Abstract
There are fewer studies on Trichoderma diversity in agricultural fields. The rhizosphere of 16 crops was analyzed for Trichoderma species in 7 districts of Rajasthan state of India. Based on DNA sequence of translation elongation factor 1α (tef-1α), and morphological characteristics, 60 isolates were identified as 11 species: Trichoderma brevicompactum, species in Harzianum clade identified as T. afroharzianum, T. inhamatum, T. lentiforme, T. camerunense, T. asperellum, T. asperelloides, T. erinaceum, T. atroviride, T. ghanense, and T. longibrachiatum. T. brevicompactum is the most commonly occurring strain followed by T. afroharzianum. No new species were described in this study. T. lentiforme, showed its first occurrence outside the South American continent. The morphological and cultural characteristics of the major species were observed, described, and illustrated in detail. The isolates were tested for their antagonistic effect against three soilborne plant pathogens fungi: Sclerotium rolfsii, Rhizoctonia solani, and Fusarium verticillioides in plate culture assays. One of the most potent strains was T. afroharzianum BThr29 having a maximum in vitro inhibition of S. rolfsii (76.6%), R. solani (84.8%), and F. verticillioides (85.7%). The potential strain T. afroharzianum BThr29 was also found to be efficient antagonists against soil borne pathogens in in vivo experiment. Such information on crop selectivity, antagonistic properties, and geographic distribution of Trichoderma species will be beneficial for developing efficient Trichoderma-based biocontrol agents.
Similar content being viewed by others
Introduction
Species in the fungal genus Trichoderma (Hypocreales, Ascomycota) are known for rapid growth and high-stress tolerance. Many of the Trichoderma species have a cosmopolitan distribution and inhabit a wide range of ecological niches such as soil, decayed wood, endophytes of plants, mushroom compost, and marine habitats1,2. Currently, there are about 500 species based on legitimate names in the Mycobank ((https://www.mycobank.org.) the majority of them have been described during the past two decades based on multi-locus phylogeny analyses of DNA sequences3,4,5,6,7. Native Trichoderma strains from crop rhizosphere are likely to be more effective biocontrol agents as they are in close contact with plant roots and have higher chances to establish endophytic relationships and exert the benefits associated with the relationship such as induced resistance to pathogens and enhance plant growth8. The occurrence and prevalence of Trichoderma in different ecological niches have contributed to the evolution of the species and genetic and metabolic diversities. This immense ecological importance of Trichoderma spp. and plant association makes it pertinent to understand their biodiversity in the crop rhizosphere.
Taxonomic classification of Trichoderma species remained obscure until 1969, about 200 years after the genus name Trichoderma was introduced. Rifai9 used morphological characters plus characters from the teleomorphs and distinguished nine aggregate species and emphasized that each aggregate species could harbour more species. Bissett10,11,12,13 revised Rifai’s aggregate species by looking at them as sections and recognized four sections and 27 biological species. Bissett rarely used characters from teleomorphs. In the early 1990s phylogenies based on DNA sequencing data started and revealed some important achievements in Trichoderma taxonomy. Phylogeny based on sequences of the ribosomal large subunit revealed that Gliocladium comprised three paraphyletic groups distinct in morphology and that G. virens is Trichoderma virens. Rehner and Samuels14 also revealed that phylogenetically Trichoderma and Hypocrea are indistinguishable, and they both fit into identical clades. Later, inaccuracies in Trichoderma taxonomy based on morphological characters alone were shown in the study of Kullnig et al.15, which confirmed that the phylogenies based on sequencing data are indispensable. This led to an explosion in the number of Trichoderma species. Currently, the DNA sequences of ribosomal internal transcribed spacer (ITS), translation elongation factor 1α (tef-1α), and RNA polymerase subunit2 (rpb2) are used for the identification of new species when used in multi-locus phylogeny16.
Previously, researchers explored crop rhizospheres in different parts of India. Kumar et al.17 characterized the isolates of Trichoderma spp. from south Andaman for their cultural, morphological, and antagonistic activity against soilborne and foliar pathogens such as Sclerotium rolfsii, Colletotrichum gloeosporioides, and C. capsici. Kamala et al.18 isolated 193 Trichoderma strains from cultivated fields of the Indian region of the Indo-Burma Biodiversity hotspot. Meena and Meena19 did morphological and physiological characterization of eight Trichoderma spp isolates collected from cluster bean fields in western Rajasthan. They studied their antagonism against Macrophomina phaseolina, Fusarium oxysporum, and Sclerotinia sclerotiorum. Rai et al.20 collected Trichoderma spp. from tomato rhizosphere and characterized it phenotypically, biochemically, and genetically to screen the most efficient Trichoderma spp. against pathogens causing disease in tomato crops. Overlapping morphological characters of T. pseudokoningii and T. longibrachiatum were characterized by Prameeladevi et al.21 using ITS and tef-1α genes and confirmed that isolates identified as T. pseudokoningii in India are genuinely T. longibrachiatum. The value of Trichoderma spp. depends on their applicability in different crops growing in varied ecozones. Therefore, collecting Trichoderma isolates from different crop rhizosphere is essential, which will help select the most efficient antagonistic strains of Trichoderma spp. Moreover, the southern part of Rajasthan was untouched to explore genetic diversity and utilize the potential of its Trichoderma spp. and strains; thus, it was necessary first to isolate, identify and characterize the isolates of Trichoderma. Growth media markedly affects the colony formed22. Various studies were conducted to evaluate different culture media for the growth of T. harzianum23,24, Several selective media for Trichoderma spp. were previously described25,26. In the present study, we emphasized on selection of the appropriate growth media to facilitate efficient isolation of potential Trichoderma spp. from the soil.
To utilize the full potential of the Trichoderma species in specific applications, precise identification and characterization of this fungus are vital27. The present study was carried out to characterize and identify Trichoderma species isolated from 16 different crop rhizosphere soils in southern Rajasthan by using morphological characterization and sequence analysis of translation elongation factor (tef-1α) section that encompasses few introns (Fig. 1). Potentiality of the Trichoderma strains was also evaluated in in vitro and in vivo assays.
Result
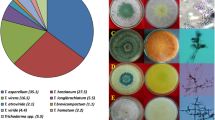
The isolation rate of Trichoderma isolates from 273 soil samples was 22%. Details of the strains isolated from two agroclimatic zones namely, Zone IVA-Sub-humid southern plains and IVB-Humid southern plains of southern Rajasthan in India, are given in Supplementary table 1. The isolates were identified using morphological characters and the DNA sequences of tef-1α section that encompass a few introns. The total 60 isolates were classified into 11 species: Trichoderma afroharzianum (11), T. asperellum (10), T. asperelloides (3), T. inhamatum (1), T. camerunense (2), T. erinaceum (11), T. lentiforme (1), T. atroviride (1), T. ghanense (1), T. longibrachiatum (1), T. brevicompactum (18). These eleven species are prevalent in the crop rhizosphere of southern Rajasthan (Fig. 2).
Distribution of Trichoderma species in the different districts of southern Rajasthan, where, (a) T. afroharzianum; (b) T. asperellum; (c) T. asperelloides; (d) T. inhamatum; (e) T. lentiforme; (f) T. camerunense; (g) T. erinaceum; (h) T. atroviride; (i) T. ghanense; (j) T. longibracheatum; (k) T. brevicompactum.
Phylogeny
Due to high variability of tef-1α section and the difficulty of aligning all the species of the Trichoderma genus together, we divided the alignment file into two files, one representing species within Trichoderma section and T. brevicompactum. The other comprising species in the T. harzianum complex clade and T. longibrachiatum section. The first file contained 54 taxa, including five reference sequences for five specific type species and one outgroup taxa. The tree in Fig. 3 shows that there were 18 isolates (BTbr66, BTbr20A, BTbr56, BTbr53, BTbr51iv, BTbr44, BTbr59, BTbr28, BTbr10, BTbr2, BTbr64a, BTbr52, BTbr6, BTbr54, BTbr71, BTbr55, BTbr34, and BTbr74) clustered with the type species of T. brevicompactum (AY857297) with high Bootstrap values, clearly indicating that the 18 isolates are T. brevicompactum.
Phylogenetic relationships of Trichoderma section Trichoderma isolates from India. One of most parsimonious trees generated from tef-1α sequence data. Bootstrap values are given (Parsimony/Maximum Likelihood) on the branches. T at the end of leaf label refers to type species sequence. B at the end of leaf label refers to culture used in biocontrol. Tree was rooted to T. hamatum.
