Abstract
While it is possible to detect cognitive decline before the age of 60, and there is a report indicating that certain cognitive abilities peak in one's 30s, the evidence regarding cognitive problems in populations younger than 65 years is scarce. This study aims to (1) determine the proportion of community-dwelling adults with different cognitive status, and (2) determine the prevalence of neuropsychiatric behaviors. A population-based survey was conducted in Chiang Mai, Thailand. Individuals aged 30 to 65 were recruited and assessed for demographic data, memory complaints, cognitive performance, and neuropsychiatric symptoms using self-reported questionnaires. In a total of 539 participants, 33.95% had mild cognitive impairment (MCI), 7.05% had subjective cognitive decline (SCD), and 52.50% had neuropsychiatric symptoms. The risk of MCI increased with age, and neuropsychiatric symptoms were significantly higher in those with MCI or SCD than in those without (p < 0.001). The most common complaints were sleep problems, anxiety, and irritability. Screening for MCI in adults aged < 65 years might be useful. However, further investigation on the appropriate age to screen and the program’s cost-effectiveness is suggested.
Similar content being viewed by others
Introduction
Dementia, or major neurocognitive disorder, is a progressive neurological disorder with deterioration in cognitive function. It is one of the major causes of disability and dependency among older people globally1. Nearly 60% of people with dementia live in low- and middle-income countries, including Thailand. The progression of the disease has, not only physical and psychosocial impact to the people with dementia, but also on the family and caregivers. Screening for cognitive impairment might help patients and caregivers prepare for managing the symptoms and consequences of future dementia.
Before the patient develop dementia, there are two clinical stages of cognitive impairment that might occur include subjective cognitive decline (SCD) and mild cognitive impairment (MCI). SCD refers to a self-reported experience of worsening in their cognition with the absence of objective cognitive deficits2. Mild cognitive impairment (MCI), or the recent term is minor neurocognitive disorder, is a term describing a stage of having some degree of neurodegenerative disorder involving cognitive function but the daily function is not sufficiently disrupted to meet the criteria of dementia3. MCI has been proposed as an initial or prodromal stage of dementia. These two stages of clinical stage of cognitive decline are gradually gaining in interest as both of them could impact on the self-care behaviors and quality of life of people who are living with these conditions4,5,6,7 and could progress to dementia in later years8,9.
Evidence has shown that people with subjective cognitive decline and mild cognitive impairment are at increased risk of progression to dementia. The incidence rate of individuals with SCD to develop dementia is 8.59 per 1000 person-years when compared with the rate of 5.66 per 1000 person-years among subjects without SCD10. At the stage of MCI, there were reports that about 10–20% of the people with MCI could progress to dementia in a year few years later when compared to 1–2% in general population11,12,13,14.
Apart from cognitive symptoms, individual with cognitive impairment could have neuropsychiatric symptom which have been reported in those with MCI and dementia15. About 35–85% of patients with MCI have at least one neuropsychiatric symptom16. The presence of these symptoms in MCI is also the risk factors for progression to dementia17. These symptoms usually play role as one of the leading causes of caregiver burden18.
Internationally, the prevalence of MCI ranged from 0.5 to 41.8%19. In Asia, a recent meta-analysis and systematic review study has been undertaken to determine the prevalence of MCI among community-dwelling Chinese populations20. The number were ranging from 1.21 to 33.03%. More than half of the studies restricted participants aged over 60 years. A few studies in Thailand reported the prevalence which were slightly higher than the international reports. They were ranging from 16.7 to 43.5% and even higher in the Northern part which was high at around 71.4%21. While cognitive decline can be identified before aged 60 years20, and there is a report suggesting that some cognitive performance peaks in one’s 30s22, the evidence about MCI and SCD in population younger than 65 years is scarce. A report among congenital heart disease survivors found that 5% had MCI23. Knowing prevalence of these spectrum of cognitive changes and prevalence of neuropsychiatric symptoms in community dwelling adults is important to estimate the potential number of people who having increased risk of developing dementia and number of family living with individual with potential cognitive decline.
