Abstract
Gold nanoparticles (AuNPs) are often used as vehicles to deliver drugs or biomolecules, due to their mild effect on cell survival and proliferation. However, little is known about their effect on cellular metabolism. Here we examine the in vivo effect of AuNPs on metabolism using Drosophila as a model. Drosophila and vertebrates possess similar basic metabolic functions and a highly conserved PI3K/Akt/mTOR signaling pathway plays a central role in the regulation of energy metabolism in both organisms. We show that dietary AuNPs enter the fat body, a key metabolic tissue in Drosophila larvae. Significantly, larvae fed with AuNP show increased lipid levels without triggering stress responses. In addition, activities of the PI3K/Akt/mTOR signaling pathway and fatty acids synthesis are increased in these larvae. This study thus reveals a novel function of AuNPs in influencing animal metabolism and suggests its potential therapeutic applications for metabolic disorders.
Similar content being viewed by others
Introduction
Gold nanoparticles (AuNPs) possess many attractive optical and electronic properties that have proven to be of high utility in biomedical applications1. Biomolecular and cellular interactions of AuNPs are particularly interesting and versatile, which have led to numerous AuNPs-based diagnostic, imaging and therapeutic tools2,3,4,5,6,7. More recently, AuNPs were found to be cell permeable8,9 in addition to their well known low cytotoxicity and high biocompatibility10,11. Consequently, several groups including ours have shown that AuNPs can serve as nanoscale vehicles for drug delivery2,8,12. Despite the well-documented progress, direct dietary use of AuNPs in living organisms and possible consequences on cellular metabolism are little explored.
Living organisms can sense nutritional conditions to coordinate cellular metabolism. Interestingly, Drosophila and vertebrates are very similar in their basic metabolic functions, i.e., sugar, lipid and amino acid homeostasis13,14. In both organisms, a highly conserved phosphatidylinositol 3-kinase (PI3K) pathway has a pivotal role in nutrition uptake and storage15,16,17,18. Aberrant PI3K signaling in human results in metabolic disorders and often occurs in type-2 diabetes or cancers15,17. As a response to dietary nutrient, the upstream signaling molecule PI3K is activated, leading to re-localization and activation of Akt15. The Akt kinase has two major metabolic target molecules, FOXO and mTOR19,20. Phosphorylation by Akt inactivates FOXO21, a key transcription factor involved in catabolism, while activates the mTOR complex and its downstream targets. Activation of the mTOR complex in turn stimulates the activity of sterol regulatory element binding protein (SREBP)22,23, a transcription factor that controls expression of key enzymes of lipogenesis including Fatty Acid Synthase (FAS) and Acetyl-CoA Carboxylase (ACC)24.
In this work, we aimed to explore whether dietary AuNPs affect cellular metabolism and used Drosophila larvae as an in vivo model. We fed larvae with several concentrations of AuNPs and found that they were absorbed by the fat body, a key metabolic tissue in Drosophila larvae. In these larvae, stress responses including expression of heat shock genes, activation of the JNK pathway or induction of autophagy were not induced compared to those in control larvae. Despite their little effects on growth or stress responses, lipid levels were increased in larvae fed with AuNPs. By examining metabolic pathways that control lipid anabolism, we found that dietary AuNPs increased activities of the PI3K/Akt/mTOR signaling pathway and fatty acids synthesis in these larvae. In addition, inhibition of mTOR abolished these effects of AuNPs, suggesting that dietary AuNPs could affect Drosophila lipid metabolism by promoting the PI3K/Akt/mTOR signaling pathway.
Results
AuNPs of 15 nm (Fig. 1A,B, supplementary Fig. S1) were synthesized and formulated in the diet. Drosophila larvae fed with AuNPs were dissected to determine the distribution of AuNPs among different tissues. Quantitative analysis with inductively coupled plasma mass spectrometry (ICP-MS) detected localization of AuNPs in the fat body (equivalent to mammalian white adipose tissue and liver), but not in brain, salivary gland or imaginal discs (Fig. 1C). Further analysis by transmission electron microscopy (TEM) showed that AuNPs localized to lipid droplets of fat body cells (supplementary Fig. S4C, D).