Three taxa (BTas41, BTas51, and BTas38) formed a highly supported clade with type species of T. asperelloides (GU198294). Another 10 strains (BTas1, BTas33, BTas23, BTas26, BTas24, BTas25, BT30, BTas21, BTas18, and BTas43) clustered with the type species of T. asperellum with high bootstrap value. The T. asperellum and T. asprelloides clades have sister-relationship with a high bootstrap value of 84. One isolate (BTat3) formed a clade with the type species sequence of T. atroviride which confirms the identity of the isolate as T. atroviride. T. atroviride is known to be dominant in North America and Europe but rare in tropical regions and well known for biocontrol applications. Finally, 11 strains (BTer39, BTer68, BTer27, BTer47, BTer49, BTer62, BTer48, BTer46, BTer57, BTer73, and BTer61) clustered with the type species of T. erinaceum (AY750880), clearly indicating the identity of the 11 strains as T. erinaceum. T. erinaceum has sister relationship with T. atroviride with high bootstrap values (89/87).
In the second phylogenetic tree Fig. 4 11 strains (BThr7, BThr16, BThr13, BThr1, BThr32, BThr14, BThr29, BThr37, BThr5, BThr16, and BTh4) formed a clade with type strain for T. afroharzianum (KP115273) and another reference sequence deposited in GenBank as T. harzianum (AY605770). The clade of 12 isolates had high bootstrap values of 83 and 91 for parsimony and maximum likelihood analyses, respectively. T. afroharzianum sequence was identical to sequences of five of the isolates under the study and differed from the other five isolates by one base, clearly indicating that the clade of 12 isolates represents an all T. afroharzianum clade. Another isolate BThr19 formed a highly supported clade with the type strain species of T. inhamatum (AF348099), which was obtained from Thailand. One isolate BThr67 clustered with the type species sequence for T. lentiforme. The last two strains of the T. harzianum clade (BThr12 and BThr35) formed a clade with high bootstrap value 96 /100 for parsimony and maximum likelihood analyses, respectively with type species of T. camerunense which is a dominant species of Harzianum clade species in Africa. Of the two strains from the Longibrachiatum section one (BTlg15) formed a clade with type species of T. longibrachiatum with bootstrap support of 100, clearly indicating that BTlg15 is T. longibrachiatum. The other strain BTgh8 formed a clade with the type strain of T. ghanense with high support. This indicates that BTgh8 is T. ghanense.
Phylogenetic relationships of Trichoderma harzianum complex species and section Longibrachiatum isolates from India. One of most parsimonious trees generated from tef-1α sequence data. Bootstrap values are given (Parsimony/Maximum Likelihood) on the branches. T at the end of leaf label refers to type species sequence. B at the end of leaf label refers to culture used in biocontrol. Tree was rooted to T. virens.
Relative abundance of different Trichoderma species in different crops
The Trichoderma strains were isolated from the rhizosphere of 16 crops cultivated in fields of southern Rajasthan. The 60 isolates are classified into eleven species that have varied abundance in different crop rhizosphere. It was observed that a maximum of 10 Trichoderma strains, three T. erinaceum, three T. brevicompactum, one each species of T. afroharzianum, T. asperelloides, T. inhamatum, and T. lentiforme were isolated from the soybean crop rhizosphere. This was followed by maize rhizosphere, from which seven strains of Trichoderma were isolated, including three strains of T. afroharzianum, two strains of T. erinaceum, and one each strain of T. asperelloides, and T. brevicompactum (Fig. 5). Eighteen strains of T. brevicompactum occurred in 12 crop rhizospheres, while eleven strains each of T. afroharzianum and T. erinaceum were isolated from crop rhizosphere of nine and eight crops, respectively. T. inhamatum, T. ghanense, T. longibrachiatum, and T. atroviride were found in individual crop rhizospheres. The relative dominance values calculated showed that the genus Trichoderma is not dominant in the soil samples. The dominance value of T. brevicompactum, T. erinaceum, T. afroharzianum, T. asperellum was 0.016, 0.012, 0.01, and 0.008, respectively, which was very low as compared to the standard dominance value (> 0.02).
Effect of different media on growth and sporulation of different Trichoderma spp.
Representative isolates of different Trichoderma species showed different growth patterns in different media. Among all the cultured species, T. harzianum (= T. afroharzianum), T. asperellum, and T. erinaceum had the fastest growth in PDA medium (Fig. 6). Box plot analysis (Fig. 7) of three media that actively support the growth of Trichoderma strains revealed that at 2 days of incubation T. asperellum, T. erinaceum, and T. afroharzianum cover 2.2, 2.6, and 2 cm on PDA media which grows to 5.7, 5.3, and 5.8 cm after 4 days of incubation (Supplementary table 2). At 6 days of incubation, all three species of Trichoderma cover 7 cm growth on PDA media. The CMA medium is the next best medium for the growth of Trichoderma spp. The T. asperellum, T. erinaceum, and T. afroharzianum grow to 2.1, 2, and 2 cm on CMA media after 2 days of growth which rose to 4, 5.23, and 5.63 cm at 4 days of incubation (Supplementary table 2). At 6 days of incubation, T. asperellum showed a growth of 7 cm, and T. erinaceum 6.1 cm. T.afroharzianum attain a maximum growth of 7 cm. TJA media poorly support the growth of Trichoderma spp.
Morphological characterization
In this study, we have reported seven species of Trichoderma prevalent in the rhizosphere of cultivated fields of southern Rajasthan. The strains were confirmed with the general morphological characters as described below.
T. ghanense
On the PDA medium after 72 h, the colony radius was 38–41 mm, mycelium covering the plate after 5 d at 27 °C. Colony dense, circular, not finely zonate with aerial hyphae more abundant at the colony center, growing fertile. Conidiation starts after two days of incubation, effuse in aerial hyphae or small granules spread in the entire plate but absent from the colony center, and turn green (Fig. 8). On the back side of the plate appears dark brown pigmentation. Numerous conidiophores, Trichoderma-like, branches paired or unpaired, at right or acute angles with the main axis, not or re-branched once. Paired phialides, lageniform, and sometimes ampulliform, are reported to have tuberculate conidia, but in microscope preparations, many or most conidia do not have visible tubercles, and typically only one or a few tubercles are seen on individual conidia. Conidia of Banswara strain (BTgh08) were considerably smaller (1.74 ± 0.15 × 1.45 ± 0.10 µm). T. ghanense is typically a soil species and has not been linked to a teleomorph. This strain was isolated from Peepalkhunth forest soil N 23° 49′ 9.9″ E 74° 35′ 21.8″.
T. afroharzianum 9
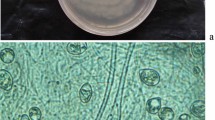
Colony on PDA medium in 72 h covers 45–50 mm, mycelium covers plate at 6th day after inoculation at 27 °C. The conidia appear green from the 2nd day onwards. The conspicuous concentric rings appear on the scale. The colony on PDA appears powdery, light pale green, and later gray-green. Light to dark brown pigmentation appeared at the bottom of the plate. A typical green mat of conidia with concentric rings also appeared on CMA. The different isolates had two types, 1. Brown color pigment producer, and 2. Non-pigment producer. Pustules are loosely arranged into flat spreading blemishes. Diffused conidia, appearing powdery due to dense conidiation, rapidly turning yellowish green to dark green. The reverse of the plate seemed colorless to dark brown. Conidiophores were irregularly branched and formed on the aerial hyphae and had constricted base and verticillate branching (Fig. 9). Phialides were short and ampulliform and arranged in whorls having swollen centers with slender peaks. The conidial shape was globose to obovoid green and smooth-walled.
T. brevicompactum
The colony of T. brevicompactum was characterized by densely aggregated conidiophores in coalescent pustules, which become greenish olivaceous to glaucous blue-green or grey olivaceous. On PDA, T. brevicompactum was fast growing, exceeding 75 mm on five days at 27 ± 1 °C. On CMA, the growth was a little slower and diffused, dispersed, and at the same time, formed concentric rings. The color of mature conidia in CMA was light green. In CZA, the colony growth was slower but had conspicuous concentric rings. In V8, no concentric rings were observed. Their conidial productions were restricted to the center of the colonies, diffused, and appeared to be yellowish green (Fig. 10). The conidiophores were hyaline, smooth-walled, pyramidally verticillately branched in the Pachybasium-type pattern. Conspicuous short sterile appendages are visible in young conidiogenous pustules but inconspicuous or absent in older cultures. Phialides were in whorls of 2–5, mostly broadly ampulliform with a short slender neck, solitary terminal with compact branches giving a compressed appearance to the conidiogenous structures. Conidia were subglobose or short ellipsoidal in some strains, mostly 2.0–3.0 mm in diameter, with a minimally protruding basal hilum, smooth-walled, appearing pale grey-green microscopically.
