabb, E. V. & Millikan, D. S. in Defensive mutualism in microbial symbiosis Vol. 27, (eds. White J. F., & RTorres M. S., eds. ) 85–98 (CRC Press, 2009).
McFall-Ngai, M., Heath-Heckman, E. A., Gillette, A. A., Peyer, S. M. & Harvie, E. A. The secret languages of coevolved symbioses: insights from the Euprymna scolopes-Vibrio fischeri symbiosis. Semin. Immunol. 24, 3–8 (2012).
Koropatnick, T. A. et al. Microbial factor-mediated development in a host-bacterial mutualism. Science 306, 1186–1188 (2004).
Foster, J. S. & McFall-Ngai, M. J. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev. Genes Evol. 208, 295–303 (1998).
Montgomery, M. K. & McFall-Ngai, M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120, 1719–1729 (1994).
Wolf, D. A. & Schwarz, R. P. Analysis of gravity-induced particle motion and fluid perfusion flow in NASA-designed rotating zero-head-space tissue culture vessel. NASA Tech. Paper 3143, 1–12 (1991).
Schwarz, R. P., Goodwin, T. J. & Wolf, D. A. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J. Tiss. Cult. Meth. 14, 51–58 (1992).
Fang, A., Pierson, D. L., Koenig, D. W., Mishra, S. K. & Demain, A. L. Effect of simulated microgravity and shear stress on microcin B17 production by Escherichia coli and on its excretion into the medium. Appl. Environ. Microbiol. 63, 4090–4092 (1997).
Nauman, E. A. et al. Novel quantitative biosystem for modeling physiological fluid shear stress on cells. Appl. Environ. Microbiol. 73, 699–705 (2007).
Nickerson, C. A. et al. Low-shear modeled microgravity: a global environmental regulatory signal affecting bacterial gene expression, physiology and pathogenesis. J. Microbiol. Methods 54, 1–11 (2003).
Nickerson, C. A., Richter, E. G. & Ott, C. M. Studying host-pathogen interactions in 3-D: organotypic models for infectious disease and drug development. J. Neuroimmune Pharmacol. 2, 26–31 (2007).
Barrila, J. et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat. Rev. Microbiol. 8, 791–801 (2010).
Wilson, J. W. et al. Media ion composition controls regulatory and virulence response of Salmonella in spaceflight. PLOS ONE 3, e3923 (2008).
Vukanti, R., Model, M. A. & Leff, L. G. Effect of modeled reduced gravity conditions on bacterial morphology and physiology. BMC Microbiol. 12, 4 (2012).
Wilson, J. W. et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. USA 104, 16299–16304 (2007).
Doino, J. A. & McFall-Ngai, M. Transient exposures to competent bacteria initiates symbiosis-specific squid light organ morphogenesis. Biol. Bull. 189, 347–355 (1995).
Volkmann, D. & Baluska, F. Gravity: one of the driving forces for evolution. Protoplasma 229, 143–148 (2006).
Morey-Holton, E. R. in Evolution on planet Earth: the impact of the physical environment (eds. Rothschild L. J., & Lister A., eds. ) 143–159 (Academic Press, 2003).
Mattoni, R. Space flight effects and gamma radiation interaction on growth and induction of lysogenic bacteria: a preliminary report. Bioscience 18, 602–608 (1968).
Thevenet, D., D'Ari, R. & Bouloc, P. The SIGNAL experiment in BIORACK: Escherichia coli in microgravity. J. Biotechnol. 47, 89–97 (1996).
Ruby, E. G. & Asato, L. M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159, 160–167 (1993).
Benoit, M. & Klaus, D. M. Microgravity, bacteria and the influence of motility. Adv. Space Res. 39, 1225–1232 (2007).
Guadarrama, S., Pulcini, E., Broadaway, S. C. & Pyle, B. H. Pseudomonas aeruginosa growth and production of Exotoxin A in static and modeled microgravity environments. Grav. Space Biol. 18, 85–86 (2005).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Nyholm, S. V., Stewart, J. J., Ruby, E. G. & McFall-Ngai, M. J. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 11, 483–493 (2009).
Koropatnick, T. A., Kimbell, J. R. & McFall-Ngai, M. J. Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol. Bull. 212, 29–39 (2007).