Uptake of AuNPs by fly larva tissues.
(A) Hydrated diameter of AuNPs (23 nm) were measured by dynamic light scattering (DLS). (B) 15 nm AuNPs characterized by transmission electron microscopy (TEM). (C) Uptake of AuNPs by fly larva tissues, as measured by quantitative inductively coupled plasma mass spectrometry (ICP-MS). This bar graph shows representative data of four individual sample analyses.
We first examined whether cellular stress was up-regulated by dietary AuNPs. Cellular stress could increase expression of heat shock proteins25, activate the c-Jun N-terminal Kinase (JNK) pathway26 and trigger autophagy27,28. In larvae fed with 0.5 or 2 nM AuNPs, expression of hsp83 or hsp70 was unchanged (Fig. 2A, B, supplementary Fig. S2A, B); activity of the JNK pathway was unchanged or slightly reduced, as measured by expression of its downstream gene puckered (Fig. 2C, supplementary Fig. S2C)29; autophagy was also unchanged or slightly reduced, as measured by lysotracker staining (Fig. 2D, supplementary Fig. S2D). However, 5 nM dietary AuNPs did slightly increase expression of hsp70 and puckered, but not that of hsp83 (supplementary Fig. S2A-C). As a conclusion, up to 2 nM dietary AuNPs did not trigger cellular stress responses in larvae, although higher concentration of AuNPs might have a mild effect. For this reason we used 2 nM AuNPs for the rest of experiments.
Dietary AuNPs do not trigger stress responses in fly larvae.
In larvae fed with control food or 2 nM AuNPs, stress responses were measured by quantitative RT-PCR (A–C) or lysotracker staining (D). (A), (B) Dietary AuNPs did not affect expression of heat shock protein genes hsp83 (A) or hsp70 (B). ns, not significant. (C) Dietary AuNPs slightly reduced activation of JNK pathway downstream gene puckered. *, P<0.05. (D) Dietary AuNP slightly reduced autophagy in fat body.
The localization of AuNPs to lipid droplets of fat body cells triggered our interest to examine its possible consequences on lipid metabolism. Drosophila and mammals regulate lipid metabolism via the highly conserved SREBP pathway30, which activates expression of key enzymes of fatty acid synthesis including FAS and ACC24. In larvae fed with AuNPs, triacylglycerol (TAG, the main stored form of lipid) levels were slightly higher (∼10%, Fig. 3A). In addition, expression of FAS and ACC were increased, suggesting that enhanced fatty acid synthesis was responsible for the change of lipid levels (Fig. 3B,C). The fly food provided excessive nutrition and lipid anabolism in larvae already reached a high level. Thus we also fed larvae in the condition of calorie restriction (CR), where lipid anabolism was limited. Under this background, larvae fed with AuNPs showed even higher TAG levels (∼20%) compared to the CR control (Fig. 4A).
Calorie restriction enhances effects of AuNPs on fat metabolism and Akt signaling.
(A) Dietary AuNPs increased larva TAG levels by 10% in standard food and by 20% under the condition of calorie restriction (CR). *, P<0.05. (B) Under CR, larvae fed with AuNPs showed increased Akt activity compared to the control (lane 4 vs. lane 3). (C) (D) Under CR, larvae fed with AuNPs (D) had higher PI3K activity in the fat body compared to the control (C).