T. erinaceum
Colony on PDA medium was moderately slow growing covering 25–30 mm in 72 h; mycelium covers plate on the 9th day after inoculation at 27 °C. Culture grows slowly, forming cottony white growth with concentric rings and turning green. Conidiophores were erect and arose at a right angle or less concerning the main branch. The phialides form a whorl of 2–3, having almost cylindrical to little swollen in the middle (6.0 to 8.0 µm long), conidia ellipsoidal to broadly ellipsoidal with a smooth margin (Fig. 11).
T. longibrachiatum
Colonies on PDA grow well and rapidly change from white to green in the frequent occurrence of concentric ring-like zones. The culture produces lemon yellow pigment with this mycelial colony. Culture grows slowly; conidiophores form scanty aerial mycelium, cottony blue-green pustules. Conidiophores with a central axis on which phialides arise singly over several levels below the tip. Spur-like phialides occur as an outgrowth of the basal cell at the septum. Lageniform phialides are often cylindrical with a hook at the apex. Conidia are smooth, thick-walled, typically oblong, ellipsoidal, and smooth (Fig. 12).
T. asperellum
Colony on PDA medium was fast growing, covering 45–50 mm in 72 h, mycelium covers plate on 6th day after inoculation at 27 °C. The colony was dark green, and the mycelium color was white at the bottom. Mycelial morphology was coarse and formed concentric rings of dense conidial production, with conidia towards the center, dark green spores, and aerial mycelium lacking. Fertile, long, regularly branched conidiophores with more or less uniformly paired lateral branches and the most extended branch occurring at the distal end from the tip. Phialides form at the tip of branches in verticillate whorls. Phialides are long and slightly wider in the middle than at the base. Conidia are globose to slightly ovoidal (Fig. 13).
T. atroviride
The colony character of the T. atroviride appeared as a tuft of dark greenish conidial mass having mature conidia in a central disk about 1.5 cm diameter and two pronounced, continuous white fluorescent concentric rings alternating with rings of sterile, felty white mycelium producing a single, terminal phialide (Fig. 14). Fertile branches arising at right angles from the base of setae, branches proximal to the tip of the seta typically comprising one or a few cells, terminating in a single phialide or a terminal whorl of 3–5 phialides; fertile branches produced lower, longer, and re-branching, each branch is having a whorl of phialides and solitary phialides arising directly from the branch axis. Conidia were subglobose to ovoidal.
In vitro inhibition of Sclerotium rolfsii, Rhizoctonia solani, and Fusarium verticillioides
Antifungal activity of Trichoderma strains against S. rolfsii
The results of dual culture indicated that all the isolates of Trichoderma spp. significantly (p ≤ 0.05) inhibited the growth of S. rolfsii from 44.8% (T. afroharzianum BThr67) to 74.8% (T. afroharzianum BThr29) on 6th day, restricting the pathogen almost completely on plates as compared to control consisting of S. rolfsii growing alone (Fig. 15A). Among all the Trichoderma isolates, the action exerted by T. afroharzianum isolates was relatively more potent (P ≤ 0.05) than that of other species of Trichoderma (BThr5 71.4%, BThr16 71.0%, BThr21 72.4%, BThr13 59.0%, BThr19 59.0%, BThr32 66.2% BThr35 62.4%, BThr37 67.1%) (Supplementary table 3). T. asperellum also showed at par efficacy to control S. rolfsii in confrontation assay. The range of S. rolfsii inhibition by T. asperellum was between 51.0% (T. asperelloides BTas51) to 72.4% (T. asperellum BTas21) (Supplementary table 3). Box plot model (Fig. 16C) depicts all data points of 60 Trichoderma strains causing % inhibition of S.rolfsii. The R-value (Z/M) indicated the radial growth of the pathogen in the presence of Trichoderma strains emphasizing the restricting potential of Trichoderma isolates. Lower the R value, higher the restricting potential. In the present study, the minimum radial growth with maximum restricting potential of S. rolfsii was 0.67 ± 0.02 showed by T. camerunense BThr12 and T. brevicompactum BTbr52. Other Trichoderma strains with lower R-value includes T. brevicompactum BTbr28 (0.70 ± 0.13), T. brevicompactum BTbr44 (0.70 ± 0.09), and T. afroharzianum BThr1 (0.71 ± 0.09). The Box plot model (Fig. 16A) indicates that Z/M values for all 60 isolates are below 1, a trend which was also indicated in the heat map (Fig. 17). Thus, all those isolates showed the good restricting potential of Trichoderma strains to prevent radial growth of the pathogen in plate culture and therefore found to be more aggressive towards the pathogen. However, the Trichoderma strains BThr29 with the maximum in vitro inhibition of S. rolfsii has R-value of 0.74 ± 0.02 (Supplementary table 33). Pakdaman’s biological control index (PBCI) of BThr 29 was recorded to be the highest at 0.77 which clearly indicates the highest antagonistic potential of the strain against S. rolfsii. Other strains BThr 5 (0.68) and BTas25 (0.65) also showed their antagonistic potential against S. rolfsii.
Antagonistic activity of Trichoderma spp. against Rhizoctonia solani
Dual culture assay of Trichoderma strains significantly inhibited the growth of Rhizoctonia solani from 61.9 ± 5.95% (T. erinaceum BTer73) to 84.8 ± 1.65% (T. afroharzianum BThr29) on 6th day after incubation (Fig. 15B). Heat map (Fig. 17) also shows the highest value near 3 to depict the intensity of inhibition of R. solani. The R-values of T. afroharzianum BThr13 and T. erinaceum BTer27 were very low 0.64 ± 0.04 and 0.67 ± 0.07, respectively (Supplementary table 4), indicating that there was less resistance from R. solani to the radial growth of Trichoderma strains which shows their antagonistic potential. Pakdaman’s biological control indices (PBCIs) of Trichoderma strains displayed in the Box plot model (Fig. 16B) and Heat map (Fig. 17) unambiguously indicated that among tested T. afroharzianum strains, the BThr29 has the highest PBCI value of 1.11 ± 0.12 followed by T. asperellum BTas25 (1.06 ± 0.24) which indicates its higher biological potential than other isolates against R. solani.
Antagonistic activity of Trichoderma spp. against Fusarium verticillioides
Notable differences were observed for the radial growth of F. verticillioides in the presence of T. afroharzianum isolates. T. afroharzianum BThr29 caused maximum inhibition (89.5%) of F. verticillioides under in vitro conditions followed by other Trichoderma strains (BThr7, BTas30) (Fig. 15C). More inhibition of F. verticillioides by 60 Trichoderma isolates in comparison to other pathogens is clearly depicted by the median line in the Box plot model which is above 80% (Fig. 16C). The parameter of R-value (calculated as the ratio of Z/M) did not exhibit significant differences in resistance of F. verticillioides against T. afroharzianum isolates. However, Box plot model proved that all 60 isolates showed resistance lower than one (Fig. 16A), which suggested that growth media (PDA) facilitates more growth of Trichoderma isolates in the presence of F. verticillioides. BThr29, BTas23, BThr30, BTbr10, and BTer39 resulted in lower R values 0.78 ± 0.10, 0.77 ± 0.06, 0.77 ± 0.03, 0.73 ± 0.12 and 0.75 ± 0.13 respectively (Supplementary table 5) showing maximum resistance to the growth of F. verticillioides.
Pakdaman’s biological control indices (PBCIs) statistically differed highly, and it showed that BThr29, which has the highest PBCI value of 1.83 ± 0.58 was more effective than other Trichoderma strains in the biological control of F. verticillioides (Supplementary table 5). Box plot model representing that median of PBCI value for all 60 Trichoderma isolates is above 1 (Fig. 16B) which showed that Trichoderma isolates outgrowth over slow-growing F. verticillioides.
In vivo evaluation of the efficacy of potent Trichoderma strains to control damping off caused by Sclerotium rolfsii and Rhizoctonia solani in pot culture experiment
The most potential Trichoderma strains (BThr29, BTas25 and BTer43) obtained in the in vitro experiment were tested in pot culture experimenst (Fig. 18). Data in Table 1 showed the effect of potential strains on germination %, disease incidence, and plant growth promotion when inoculated with pathogens S. rolfsii and R. solani. The effects of Trichoderma treatments were significant when compared with the respective controls. In all the treatments in Table 1, the Trichoderma strains increased seed germination significantly (84.5–93.6%) compared to control treatments (66.3–67.1%). The Trichoderma treatments also reduced disease incidence in the tomato plants (5.2–11.2%) compared to 18.5–21.7% in control treatments. The beneficial effect of Trichoderma treatments also extended to plant growth promotion as measured by shoot length and weight. T. asperellum strain was slightly better than the other two strains for seed germination. On the other hand, T. afroharzianum strain was slightly better in controlling diseases caused by S. rolfsii and R. solani. The two strains of T. asperellum and T. erinaceum performed slightly better than T. afroharzianum strain in growth promotion in the tomato plants.