Therefore, this study aims to determine the proportion of individuals with normal cognition, subjective cognitive complaint, and mild cognitive impairment, and also to determine the prevalence of neuropsychiatric behaviors among community-dwelling adults aged between 30 and 65 years in urban area of Chiang Mai, Thailand.
Methods
Study design
A population-based survey was conducted in Chiang Mai province, the largest province in the Northern region of Thailand.
Sampling and sample size calculations
A stratified two-stage cluster sampling technique was done. The enumeration areas (EAs) were districts in the city center of Chiang Mai Province (the primary sampling units). Thirty-six EAs were randomly selected using probability proportional to the size of the population in each unit. Twenty households (the secondary sampling units) for each cluster were randomly selected using systematic sampling. This allowed 720 households to be included in the study. All family members aged between 30 and 65 years were recruited.
Data collection and measurements
Demographic and sociodemographic data (age, gender, education, underlying diseases, functional status and physiological parameters) were obtained from interviews and physical examination performed by three trained research assistants between September 2020 and December 2020. For functional status, in this household survey we used a brief question to categorize the participants into (1) total dependence (all ADLs requiring assistance), (2) partial dependence (at least 1 ADL requiring assistance but not all), and (3) independence24. The population with independent living and no previous diagnosis of dementia were further recruited for the analysis. In addition to general demographic data, the survey obtained information on self-reported memory complaint from participants and family member. The cognitive performance and neuropsychiatric symptoms were assessed. The questionnaires were included in the Supplementary file 1.
Objective cognitive assessment was performed by using the Thai version of Montreal Cognitive Assessment (MoCA) which has a high sensitivity and specificity for detecting mild cognitive impairment25. The battery consists of many dimensions of cognitive function including memory, attention, language, orientation, executive function, naming, and abstract thinking. In this version, the sum score of 25 and over refers to negative for testing. This MoCA-Thai version was validated in 200926 and has been used in recent studies27,28,29,30, The tool is available online at https://mocacognition.com/paper for any languages, including Thai and English.
SCD was diagnosed by a self-report on experiencing memory decline but cognitive test was negative (MoCA score ≥ 25). MCI diagnostic criteria based on revised criteria in 201131 included the following: (1) concern by participant or family member regarding cognitive change, (2) cognitive impairment in one or more domains, (3) normal activities of daily living, and (4) absence of dementia. As mentioned earlier, in this study, the cognitive performance was assessed by MoCA test. Participant who received MoCA score ≥ 25 and had no report of memory impairment were classified as normal.
Neuropsychiatric symptoms were assessed using a self-reported questionnaire adapted from the Neuropsychiatric Inventory-Questionnaire (NPI-Q) by simply asking that “In the past month, have you been feeling yourself change in any of these kinds of behaviors or moods?” The 12 domains of symptoms included delusion, hallucination, depression, anxiety, euphoria, apathy, agitation or aggression, disinhibition, irritability, repetition, sleep problem, and eating problem. Questions can be answered with “yes” or “no”. The answer “yes” to any symptom reflect that the participant had that neuropsychiatric symptom.
Statistical analysis
The data were analyzed with STATA version 15.1. Descriptive statistics including frequency, percentage, mean and standard deviation (SD), were used to analyze demographic data. Descriptive statistics were used to describe the sampled population in terms of age, gender, education, underlying diseases, and physiological parameters. To infer back to the source population, weighted analysis were conducted to account for a clustering effect using the survey commands. Univariate analysis included Pearson’s chi-square, student t test, and relative risk (95% CI). To assess the association between the presence of at least one symptom of neuropsychiatric symptom (independent variable) and a cognitive problem combining MCI and SCD as a dependent variable, a multivariable logistic regression analysis was performed. This analysis was adjusted for covariates obtained from the univariable analysis, followed by a backward stepwise reduction, with the reduced model presented as the final result. A p value < 0.05 was considered as statistically significant.