The SREBP pathway is a downstream effector of the PI3K/Akt/mTOR pathway22,23. To test whether this signaling pathway was activated by dietary AuNPs, we examine the activity of PI3K, Akt and expression of their downstream genes. In standard diet, the activity of Akt in larvae fed with AuNPs was comparable to that in control, consistent with the result that AuNPs only had a mild effect on lipid metabolism under this condition. In the presence of CR, dietary AuNPs significantly enhanced Akt activity (Fig. 4B). We also measured membrane bound tGPH in the fat body, whose levels reflected the activity of PI3K16. Larvae fed with AuNPs showed higher activity of PI3K in the fat body than the control (Fig. 4C,D). These results confirmed that the upstream signaling molecules (PI3K and Akt) were activated by dietary AuNPs. Activated Akt can enhance expression of FAS and ACC via the mTOR/SREBP cascade22 and repress expression of InR and 4EBP via FOXO (supplementary Fig. S3A)21. In both standard diet and CR diet, larvae fed with AuNPs showed increased expression of FAS and ACC and reduced expression of InR and 4EBP (supplementary Fig. S3B-E), confirming that dietary AuNPs activated the Akt pathway and affected Akt target gene expression.
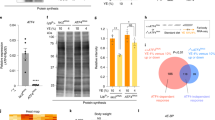
Dietary AuNPs may affect other metabolic pathways besides Akt signaling. To test whether the effect of AuNPs on Drosophila fat metabolism was dependent on the PI3K/Akt/mTOR pathway, we blocked Akt signal transduction by rapamycin, an inhibitor of mTOR31 and examined the effect of AuNPs on fat levels and fatty acid synthesis. In the presence of rapamycin, larvae fed with AuNPs had similar fat levels compared to the control, either in standard diet or CR diet (Fig. 5A,B). In addition, dietary AuNPs no longer increased expression of FAS or ACC (Fig. 5C,D), suggesting that rapamycin blocked the stimulation of fatty acid synthesis by AuNPs. Therefore, dietary AuNPs possibly affect Drosophila fat metabolism via the PI3K/Akt/mTOR pathway (Fig 5E).
The effect of AuNPs on larva fat metabolism is dependent on Akt/mTOR signaling.
(A) (B) In standard diet (A) and CR (B), the increase in larva TAG levels by dietary AuNPs was abolished when the Akt/mTOR signaling pathway was blocked by rapamycin. *, P<0.05. ns, not significant. (C) (D) Increased expression of FAS (C) and ACC (D) by dietary AuNPs was abolished when the Akt/mTOR signaling pathway was blocked. **, P<0.01. ns, not significant. (E) A diagram for the effect of dietary AuNPs on larva fat metabolism.
Discussion
There are several ways that AuNPs can affect PI3K/Akt signaling. PI3K/Akt signaling generally senses nutritional conditions to coordinate metabolism. Although larvae fed with or without AuNPs consume the same amount of food (supplementary Fig. S4A), it is possible that AuNPs may promote nutrient uptake at cellular levels. To test this possibility, we examined total sugar levels as sugar catabolism provided acetyl-CoA precursors (pyruvate and citrate) for fatty acid synthesis. We found that larvae fed with AuNPs had higher sugar levels than the control in CR food, while this difference was insignificant in standard food (supplementary Fig. S4B). These data suggest that AuNPs enhance nutrient uptake in CR food and may at least partially explain our observations. Alternatively, AuNPs may affect activities of signaling molecules of the PI3K/Akt pathway. Activations of both PI3K and Akt occur on the cell membrane. It is also likely that endocytosis of AuNPs facilitates recruitment of PI3K and Akt on the cell membrane and promotes their activations. Consistent with this model, higher levels of tGPH are detected at the cell membrane (Fig. 4C,D), reflecting that higher levels of PI3K are localized to the cell membrane. Therefore this mechanism may also contribute to the effect of AuNPs.
AuNPs have shown great promise in biomedicine, e.g. they may serve as vehicles to deliver chemical drugs or biomolecules. While it is generally considered that AuNPs are biocompatible and non-toxic for cell growth, other biochemical activities of AuNPs are little studied. In this work, we focus on the metabolic effects of dietary AuNPs in Drosophila larvae. We find that dietary AuNPs promote the Akt signaling pathway and alter lipid metabolism, an unexpected in vivo effect of nanomaterials on cellular metabolism. Given that metabolic diseases such as diabetes have become worldwide epidemic, our new observations shed new light on using AuNPs, either by themselves or coupled with other functional drugs, as nanomedicine against metabolic disorders.