In vivo assay to assess potentiality of the effective Trichoderma strains in pot culture experiment. Stem length was measured 30 days post inoculation with Trichoderma strains. (A) Efficacy of T. afroharzianum BThr29, T. asperellum BTas25 and T. erinaceum BTer43 against Sclerotium rolfsii challenge inoculated in pots having tomato plants; (B) Efficacy of T. afroharzianum BThr29 and T. asperellum BTas25 against Rhizoctonia solani challenge inoculated in pots having tomato plants.
Discussion
Correct species identification is essential in selecting and validating microbial biocontrol agents6 thus, it is pertinent to assess intraspecific variation to understand the species diversity available in the crop rhizosphere. The phylogenetic species concept has added benefits over traditional morphology-based identification. In the present study, the Trichoderma species isolated from the crop rhizosphere of southern Rajasthan were identified based on morphology and tef-1α sequence analysis to delineate the species and phylogenetic analysis to report the level of Trichoderma biodiversity in agricultural soil. Of the 11 species identified, 4 dominant species T. brevicompactum (18), T. afroharzianum (12), T. erinaceum (11), and T. asperellum (10) showed ubiquitous distribution in studied districts of southern Rajasthan. The higher abundance of these four species may be due to high genetic diversity which shows their potential to adapt to varied environments. Recently, the biodiversity of Trichoderma has been widely studied in different parts of the world. But those studies were designed to discover newer species from non-agricultural soils28,29,30,31. However, there are very few studies that focus on Trichoderma diversity in agricultural soils internationally and in India32 in China33, in Ethiopia18, in Manipur20, in Uttarakhand), and little effort was made to study the actual distribution and association of Trichoderma species in various crop rhizosphere. The biodiversity evaluation is important for agricultural soil to find the most potential isolate of Trichoderma species which can be further developed as a bioformulation to manage various soilborne pathogens. In this study 60 Trichoderma isolates were isolated from seven districts of southern Rajasthan covering 16 crops in the rhizosphere. These seven districts are representative of two agroclimatic zones namely, Zone IVA-Sub-humid southern plains and IVB-Humid southern plains. Agriculturally fertile land of the southern humid zone (Zone IVB) has 72% isolated strains as compared to the remaining 28% from Sub-humid southern plains (Zone IVA). Agro-ecological condition of collection sites in zone IV B has 900 to 1000 mm average rainfall with a thick density of forests, shrubs, and cropping intensity of more than 100%. Zone IV A sub-humid southern plan has 700–900 mm average rainfall with relatively thinner vegetation34. This suggests that the isolation frequency of Trichoderma isolates largely depends on a better ecological environment (minimum–maximum temperature, relative humidity, rainfall) and higher cropping intensity. Researchers28,35,36,37 also support that the Trichoderma diversity is more in areas with higher vegetation and fertile and as compared to the region with poor ecological environment. T. brevicompactum, T. afroharzianum, and T. asperellum isolates were mainly restricted to the irrigated regions of southern Rajasthan including Banswara, Dungarpur Pratapgarh, and Chittorgarh. Previously, it was reported by Maina et al.38 that T. brevicompactum was restricted to the irrigated regions, in addition, our report suggests that not only T. brevicompactum but T. afroharzianum, and T. asperellum also predominance in irrigated regions. Trichoderma brevicompactum was described by39 and later revisited by Degenkolb et al.40 and was shown to be in a new lineage with few other closely related species. They also reported that T. brevicompactum has no close relationship to species used in biological applications. T. ghanense was isolated from the undisturbed forest area of Pratapgarh during the summer season. T. erinaceum is isolated from areas with lesser humidity and dry regions of Pratapgarh, Udaipur, Chittorgarh, Rajsamand, and Sirohi districts. Maina et al.38 also reported the prevalence of T. ghanense, and T. erinaceum in hot areas.
In this study, we observed T. brevicompactum is the most commonly occurring species as compared to many previous reports which suggest T. harzianum is a commonly reported species28,41. T. afroharzianum is the next most commonly occurring species in the present study. We found T. afroharzianum, T. inhamatum, and T. camerunense encompassing Harzianum clade as has been reported by Chaverri et al.6 that T. harzianum is a species complex. Next dominant species including 12 strains reported in our study was T. afroharzianum. This species has worldwide distribution and well-known in biocontrol applications. In fact, the famous commercial isolate T-22 was found to be T. afroharzianum (KP008850)6. T. harzianum was split into more than 14 species with T. harzianum representing only one of those 15 species6. There are two other reference sequences in the T. harzianum clade which have been used in biocontrol but deposited in GenBank under the old name (T. harzianum). The newly assigned T. harzianum has rare distribution in Europe and North America. Another closely related strain in Harzianum clad was T. lentiforme BThr67. In fact, BThr67 had an identical sequence to T. lentiforme from Mexico. T. lentiforme is known as South American species and mostly as endophyte6. To our knowledge this is the first report of the species outside the South American continent. T. erinaceum and T. asperellum were the next dominant species in southern Rajasthan. Samuels et al.7 described T. asperelloides as new species out of T. asperellum. Our study indicated 10:3 ratio among T. asperellum and T. asperelloides within the strains isolated in India indicating the dominance of T. aseprellum over T. asperelloides in the isolation region. Both of these species have been used in biocontrol applications42,43,44. Longibrachiatum section consisting of two strains, T. longibrachiatum BTlg15 and T. ghanense BTgh8 known producers of cellulose hydrolyzing enzymes (particularly T. reesei45,46), as cause of opportunistic infections of man and animals47,48.
The identities of 60 isolates were established by morphological and PCR amplification of the rDNA tef-1α primer sequence. Many researchers demonstrated the effectiveness of tef-1α marker delineating variations to discriminate among different Trichoderma species30,49,50. Trichoderma species included among the 60 identified isolates were Trichoderma afroharzianum, T. asperellum, T. asperelloides, T. inhamatum, T. camerunense, T. erinaceum, T. atroviride, T. ghanense, T. lentiforme, T. longibrachiatum, and T. brevicompactum. T. atroviride is known to be cosmopolitan in nature but more common in northern temperate regions51. Moreover, among strains identified in this study, T. afroharzianum, T. asperellum, T. asprelloides, T. atroviride, T. brevicompactum, and T. erinaceum are well-known as biocontrol species52,53.
However, these species can be distinguished from other species by having species-specific features, viz., different colony growth characteristics, colony color, shape, size, and arrangements of phialides. In our study, these species were grown on different media to study colony morphology. The T. afroharzianum, T. asperellum, T. ghanense, T. erinaceum, T. longibrachiatum, and T. atroviride, T. hamatum formed distinguished concentric rings of conidia on different media. The rings were thicker and denser on PDA and CMA media under alternating light and dark conditions of photoperiod. T. brevicompactum under these photoperiod conditions showed broken rings while T. erinaceum and T. atroviride showed wavy conidial rings. Steyaert et al.54 reported that 15 min burst of blue light could produce clearly defined concentric rings. The authors also suggested that in addition to primary N present in media, the supply of secondary N produced distinguished rings in PDA media. The formation of concentric rings of conidia is the response of alternating light and dark photoperiod, and in single light exposure, a single ring of profuse conidiation will be observed at the colony margin55.
In the present study, it was observed that Trichoderma species communities in various crop rhizosphere varied significantly. The crop rhizosphere of soybean represents the maximum number of 10 strains covering four species including T. erinaceum, T. brevicompactum, T. afroharzianum, T. asperelloides, T. inhamatum, and T. lentiforme. Maize crop rhizosphere represents nine strains covering three species seven strains of Trichoderma, including T. afroharzianum, T. erinaceum, T. asperelloides, and T. brevicompactum. The paddy crop represents only one species T. afroharzianum. The cucumber crop rhizosphere represents four species, namely T. afroharzianum, T. brevicompactum, T. asperellum, and T. longibrachiatum. Crop rhizosphere of Cluster bean represents only T. atroviride, sugarcane represents only T. brevicompactum and okra crop represents only T. asperellum. This variation may be due to the composition of a complex mixture of bioactive root exudates to modify the composition of the rhizospheric microbiome56. Lombardi et al.57 demonstrated that different biotic and abiotic stress elicit root exudates from tomato crops to increase the chemo-attractive effect on T. harzianum. Thus, it is considered that root exudation in different crop rhizosphere is responsible for variation in Trichoderma communities. Our study showed that the rhizosphere of soybean and maize crops harbor more isolates and species than other hosts. That may not only be due to the root exudates of these crops but also due to the existence of phenolic acids in their rhizosphere having synergistic antimicrobial activity by modulating microbial proliferation58.