Ethical approval and consent to participate
This study came from the study of health service management for patients with hypertension in Thailand. Sub-study in Chiang Mai province was conducted to understand to consequence of hypertension in elderly such as dementia and mild cognitive impairment. The study was approved by the Faculty of Medicine, Chiang Mai University ethic committee (Research ID: 7528, IRB Number 308/2563). Research involving human research participants must have been performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Results
The total of 539 participants were recruited in the analysis of study. Most of them were female (67.90%) and the average age was 51.49 years-old (SD 10.25). Around 64% of the population has highest education at high school or higher and about 56% has at least one underlying disease. Demographic data of the study samples was shown in Table 1.
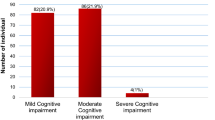
Figure 1 showed the proportion of adults with normal cognition, SCD, and MCI. The prevalence of MCI among adult population in our study who aged between 30 and 65 years old was 33.95% and prevalence of SCD was 7.05%. When stratified by age, the proportion of adults with MCI was increased with age (Table 2). While the prevalence was 9.57% in age group 30–34 years, it was high as 47% in age group 60–65 years. The proportion of SCD were range between 1.5 and 15.5%.
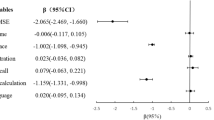
Among 183 participants with MCI and 38 participants with SCD, neuropsychiatric symptoms have been reported as high as over 60% in both groups which were significantly higher than found in population with normal cognition (p < 0.001). As shown in Table 3, sleep problems, anxiety, and irritability were the top three neuropsychiatric symptoms that has been reported in this population. The symptoms that were significantly different in number of self-reports between group included depression (p 0.001), anxiety (p < 0.001), apathy (p 0.001), agitation (p 0.026), irritability (p < 0.001), repetitive behaviors (p 0.003), sleep problem (p < 0.001), and eating change (p 0.015). When including MCI and SCD together, the multivariable logistic regression analysis, adjusted for age and sex, showed a significant association between neuropsychiatric symptoms and cognitive impairment as shown in Table 4.
Discussion
Our study found that MCI was also prevalent in age younger than 65 years and the prevalence was increased with age, while this phenomenon was not found in SCD. About one third of adults with SCD and MCI reported having at least one neuropsychiatric symptoms. Most neuropsychiatric symptoms reported by individual with SCD were anxiety, irritability and sleep problem whereas most individuals with MCI reported of having sleep problem, anxiety, and irritability, respectively.
The prevalence of MCI in adult population aged between 35 and 65 years was around 34%. This was high when compared to prior studies in older adults in community-dwelling population in China20. Even we excluded the population aged younger than 60 years, the prevalence (47%) was still higher than results from most of previous community-based researches which was usually less than 20%32,33,34,35. However, the prevalence is still within the upper range when compared to the review study of studies worldwide36. Our study employed the MoCA as the assessment tool, known for its superior ability to detect MCI compared to the MMSE. This heightened sensitivity of MoCA may have contributed to a broader range of identified cases when compared to the tools used in the most frequently referenced studies. When compare to the results from research in Thai population, the result was still lower than the prior study in Northern Thai community-dwelling older adults21. This could be from the diagnostic test were slightly different and they studied in older population living in rural area which could have lower education and different cognitive related lifestyle when compared to the younger population living in urban area as in our study. Therefore, they potentially have higher prevalence of MCI.
The higher prevalence of MCI could be associated with increasing age which is not surprising. This result was consistent with many studies20,35,37. Increasing age may increase the number of underlying disease which is associated with cognitive impairment38. Moreover, the factors associated with the progression of cognitive impairment through aging process includes systemic inflammation, cerebral ischemia, toxins, or neurodegeneration39. These leads to both molecular and structural brain change and then cognitive decline.
For SCD, our study reported that the prevalence is about 1.5–15.5% while the recent review reports the prevalence of SCD in population with old age is ranged from 10 to 88%40. In younger adults, aged 65 and lower, tend to have lower prevalence compared to the older age. However, in between age 30 to 65, as in our study, we could not observe this trend. Maybe in this age group, there could be some degree of cognitive change due to other causes that could occasionally occur from lifestyle and is not directly related to age change, such as, emotional disturbance or psychiatric disorder41, substance use42, or head trauma43.