Methods
Preparation and characterization of AuNPs
AuNPs of 15 nm were synthesized by reduction of HAuCl4 as previously reported32 and their sizes were characterized with transmission electron microscopy and dynamic light scattering.
Fly strain and culture
yw D. melanogaster was used in this study. Flies were raised in cages. 0–1 hour embryos were collected from fruit juice agar plates, counted and distributed on SY food (10% sucrose, 10% yeast, 1.5% agar)33 with or without AuNPs. AuNPs were coated with 2 ug/ul BSA for 10 minutes and mixed with the food before solidification. Same amount of BSA is also included in the control food. Larvae were harvested 108 hours later for analysis. For CR, the fly food contained 25% nutrients of standard food and larvae were harvested 156 hours after egg laying. To prepare larval tissues for detection of AuNPs distribution, larvae fed with AuNPs were transferred to control food for 8 hours, allowing evacuation of AuNP food. Larvae were then transferred to 6 cm dishes and washed 10 times in 1×PBST before dissection. Dissected tissues were transferred to microcentrifuge tubes and washed 10 times in 1×PBST.
Measurement of mRNA, protein and lipid levels
Total RNAs were extracted from larvae with TRIzol reagent (Invitrogen). The first-strand cDNAs were generated by iScript™cDNA Synthesis Kit (Bio-Rad). Quantitative RT-PCR assays were performed with SYBR Green PCR Master Mix and the Applied Biosystems ABI Prism 7000 Sequence Detection System (Applied Biosystems; Foster City, CA). The average expression levels of actin, tubulin and TBP were used as internal controls for normalization of mRNA levels. For western blotting, anti-phospho-Akt and anti-Tubulin antibodies (Cell Signaling) were used. For measurement of TAG, larvae were suspended in 400 ul chilled lysis buffer (0.01 mM KH2PO4, 1 mM EDTA, protease inhibitors cocktail, 0.05% Tween 20), lysed by Tissuelyser II (Qiagen, set at 30 Hz for 3 min) and heated at 70°C for 5 min. Cleared supernatant was used to measure TAG with TAG determination kits (Sigma,TR0100). Lipid levels were normalized to total protein levels.
References
Daniel, M. C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties and applications toward biology, catalysis and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Ghosh, P., Han, G., De, M., Kim, C. K. & Rotello, V. M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 60, 1307–1315 (2008).
Zhang, J., Song, S., Wang, L., Pan, D. & Fan, C. A gold nanoparticle-based chronocoulometric DNA sensor for amplified detection of DNA. Nat. Protoc. 2, 2888–2895 (2007).
Liu, J. & Lu, Y. Fast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles. Angew. Chem. Int. Ed. 45, 90–94 (2005).
Xiao, Y., Patolsky, F., Katz, E., Hainfeld, J. F. & Willner, I. "Plugging into Enzymes": nanowiring of redox enzymes by a gold nanoparticle. Science 299, 1877–1881 (2003).
Zhang, J., Liu, Y., Ke, Y. & Yan, H. Periodic square-like gold nanoparticle arrays templated by self-assembled 2D DNA Nanogrids on a surface. Nano Lett. 6, 248–251 (2006).
Chen, P. et al. Gold nanoparticles for high-throughput genotyping of long-range haplotypes. Nature Nanotechnol. 6, 639–644 (2011).
Dhar, S., Daniel, W. L., Giljohann, D. A., Mirkin, C. A. & Lippard, S. J. Polyvalent Oligonucleotide Gold Nanoparticle Conjugates as Delivery Vehicles for Platinum(IV) Warheads. J. Am. Chem. Soc. (2010).
Rosi, N. L., Giljohann, D. A., Thaxton, C. S., Lytton-Jean, A. K., Han, M. S. & Mirkin, C. A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006).
Rosi, N. L. & Mirkin, C. A. Nanostructures in biodiagnostics. Chem. Rev. 105, 1547–1562 (2005).