To study the morphological and cultural variability of isolates, it is pertinent to grow them in different mediums. The growth medium has a significant effect on the growth rate and morphology of fungi. The respective media (i.e., PDA, CMA, TJA) were chosen in this study partly because of their widespread adoption in similar studies by other workers59,60 and because of their inherent variability in composition. It was observed that Trichoderma isolates grew poorly on TJA media but showed luxuriant growth on PDA and CMA. The growth performance of T. harzianum was studied by Jahan et al.59, and the highest linear growth, fresh weight, and dry weight were found in potato dextrose agar followed by carrot agar and corn meal agar (CMA). There are several reports which support our observation that PDA is the most suitable media for the growth of Trichoderma60,61. Our results showed that minimal media TJA did not sustain the luxuriant growth of different Trichoderma species; this was also supported by the work of Harman et al.23 who reported high conidial production of T. harzianum but with an overall low yield.
A confrontation assay of Trichoderma strains against S. rolfsii, R. solani, and F. verticillioides was based on the direct radial growth of the pathogen in the presence of Trichoderma strains, and in the assay, the parameters of pathogen resistance against Trichoderma strains were evaluated. R value (Z/M) of the confrontation assay against all three pathogens was less than 1, which clearly indicates that none of the pathogens resisted but permitted the growth of Trichoderma strains62. That implies that the strains with lower R values are more potent. Nevertheless, the strain T. afroharzianum BThr29 caused maximum inhibition of S. rolfsii (74.8%), R.solani (84.8%), and F. verticillioides (89.5%) with comparatively higher R values. This shows that R-value is not the only parameter to decide the potentiality of Trichoderma strains. The Pakdaman’s biological control indices (PBCIs) of Trichoderma strains evidently indicated that the tested T. afroharzianum BThr 29 has the highest PBCI values of 0.77 ± 0.02, 1.11 ± 0.12, and 1.83 ± 0.58 respectively against S. rolfsii, R. solani, and F. verticillioides, indicating that the BThr29 strain of Trichoderma has more biological control potential than other strains against S. rolfsii. Based on these PBCI values obtained in the study T. afroharzianum BThr29 is the most potential Trichoderma strain. A good amount of Trichoderma biodiversity in the crop rhizosphere of southern Rajasthan was identified in this study. Our study was based on isolates collected during the summer season. Some species sporulate or proliferate in some other seasons. Thus, seasonal variability must be considered to explore other seasons of crop rhizosphere to collect more information in future surveys of Trichoderma diversity in crop rhizosphere.
Potentiality of the effective Trichoderma strains were assessed by their efficacy evaluation in the in vivo pot culture experiments.Several studies have shown that Trichoderma prevented diseases caused by soil-borne pathogens such as Fusarium spp63,64, Pythium spp.65, R. solani66,67, and Sclerotium rolfsii68,69. In our previous study, we evaluated the efficacy of Trichoderma strains BThr29, BTer43 and BTas25 against Fusarium species complex causing post flowering stalk rot in maize. These Trichoderma strains not only reduced disease severity from 17.58 to 62.37% but also improved crop stand by reducing lodging from 42.1 to 71.1%43. In the present study, it was found that T. afroharzianum BThr29 not only effective in managing pathogen growth in in vitro experiments but also was found to reduce the disease incidence by S. rolfsii and R. solani when challenge-inoculated in pot culture experiments. This may be because T. harzianum has high chitinase- and β 1,3 glucanase-producing ability70. Though, T. afroharzianum BThr29 was found to be most effective in controlling diseases incidence, treatments with T. asperellum and T. erinaceum BTer43 were also observed to improve plant growth promotion. There are reports which suggest that Trichoderma, promotes plant growth and development through several mechanisms including stem and root growth, increased nutrient uptake, and expression of plant defense-related genes71. The biopriming of tomato plants with T. erinaceum induces profuse growth in plants by inducing stem and root growth72.
In conclusion, the findings of our study present detailed information on the diversity of Trichoderma species in the crop rhizosphere of southern Rajasthan, India. A total of 60 isolates of Trichoderma were identified up to species level based on tef-1α primer sequence analysis. T. afroharzianum (BThr29) showed high antagonistic potential against three soil-borne pathogens S. rolfsii, R. solani, and F. verticillioides. To the best of our knowledge, this study first time reports the occurrence of three cryptic species of T. harzianum species complex from India. They include T. lentiforme, T. camerunense, and T. inhamatum. The strains T. afroharzianum BThr29 and T. asperellum BTas25 were found to be efficient antagonists, while T. asperellum BTas25 and T. erinaceum BTer43 have good growth promotion potentials.
Materials and methods
Geography of sampling sites
Trichoderma isolates in this study were obtained from soils of cultivated lands and orchards in 7 districts (Banswara, Udaipur, Dungarpur, Pratapagarh, Rajsamand, Sirohi, Chittorgarh, Pali) of southern Rajasthan (Fig. 1). The sampling sites were located in two agro-climatic zones, viz. Zone IVa-Sub Humid Eastern Plains, and Aravalli Hills and Zone IVb-Humid Southern Plain. We collected 273 soil samples during March, April, and May of 2017. The geographical coordinates of the places from where samples were collected are depicted in Supplementary table 1, and the soil samples were collected from diverse crop rhizosphere of cereals, pulses, vegetables, fruit plants, and forest soil as depicted in Fig. 1. Each sample contained about 200 g of soil from a depth of approximately 20 cm. The samples were kept in clean polythene bags, transported to the laboratory, and stored at 4 °C until isolation.
Isolation and storage of Trichoderma strains
Five-fold serial dilutions of soil samples were prepared in sterilized distilled water and 1 ml of diluted sample was poured on the surface of Trichoderma selective Medium (TSM) (MgSO4*7H2O 0.2 g + K2HPO4*3H2O 0.9 g + NH4NO3 1.0 g + KCl 0.15 g + rose Bengal 0.15 g + glucose 3 g + agar 20 g liter dissolved in 1 L of sterile distilled water. Then added 1 ml of a solution containing FeSO4.7H2O 1 g, MnSO4.4H2O 0.65 g, and ZnSO4.7H2O 0.9 g. Autoclaved the TSM at 121 °C, 15psi for 15 min. Before pouring TSM 0.5 ml of Chloramphenicol @ 5 mg ml−1 was added as a bacterial inhibitor. Plates were incubated at 28 ± 2 °C in the dark, and on the second day, characteristic distinct colonies were spotted on the plates. After spotting the desired Trichoderma colonies, they were confirmed under a microscope (20 × and 40 ×) for the presence of phialides. Putative Trichoderma colonies were single spore purified by two rounds of sub-culturing on potato-dextrose agar (PDA) and kept in a refrigerator (4 °C) for further studies.
Morphological characterizations
Growth rate
For morphological analysis, the growth of Trichoderma isolates was observed on grown on three different culture media. The composition of each for 1 L was: (1) Corn Meal Agar (CMA) media (Corn meal infusion 50 g + Dextrose 2 g + Agar 15 g), (2) Potato Dextrose Agar (PDA) media, and (3) Tomato juice agar (TJA) media (Tomato juice 100 ml + L-Asparagine 10 g + Yeast extract 2 g + calcium carbonate 2 g + glucose 2 g agar 20 g). Mycelial plugs (5 mm) from the margin of growing fungal colonies were cut and placed 1 cm away from the periphery of 9 cm agar plates. The culture plates were then incubated in the dark at 28 ± 2 °C. A duplicate plate for each isolate was prepared. The diameter of each colony in mm was measured every 24 h-interval until the agar plates were fully colonized. The cultural phenotypic characteristics were observed using digital camera (Canon DSLR- EOS 80D).
Cultural characteristics
Morphological observations were based on fresh cultures grown on PDA for up to one week in an incubator at 28 ± 2 °C in the dark. Cultural descriptions are based on growth observations on PDA because of superior sporulation and more normal development of conidiophores. Characteristics of conidium-bearing structures and conidia were assessed from cultures grown on PDA. Trichoderma isolates were identified up to the genus level based on colony characters, growth, and structure of mycelium, conidiophores, phialides, and conidia as described by Kubicek and Harman73. The microscopic observations of 2–3 days from young culture were made from slides by mounting mycelia from the edges of actively growing culture on the sterile distilled water drop. Observations of the conidiophores and structure of phialides were made under a Leica DM LB microscope (Leica Microsystems, Wetzer, Germany), and an ES4 Leica microscope camera (Leica Microsystems, Wetzer, Germany) was used for microscopic photographs.