The prevalence of neuropsychiatric symptoms were high as over 60% for both MCI and SCD. Our finding was still in line with the previous study that report the prevalence of neuropsychiatric symptoms ranging from 35 to 85% in MCI15. However, we could not compare the prevalence of neuropsychiatric symptoms in SCD as the evidence is still limited. It is important to assess these symptoms because they could be early symptoms/signs of dementia, especially depression, anxiety, apathy, irritability, and sleep disorder40,44,45,46. We also found that anxiety, irritability and sleep change were most reported by adults with SCD and MCI. The finding in our study could be supported by this statement. Moreover, the overall characteristic of self-reported neuropsychiatric symptoms was similar between SCD and MCI which was different from normal cognition. There might be some brain pathologies occur in these two prodromal groups but not severe enough to cause dementia. Early detection of these symptoms and provide appropriate treatment would be beneficial for patients and also allow physician to detect early dementia.
The strength of our study was that we could include a large number of participants in the study in an urban northern Thailand community-based setting. To our knowledge, this is the first study that assess the prevalence of MCI in a community-dwelling non-elderly population in Thailand. Nonetheless, the study is not without its limitations. This study used cognitive assessment tool, MoCA, which could be interfered by education level of the subject which might lead to the overestimation of the prevalence. However, MoCA is the prominent tool to detect MCI in many studies worldwide20 and the scoring system add up the score for those who have lower education to adjust this confounding effect. The diagnosis of MCI was not based on the most recent criteria in DSM-V, which requires physicians to exclude some symptoms that mimic MCI. However, this criterion has been widely used and cited in many research articles. In our study, neuropsychiatric symptoms were measured based on participants’ self-perception. We did not use the standard screening tool which consist of more symptoms and mostly use to interview informants, not the participants themselves which could affect the result.
In conclusion, this study reported the slightly high prevalence of SCD and MCI among middle aged adults. Even though there is no current effective treatment for MCI population, it is suggested that health care provider can start providing intervention to people with MCI believing that it could delay the cognitive decline and improve symptoms, which include neuropsychiatric symptoms47. Therefore, it might be useful to detect MCI in population age lower than 65 years for early detection of these cases. However, further study to find the appropriate age to start screening is still needed. The cost-effectiveness study might be useful to help design the method of screening at the community level.
Data availability
Data sets are available from the corresponding author on request due to ethical commitment.
References
World, Health & Organization. Global action plan on the public health response to dementia 2017 - 2025, <https://www.who.int/news-room/fact-sheets/detail/dementia> (2020).
Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852. https://doi.org/10.1016/j.jalz.2014.01.001 (2014).
Smith, G. E. & Bondi, M. W. Mild Cognitive Impairment and Dementia: Definitions, Diagnosis, and Treatment (Oxford University Press, 2013).
Goda, A. et al. The relationship between subjective cognitive decline and health literacy in healthy community-dwelling older adults. Healthcare (Basel) 8, 567. https://doi.org/10.3390/healthcare8040567 (2020).
Uchmanowicz, I., Jankowska-Polańska, B., Mazur, G. & Sivarajan Froelicher, E. Cognitive deficits and self-care behaviors in elderly adults with heart failure. Clin. Interv. Aging. 12, 1565–1572. https://doi.org/10.2147/cia.S140309 (2017).
Świątoniowska-Lonc, N., Polański, J., Tański, W. & Jankowska-Polańska, B. Impact of cognitive impairment on adherence to treatment and self-care in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 14, 193–203. https://doi.org/10.2147/dmso.S284468 (2021).
Wei, Y. C. et al. Subjective cognitive decline in the community is affected at multiple aspects of mental health and life quality: A cross-sectional study of the community medicine of Keelung Chang Gung memorial hospital. Dement. Geriatr. Cogn. Disord. Extra 9, 152–162. https://doi.org/10.1159/000497222 (2019).