Connor, E. E., Mwamuka, J., Gole, A., Murphy, C. J. & Wyatt, M. D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1, 325–327 (2005).
Wei, M. C., H., Li, J., Yin, M., Liang, L., He, Y., Song, H., Fan, C. & Huang, Q. Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide-conjugated gold nanoparticles. Angew. Chem. Int. Ed. 51, 1202–1206 (2012).
Baker, K. D. & Thummel, C. S. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metabolism 6, 257–266 (2007).
Leopold, P. & Perrimon, N. Drosophila and the genetics of the internal milieu. Nature 450, 186–188 (2007).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002).
Britton, J. S., Lockwood, W. K., Li, L., Cohen, S. M. & Edgar, B. A. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2, 239–249 (2002).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 (2006).
Huang, H. & Tindall, D. J. Dynamic FoxO transcription factors. J. Cell. Sci. 120, 2479–2487 (2007).
Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8, 179–183 (2005).
Puig, O., Marr, M. T., Ruhf, M. L. & Tjian, R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006–2020 (2003).
Porstmann, T., Santos, C. R., Griffiths, B., Cully, M., Wu, M., Leevers, S., Griffiths, J. R., Chung, Y. L. & Schulze, A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 146, 408–420 (2011).
Magana, M. M., Lin, S. S., Dooley, K. A. & Osborne, T. F. Sterol regulation of acetyl coenzyme A carboxylase promoter requires two interdependent binding sites for sterol regulatory element binding proteins. J. Lipid Res. 38, 1630–1638 (1997).
Singh, M. P., Reddy, M. M., Mathur, N., Saxena, D. K. & Chowdhuri, D. K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: role of ROS generation. Toxicol. Appl. Pharmacol. 235, 226–243 (2009).
Stronach, B. E. & Perrimon, N. Stress signaling in Drosophila. Oncogene. 18, 6172–6182 (1999).
Simonsen, A., Cumming, R. C. & Finley, K. D. Linking lysosomal trafficking defects with changes in aging and stress response in Drosophila. Autophagy. 3, 499–501 (2007).
Scott, R. C., Schuldiner, O. & Neufeld, T. P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 7, 167–178 (2004).
McEwen, D. G. & Peifer, M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 132, 3935–3946 (2005).
Rawson, R. B. The SREBP pathway--insights from Insigs and insects. Nat. Rev. Mol. Cell. Biol. 4, 631–640 (2003).
Brown, E. J., Albers, M. W., Shin, T. B., Ichikawa, K., Keith, C. T., Lane, W. S. & Schreiber, S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 369, 756–758 (1994).
Hayat, M. A. Colloidal gold : principles, methods and applications (Academic Press, 1989).
Magwere, T., Chapman, T. & Partridge, L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J. Gerontol. A-Biol. Sci. Med. Sci. 59, 3–9 (2004).
Acknowledgements
This work was supported in part by grants from the Chinese Ministry of Science and Technology (MOST, 2011CB943900 and 2012CB932600), the National Natural Science Foundation of China (NSFC, 31071145, 90913014). H.S. is a scholar of the “Hundred Talent Program.”
Author information
Authors and Affiliations
Contributions
CF and HS designed the project, BW, NC and YW performed experiments, JL, LS, JW, QH and CL provided new materials, BW, NC, CF and HS wrote the main manuscript text and BW prepared figures 1–5. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wang, B., Chen, N., Wei, Y. et al. Akt signaling-associated metabolic effects of dietary gold nanoparticles in Drosophila. Sci Rep 2, 563 (2012). https://doi.org/10.1038/srep00563
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00563
This article is cited by
-
Tailoring of graphene quantum dots for toxic heavy metals detection
Applied Physics A (2019)
-
The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster - a 20 generation dietary exposure experiment
Scientific Reports (2018)
-
Deciphering active biocompatibility of iron oxide nanoparticles from their intrinsic antagonism
Nano Research (2018)
-
Mapping the Glyco-Gold Nanoparticles of Different Shapes Toxicity, Biodistribution and Sequestration in Adult Zebrafish
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.