Molecular characterization
DNA extraction, amplification, and sequencing-
Trichoderma mycelia were obtained from cultures on PDA plates grown at 28 ± 2 °C for 4–5 days. An approximately 5 mm2 agar plug with mycelium was cut and transferred to a 1.5 mL Eppendorf tube. The agar plug was ground with motor driven tissue grinder, and 600 µL CTAB (cetyl trimethyl ammonium bromide) buffer [Tris- HCL 100 mM, EDTA (Ethylene diamine tetra acetic acid) 20 mM, NaCl 1.4 M, CTAB 2% (w/v), β-mercaptoethanol 0.2% (v/v), pH 8.0] was added. The homogenate was incubated at 65 °C for 30 min. Subsequently, 600 µL chloroform-isoamyl alcohol (24:1) was added to the solution, mixed, and centrifuged (Eppendorf™ 5727R) for 10 min at 17,709×g. The upper aqueous layer was transferred to a new tube. 600 µL chloroform-isoamyl alcohol (24:1) was added, repeating the previous step. 300 µL isopropanol was added to the aqueous phase obtained from the previous step and incubated at − 20 °C for 30 min. Then, the mixture was centrifuged for 10 min at 12,000 rpm (16,128×g). The precipitate was washed with ice-cold 70% ethanol, dried at room temperature, re-suspended with 150µL double distilled water, and stored at − 20 °C.
For amplification of the tef-1α section, the forward primer tef1 F5′-CAT YGA GAA GTT CGA GAA GG and the reverse primer tef1 R5′-GGA RGT ACC AGT SAT CAT GTT74 were used. The PCR reaction was performed in a thermal cycler (Bio-Rad® Laboratories India Pvt Ltd, Gurugram) using a program with the following parameters: 5 min initial denaturation at 95 °C, followed by 35 cycles of 1 min denaturation at 94 °C, 45-s primer annealing at 54.3 °C, 30-s extension at 72 °C. The program was concluded with a final extension at 72 °C for 5 min. Aliquots of 2 µl of PCR reactions were analysed by electrophoresis in a 1% agarose gel, stained with ethidium bromide for visualization under the Gel Doc EZ system Bio-Rad® (Bio-Rad® Laboratories India Pvt Ltd, Gurugram). PCRs positive for products were purified using a QIAquick® PCR purification kit (Qiagen Inc., Chatsworth, CA, USA). Purified PCR products were sequenced in both directions using the primers used to generate them, on an Agile BioScience (Mumbai) sequencer.
Phylogenetic analysis
The tef-1α sequences were assembled and edited using the software Sequencher from Gene Codes Corporation, Ann Arbor, MI USA (http://www.genecodes.com). The sequences were placed in one FASTA file with reference sequences obtained from GenBank based on homologies with our isolates and aligned all the sequences using the online tool (Guidance server) available at http://guidance.tau.ac.il/. The aligned file was used to generate phylogenetic trees using Maximum Likelihood criterion in MEGA X with the substitution model predetermined with MEGA X as well. Supports for the branches were assessed with 1000 bootstrap replicates. Phylogeny trees were also constructed using PAUP4 version 4a169 available online at http://phylosolutions.com/paup-test/. The tree was obtained using parsimony in heuristic search, with starting tree obtained via random stepwise addition and TBR as branch swapping algorithm plus Multrees in effect. The maximum likelihood tree had essentially identical topology to the parsimony tree and thus we presented the parsimony trees but the bootstrap values from Maximum likelihood tree are depicted on the branches of the presented trees.
Antagonistic activity of Trichoderma strains against Sclerotium rolfsii, Fusarium verticillioides, and Rhizoctonia solani
The antagonistic potentials of Trichoderma strains against S. rolfsii, R. solani, and F. verticillioides were evaluated by following the procedure described by Comporta75. Mycelial discs of seven days old cultures of Trichoderma strains (60 no.) and fungal pathogens (S. rolfsii, R. solani, and F. verticillioides) from PDA grown at 27 ± 2 °C under dark conditions were placed at 1 cm away from the periphery at opposite ends on Petri dishes having PDA medium. Three replications were kept for each fungal-fungal interaction, and the biocontrol potential of each Trichoderma strain was studied against the three pathogens. Trichoderma strains and pathogens discs alone were kept on PDA in Petri plates as control. All these plates were incubated at 27 ± 2 °C in the dark, and the periodic growth of fungi was measured for 10 consecutive days. The inhibition was calculated at six days of incubation using the formula given by Mokhtar and Aid76.
where, %I = Inhibition percentage of pathogen growth by Trichoderma strains; C = Radial growth in control (cm); T = Radial growth of test culture (cm).
Other biocontrol parameters were determined as suggested by Pakdaman et al.62 including (i) days required for two colonies to come in contact (I),(ii) days required for the BCA to fully grow over the pathogen colony (Z); (iii) days required for the BCA to fully grow over the plate (M); (iv) the radial growth distance (in cm) of pathogen colony between the point of inoculation and the marginal point of contact with the BCA growth zone (P); (v) the pathogen resistance index to the BCA was defined as the ratio of Z/M based on the periods required for the full growth of a fungal BCA in the presence (Z) and absence (M) of the pathogen62. Pakdaman biological control index (PBCI)62 combining temporal parameters and pathogen growth parameters was calculated following the formula given below:
In-vivo efficacies of potent Trichoderma strains
The potent Trichoderma strains T. afroharzianum BThr29, T. asperellum BTas25 and T. erinaceum BTer43 obtained in the in-vitro evaluation against S. rolfsii and R. solani were evaluated for their efficacies in the pot culture experiment. The experiment was designed with four replicates of the four treatments with randomised block design. The four treatments include seed treatment and soil drenching of T. afroharzianum BThr29, T. asperellum BTas25, and T. erinaceum BTer43 in challenge-inoculated pots with S. rolfsii and R. solanii. Pathogen inoculums were prepared on maize sand meal (MSM) media77. Fifteen days old MSM media with sufficient inoculums of the two pathogens were uniformly distributed (5 g/Kg of sterilized soil) in each pot. Spore suspensions (1 × 107 cfu/ml) of freshly grown Trichoderma strains were inoculated through root irrigation. Non-inoculated pots were kept as controls. Twenty (20) surface sterilized tomato seeds per pot were treated with slurry of individual Trichoderma strains and were allowed to soak for 30 min. The treated seeds were placed in sterile 90 mm Petri plates and air dried in laminar flow bench overnight at room temperature and then used for sowing in the glass house study. Germination % was measured at 10 days after sowing. Thinning was done to maintain 5 seedlings per pot. Disease incidence was measured on the basis of infected seedlings out of a total number of seedlings. Each seedling was inoculated with 3 mL of individual Trichoderma strain suspension. Trichoderma treated seeds were evaluated for germination %, shoot length (in cm), shoot fresh weight (in g), and incidence of damping off of seedlings. Shoot length and shoot fresh weight were measured 30 days after sowing. The incidence of damping off in seedlings was expressed as a percentage of the total number of plants.
Statistical analysis
The data collected were analysed by ANOVA technique using statistical package for social science (spss version 20.0). Experimental design was completely randomised design (CRD) for in vitro experiments and for pot culture experiment, the randomised block design (RBD) was used. The data of germination percentage, disease incidence were subjected to analysis of variance with arcsine transformation of percentage values and treatment means were separated by Duncan’s Multiple Range Test (DMRT) at a probability level 0.05, where treatment effects were significant. Box plots were created to depict the growth of Trichoderma spp. in growth media at different days after inoculation. The correlation study was performed using Jamovi version 1.2.27 at 5% level of significance.
Data availability
Sequence data of identified Trichoderma strains used in the current study are available in NCBI archives https://ncbi.nlm.nih.gov/. The name of the repository and accession number can be found here; https://www.ncbi.nlm.nih.gov/genbank/, NBCI GenBank accession numbers are. OQ362340, OQ362310, OQ362336, OQ594958 OQ362342, OQ362304, OQ362337, OQ362349, OQ362302, OQ362312, OQ362351, OQ362339, OQ362344, OQ362348, OQ362338, OQ362320, OQ362350, OQ362295, OQ362319, OQ362314, OQ362316, OQ362317, OQ362315, OQ362301, OQ362345, OQ362318, OQ362341, OQ362313, OQ362308, OQ362352, OQ362346, OQ362324, OQ362325, OQ362322, OQ362321, OQ362299, OQ362329, OQ362333, OQ362335, OQ362334, OQ362298, OQ362311, OQ362323, OQ362297, OQ362305, OQ362307, OQ362296, OQ362330, OQ362300, OQ362343, OQ362331, OQ362328, OQ362303, OQ362294, OQ362347, OQ362326, OQ362306, OQ362332, OQ362309, OQ362340.