Lee, K. H. & Ruby, E. G. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 60, 1565–1571 (1994).
Baqai, F. P. et al. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 106, 1935–1942 (2009).
Sharma, C. S. et al. Simulated microgravity activates apoptosis and NF-kappaB in mice testis. Mol. Cell. Biochem. 313, 71–78 (2008).
Kang, C. Y. et al. Impact of simulated microgravity on microvascular endothelial cell apoptosis. Eur. J. Appl. Physiol. 111, 2131–2138 (2011).
Silverman, N. & Maniatis, T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15, 2321–2342 (2001).
Goodson, M. S. et al. Identifying components of the NF-kappaB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl. Environ. Microbiol. 71, (2005).
Goodson, M. S., Crookes-Goodson, W. J., Kimbell, J. R. & McFall-Ngai, M. J. Characterization and role of p53 family members in the symbiont-induced morphogenesis of the Euprymna scolopes light organ. Biol. Bull. 211, 7–17 (2006).
Zychlinsky, A. et al. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64, 5357–5365 (1996).
Anderson, K. V. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12, 13–19 (2000).
Yang, R. B. et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395, 284–288 (1998).
Kaur, I., Simons, E. R., Kapadia, A. S., Ott, C. M. & Pierson, D. L. Effect of spaceflight on ability of monocytes to respond to endotoxins of gram-negative bacteria. Clin. Vaccine Immunol. 15, 1523–1528 (2008).
Brown, B., Lindberg, K., Reing, J., Stolz, D. B. & Badylak, S. F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 12, 519–526 (2006).
Boudreau, N., Werb, Z. & Bissell, M. J. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. USA 93, 3509–3513 (1996).
Zayzafoon, M., Gathings, W. E. & McDonald, J. M. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology 145, 2421–2432 (2004).
Zayzafoon, M., Meyers, V. E. & McDonald, J. M. Microgravity: the immune response and bone. Immunol. Rev. 208, 267–280 (2005).
Infanger, M. et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell Tissue Res. 324, 267–277 (2006).
Boettcher, K. J. & Ruby, E. G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172, 3701–3706 (1990).
Heath-Heckman, E. A. C. & McFall-Ngai, M. J. The occurance of chitin in the hemocytes of invertebrates. Biol. Bull. 114, 191–198 (2011).
Acknowledgements
The authors would like to thank Jennifer Mobberley and John Warden for their comments on this manuscript. We also thank Margaret McFall-Ngai and Elizabeth Heath-Heckman for their technical advice and Michael Hadfield for providing research space at the Kewalo Marine Laboratory during animal collection. Additionally, we thank Ned Ruby for the strain V. fischeri ES114 and Wayne Nicholson for the use of his HARV chambers. Finally, we thank Nell Bekiares, Kyle Grant, Jennifer Larmore, Justin Lockhart and Oliver Lundy for their assistance in collecting and maintaining the adult animal colonies. This work was supported by the NASA Florida Space Grant Consortium award UCF01-0000232913, as well as an equipment donation from the Promega, Corporation.
Author information
Authors and Affiliations
Contributions
J.S.F. conceived and designed the experiments. C.L.M.K., S.R.A., M.L.P., J.S.F. performed the experiments and analyzed the data. J.S.F. and C.L.M.K. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Foster, J., Khodadad, C., Ahrendt, S. et al. Impact of simulated microgravity on the normal developmental time line of an animal-bacteria symbiosis. Sci Rep 3, 1340 (2013). https://doi.org/10.1038/srep01340
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01340
This article is cited by
-
Impact of modeled microgravity stress on innate immunity in a beneficial animal-microbe symbiosis
Scientific Reports (2024)
-
Modeled microgravity alters apoptotic gene expression and caspase activity in the squid-vibrio symbiosis
BMC Microbiology (2022)
-
Modeled microgravity alters lipopolysaccharide and outer membrane vesicle production of the beneficial symbiont Vibrio fischeri
npj Microgravity (2021)
-
A lasting symbiosis: how the Hawaiian bobtail squid finds and keeps its bioluminescent bacterial partner
Nature Reviews Microbiology (2021)
-
Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.