References
Migheli, Q. et al. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ. Microbiol. 11, 35–46. https://doi.org/10.1111/j.1462-2920.2008.01736.x (2009).
Atanasova, L. Ecophysiology of Trichoderma in genomic perspective. In Biotechnology and Biology of Trichoderma (eds. Gupta et al.). 25–40 (Elsevier, 2014).
Samuels, G. J. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 96, 195–206. https://doi.org/10.1094/PHYTO-96-0195 (2006).
Jaklitsch, W. M. European species of Hypocrea Part I. The green-spored species. Stud. Mycol. 63, 1–91. https://doi.org/10.3114/sim.2009.63.01 (2009).
Jaklitsch, W. M. European species of Hypocrea Part II: Species with hyaline ascospores. Fungal Divers. 48, 1–250. https://doi.org/10.1007/s13225-011-0088-y (2011).
Chaverri, P. et al. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 107, 558–590. https://doi.org/10.3852/14-147 (2015).
Samuels, G. J., Bon, M. C. & Respinis, S. D. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102(4), 944–966. https://doi.org/10.3852/09-243 (2010).
Harman, G. E. Trichoderma—Not just for biocontrol anymore. Phytoparasitica 39, 103–108. https://doi.org/10.1007/s12600-011-0151-y (2011).
Rifai, M. A. A revision of the genus Trichoderma. Mycol. Pap. 116, 1–56 (1969).
Bissett, J. A revision of the genus Trichoderma: I. Section longibrachiatum, new section. Can. J. Bot. 62, 924–931. https://doi.org/10.1139/b84-131 (1984).
Bissett, J. A revision of the genus Trichoderma: II. Infrageneric classification. Can. J. Bot. 69, 2357–2372. https://doi.org/10.1139/b91-297 (1991).
Bissett, J. A revision of the genus Trichoderma. IV. Additional notes on section longibrachiatum. Can. J. Bot. 69, 2418–2420. https://doi.org/10.1139/b91-299 (1991b).
Bissett, J. Trichoderma atroviride. Can. J. Bot. 70, 639–641. https://doi.org/10.1139/b92-082 (1992).
Rehner, S. & Samuels, G. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634. https://doi.org/10.1016/S0953-7562(09)80409-7 (1994).
Kullnig, C. M. et al. Confusion abounds over identities of Trichoderma biocontrol isolates. Mycol. Res. 105(770–772), 2001. https://doi.org/10.1017/S0953756201229967 (2001).
Cai, F. & Druzhinina, I. S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 107, 1–69. https://doi.org/10.1007/s13225-020-00464-4 (2021).
Kumar, K., Amaresen, N., Bhagat, S., Madhuri, K. & Srivastava, R. C. Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J. Microbiol. 52(2), 137–144. https://doi.org/10.1007/s12088-011-0205-3 (2012).
Kamala, T., Indira Devi, S., Sharma, C. & Kennedy, K. Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed. Res. Int. https://doi.org/10.1155/2015/285261 (2015).
Meena, A. K. & Meena, A. K. Characterization and antagonistic effect of isolated Trichoderma sp. against pathogens under Cluster bean (Cyamopsis tetragonoloba L.). Indian J. Agri. Res. 50(3), 249–253. https://doi.org/10.18805/ijare.v0iOF.8439 (2016).
Rai, S. et al. Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. Springerplus 5, 1–16. https://doi.org/10.1186/s40064-016-3657-4 (2016).
Prameeladevi, T., Prabhakaran, N., Kamil, D., Toppo, R. S. & Tyagi, A. Trichoderma pseudokoningii identified based on morphology was re-identified as T. longibrachiatum through molecular characterization. Indian Phytopathol. 71, 579–587. https://doi.org/10.1007/s42360-018-0067-2 (2018).
Johnsen, K. & Nielsen, P. Diversity of Pseudomonas strains isolated with King’s B and Gould’s S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy characterization. FEMS Microb. Lett. 173, 155–162. https://doi.org/10.1016/S0378-1097(99)00065-8 (1999).
Harman, G. E. et al. Production of conidial biomass of Trichoderma harzianum for biological control. Biol. Control 1, 23–28. https://doi.org/10.1016/1049-9644(91)90097-J (1991).
Angraeni, L. et al. Application of various species of Trichoderma spp. in composting cocoa pod husk contaminated Phytophthora palmivora. In IOP Conf. Series: Earth and Environmental Science 515, 012069. https://doi.org/10.1088/1755-1315/515/1/012069 (2020)
Askew, D. J. & Laing, M. D. An adapted selective medium for the quantitative isolation of Trichoderma species. Plant Pathol. 42, 686–690. https://doi.org/10.1111/j.1365-3059.1993.tb01553.x (1993).
Papavizas, G. C. & Lumsden, R. D. Improved medium for isolation of Trichoderma spp. from soil. Plant Dis. 66, 1019–1020. https://doi.org/10.1094/PD-66-1019 (1982).
Lieckfeldt, E., Samuels, G. J., Borner, T. & Gams, W. Trichoderma koningii: Neotypification and Hypocrea teleomorph. Can. J. Bot. 76(9), 1507–1522. https://doi.org/10.1139/b98-090 (1998).
Ma, J. et al. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 10, 362. https://doi.org/10.1007/s13205-020-02301-6 (2020).
Dou, K. et al. Trichoderma biodiversity in major ecological systems of China. J. Microbiol. 57, 668–675. https://doi.org/10.1007/s12275-019-8357-7 (2009).
Saravanakumar, K. et al. Biodiversity of Trichoderma community in the tidal flats and Wetland of South eastern China. PLoS ONE https://doi.org/10.1371/journal.pone.0168020 (2016).
du Plessis, I. L., Druzhinina, I. S., Atanasova, L., Yarden, O. & Jacobs, K. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 110, 559–583. https://doi.org/10.1080/00275514.2018.1463059 (2018).
Jiang, H., Zhang, L., Zhang, J. Z., Ojaghian, M. R. & Hyde, K. D. Antagonistic interaction between Trichoderma asperellum and Phytophthora capsici in vitro. J. Zhejiang Univ. Sci. B 17(4), 271–281. https://doi.org/10.1631/jzus.B1500243 (2016).
Mulatu, A. et al. Biodiversity of the genus Trichoderma in the Rhizosphere of coffee (Coffea arabica) plants in Ethiopia and their potential use in biocontrol of coffee wilt disease. Crops 2(2), 120–141. https://doi.org/10.3390/crops2020010 (2022).
Hussain, M. Agro-climatic zones and economic development of Rajasthan. Int. J. Humanit. Soc. Sci. Invent. 4(2), 50–57 (2015).
Zachow, C. et al. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): Relationship to vegetation zones and environmental factors. ISME J. 3(1), 79–92. https://doi.org/10.1038/ismej.2008.87 (2009).
Widden, P. & Scattolin, V. Competitive interactions and ecological strategies of Trichoderma species colonizing spruce litter. Mycologia 80, 795–803. https://doi.org/10.1080/00275514.1988.12025726 (2018).
Hu, J. D. et al. Large-scale Trichoderma diversity was associated with ecosystem, climate and geographic location. Environ. Microbiol. 22, 1011–1024. https://doi.org/10.1111/1462-2920.14798 (2020).
Maina, P. K., Wachira, P. M., Okoth, S. A. & Kimenju, J. W. Distribution and diversity of indigenous Trichoderma species in Machakos County, Kenya. Microbiol. Res. J. Int. 9(4), 1–15. https://doi.org/10.9734//BMRJ/2015/18034 (2015).
Kraus, G. F. et al. Trichoderma brevicompactum sp.nov.. Mycologia 96(5), 1059–1073. https://doi.org/10.2307/3762089 (2004).
Degenkolb, T. et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Progress 7, 177–219. https://doi.org/10.1007/s11557-008-0563-3 (2008).
Jaklitsch, W. M. & Voglmayr, H. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol. 80, 1–87 (2015).
Zhang, Y. et al. Transcriptomic and metabonomic insights into the biocontrol mechanism of Trichoderma asperellum M45a against watermelon Fusarium wilt. PLoS ONE 17(8), e0272702. https://doi.org/10.1371/journal.pone.0272702 (2022).
Jambhulkar, P. P. et al. Potential native Trichoderma strains against Fusarium verticillioides causing post flowering stalk rot in winter maize. Crop Prot. 152, 105838. https://doi.org/10.1016/j.cropro.2021.105838 (2022).
Ramírez-Cariño, H. F. et al. Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperelloides and Bacillus paralicheniformis in tomato plants. Antonie Van Leeuwenhoek 113, 1247–1261. https://doi.org/10.1007/s10482-020-01433-2 (2020).
Harman, G. E., & Kubicek, C. P. (Eds.). Trichoderma And Gliocladium, Volume 2: Enzymes, Biological Control and commercial applications (1st ed.). CRC Press. https://doi.org/10.1201/9781482267945 (1998).
Kubicek, C. P., Mikus, M., Schuster, A., Schmoll, M. & Seiboth, B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol. Biofuels 2, 19. https://doi.org/10.1186/1754-6834-2-19 (2009).
Kuhls, K., Lieckfeldt, E., Börner, T. & Guého, E. Molecular re-identification of human pathogenic Trichoderma isolates as Trichoderma longibrachiatum and Trichoderma citrinoviride. Med. Mycol. 37, 25–33. https://doi.org/10.1080/02681219980000041 (1999).
Kredics, L. et al. Clinical importance of the genus Trichoderma. A review. Acta Microbiol. Immunol. Hung. 50, 105–117. https://doi.org/10.1556/AMicr.50.2003.2-3.1 (2003).
Oskiera, M., Szczech, M. & Bartoszewski, G. Molecular identification of Trichoderma strains collected to develop plant growth-promoting and biocontrol agents. J. Hortic. Res. 23, 75–86. https://doi.org/10.2478/johr-2015-0010 (2015).
Haddad, P. E., Leite, L. G., Lucon, C. M. M. & Harakava, R. Selection of Trichoderma spp. strains for the control of Sclerotinia sclerotiorum in soybean. Pesqui. Agropecu. Bras. 52, 1140–1148. https://doi.org/10.1590/s0100-204x2017001200002 (2017).
Samuels, G. J. & Hebbar, P. K. Trichoderma: Identification and Agricultural Application (The American Phytopathological Society, 2015).
Kubheka, B. P. & Ziena, L. W. Trichoderma: A biofertilizer and a bio-fungicide for sustainable crop protection. Trichoderma Intechopen 10, 5772. https://doi.org/10.5772/intechopen.102405 (2022).
Phoka, N. et al. Role of Volatiles from the endophytic fungus Trichoderma asperelloides PSU-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 6(4), 341. https://doi.org/10.3390/jof6040341 (2020).
Steyaert, J. M., Weld, R. J. & Stewart, A. Isolate-specific conidiation in Trichoderma in response to different nitrogen sources. Fungal Biol. 114, 179–188. https://doi.org/10.1016/j.funbio.2009.12.002 (2010).
Casas-Flores, S., Rios-Momberg, M., Bibbins, M., Ponce-Noyola, P. & Herrera-Estrella, A. BLR-1 and BLR-2 are key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 150, 3561–3569. https://doi.org/10.1099/mic.0.27346-0 (2004).
Berendsen, R. L., Pieterse, C. M. & Bakker, P. A. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. https://doi.org/10.1016/j.tplants.2012.04.001 (2012).
Lombardi, N. et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant Microbe Interact. 31, 982–994. https://doi.org/10.1094/MPMI-12-17-0310-R (2018).
Zhang, H. et al. Phenolic acids released in maize rhizosphere during maize-soybean intercropping inhibit phytophthora blight of soybean. Front. Plant Sci. 11, 886. https://doi.org/10.3389/fpls.2020.00886 (2020).
Jahan, N., Sultana, S., Adhikary, S. K., Rahman, S. & Yasmin, S. Evaluation of the growth performance of Trichoderma harzianum (Rifai.) on different culture media. IOSR J. Agric. Vet. Sci. 3(4), 44–50 (2013).
Hewedy, O. A. et al. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 9, 189. https://doi.org/10.3390/biology9080189 (2020).
Pastirčáková, K. In vitro interactions between Trichoderma harzianum and pathogenic fungi damaging horse-chestnut (Aesculus hippocastanum) leaves and fruits. Biol. Lett. 54, 21–35. https://doi.org/10.2478/biolet-2019-0003 (2019).
Pakdaman, B. S. et al. Toward the quantification of confrontation (Dual Culture) Test: A case study on the biological control of Pythium aphanidermatum with Trichoderma asperelloides. J. Biofertil. Biopestic. 4, 137. https://doi.org/10.4172/2155-6202.1000137 (2013).
Emara, A. R., Ibrahim, H. M. & Masoud, S. A. The role of storage on mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Arab. J. Chem. 14(10), 103322. https://doi.org/10.1016/j.arabjc.2021.103322 (2021).
Mannai, S., Benfradj, N., Horrigue-Raouani, N. & Boughalleb-M’Hamdi, N. Prevalence of Fusarium species associated with peach decline in Tunisian nurseries. MRJI. 23, 1–162018. https://doi.org/10.9734/MRJI/2018/40746 (2018).
Naseby, D. C., Pascual, J. A. & Lynch, J. M. Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 88(1), 161–169. https://doi.org/10.1046/j.1365-2672.2000.00939.x (2000).
Abbas, A. et al. Trichoderma spp. genes involved in the biocontrol activity against Rhizoctonia solani. Front. Microbiol. 13, 884469. https://doi.org/10.3389/fmicb.2022.884469 (2022).
Yu, Y. et al. Induced systemic resistance for improving plant immunity by beneficial microbes. Annu. Rev. Phytopathol. 11(1–19), 2022. https://doi.org/10.3390/plants11030386 (2022).
Raja, M., Sharma, R. K., Jambhulkar, P. P., Pandian, R. T. P. & Sharma, P. Comparative evaluation of native Trichoderma species from groundnut rhizosphere against stem rot caused by Sclerotium rolfsii Sacc. Indian Phytopathol. 76(459–471), 2023. https://doi.org/10.1007/s42360-023-00610-3 (2023).
Jambhulkar, P. P., Sharma, P., Yadav, R. & Padghan, P. Management of groundnut stem and root rot complex by using Trichoderma harzianum Th3 at field level. Indian J. Plant Prot. 44(3), 337–344 (2016).
El-Katatny, M. et al. Characterization of a chitinase and an endo-β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl. Microbiol. Biotechnol. 56, 137–143. https://doi.org/10.1007/s002530100646 (2001).
Mayo, S. et al. Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum Rhizoctonia solani interaction. Front. Plant Sci. 7, 1109. https://doi.org/10.3389/fpls.2016.01109 (2016).
Amir, M. et al. Trichoderma erinaceum bio-priming modulates the WRKYs defense programming in tomato against the Fusarium oxysporum f. sp. lycopersici (Fol) challenged condition. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.00911 (2019).
Kubicek, C. P. & Harman, G. E. Trichoderma and Gliocladium, Basic Biology, Taxonomy and Genetics Vol. 1 (Taylor & Francis, 1998).
Samuels, G. J., Ismaiel, A., de Souza, J. & Chaverri, P. Trichoderma stromaticum and its overseas relatives. Mycol. Prog. 11, 215–254. https://doi.org/10.1007/s11557-011-0743-4 (2011).
Comporta, P. Antagonisme in vitro de Trichoderma spp. vis-à-vis Rhizoctonia solani Kühn. Agronomie 5, 613–620. https://doi.org/10.1051/agro:19850707 (1985).
Mokhtar, H. & Aid, D. Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. Agric. Biol. J. N. Am. 4(2), 145–154. https://doi.org/10.5251/abjna.2013.4.2.145.154 (2013).
Dutta, P. & Deb, L. An innovative technique for artificial inoculation of Rhizoctonia solani Kuhn for field experiments. Int. J. Curr. Microbiol. App. Sci. 9(12), 1077–1085. https://doi.org/10.20546/ijcmas.2020.912.129 (2020).
Acknowledgements
Authors greatly acknowledge the Zonal Director Research, Agricultural Research Station, Banswara for providing institutional support to carry out this work. The authors also acknowledge the funding agency Rashtriya Krishi Vivas Yojna (RKVY)- Rajasthan to provide funds to carry out this work.
Funding
This research was funded by Rashtriya Krishi Vikas Yojna (RKVY). Rajasthan RA/RKVY-AGRE/2016/595.
Author information
Authors and Affiliations
Contributions
Conceptualization: P.P.J.; Data curation: P.P.J., B.S., M.R.; Formal analysis: P.P.J., A.I., M.T.; Investigation: P.P.J., B.S., M.R.; Methodology: P.P.J., B.S., M.R., D.K.L., A.I.; Project administration and resources: P.P.J.; Software: A.I., M.T.; Supervision: D.K.L., P.S.; Writing original draft: P.P.J., A.I.; Writing review and editing: D.K.L., A.I., P.S.; Funding acquisition: P.P.J.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jambhulkar, P.P., Singh, B., Raja, M. et al. Genetic diversity and antagonistic properties of Trichoderma strains from the crop rhizospheres in southern Rajasthan, India. Sci Rep 14, 8610 (2024). https://doi.org/10.1038/s41598-024-58302-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58302-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.