Mitchell, A. J., Beaumont, H., Ferguson, D., Yadegarfar, M. & Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 130, 439–451. https://doi.org/10.1111/acps.12336 (2014).
Campbell, N. L., Unverzagt, F., LaMantia, M. A., Khan, B. A. & Boustani, M. A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 29, 873–893. https://doi.org/10.1016/j.cger.2013.07.009 (2013).
Lee, Y. C. et al. Subjective cognitive decline and subsequent dementia: A nationwide cohort study of 579,710 people aged 66 years in South Korea. Alzheimer’s Res. Ther. 12, 52. https://doi.org/10.1186/s13195-020-00618-1 (2020).
Loewenstein, D. A. et al. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3-year follow-up period. Dement. Geriatr. Cogn. Disord. 27, 418–423. https://doi.org/10.1159/000211803 (2009).
Roberts, R. & Knopman, D. S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 29, 753–772. https://doi.org/10.1016/j.cger.2013.07.003 (2013).
Koepsell, T. D. & Monsell, S. E. Reversion from mild cognitive impairment to normal or near-normal cognition: Risk factors and prognosis. Neurology 79, 1591–1598. https://doi.org/10.1212/WNL.0b013e31826e26b7 (2012).
Behrman, S., Valkanova, V. & Allan, C. L. Diagnosing and managing mild cognitive impairment. Practitioner 261, 17–20 (2017).
Martin, E. & Velayudhan, L. Neuropsychiatric symptoms in mild cognitive impairment: A literature review. Demen. Geriatr. Cogn. Disord. 49, 146–155. https://doi.org/10.1159/000507078 (2020).
Gallagher, D., Fischer, C. E. & Iaboni, A. Neuropsychiatric symptoms in mild cognitive impairment. Can. J. Psychiatry 62, 161–169. https://doi.org/10.1177/0706743716648296 (2017).
Palmer, K. et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology 68, 1596–1602. https://doi.org/10.1212/01.wnl.0000260968.92345.3f (2007).
Pinyopornpanish, M. et al. Perceived stress and depressive symptoms not neuropsychiatric symptoms predict caregiver burden in Alzheimer’s disease: A cross-sectional study. BMC Geriatr. 21, 180. https://doi.org/10.1186/s12877-021-02136-7 (2021).
Pessoa, R. M. P., Bomfim, A. J. L. & Chagas, M. H. N. Diagnostic criteria and prevalence of mild cognitive impairment in older adults living in the community: A systematic review and meta-analysis. Arch. Clin. Psychiatry 46, 72–79 (2019).
Lu, Y. et al. Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: A meta-analysis and systematic review. BMC Geriatr. 21, 10. https://doi.org/10.1186/s12877-020-01948-3 (2021).
Griffiths, J., Thaikruea, L., Wongpakaran, N. & Munkhetvit, P. Prevalence of mild cognitive impairment in rural Thai older people, associated risk factors and their cognitive characteristics. Demen. Geriatr. Cogn. Disord. Extra 10, 38–45. https://doi.org/10.1159/000506279 (2020).
Hartshorne, J. K. & Germine, L. T. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol. Sci. 26, 433–443. https://doi.org/10.1177/0956797614567339 (2015).
Rodriguez, C. P., Clay, E., Jakkam, R., Gauvreau, K. & Gurvitz, M. Cognitive impairment in adult CHD survivors: A pilot study. Int. J. Cardiol. Congenit. Heart Dis. 6, 100290. https://doi.org/10.1016/j.ijcchd.2021.100290 (2021).
Scarborough, J. E., Bennett, K. M., Englum, B. R., Pappas, T. N. & Lagoo-Deenadayalan, S. A. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann. Surg. 261, 432–437. https://doi.org/10.1097/sla.0000000000000767 (2015).
Nasreddine, Z. S. et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x (2005).
Tangwongchai, S. et al. The validity of Thai version of the montreal cognitive assessment (MoCA-T). Dement. Neuropsychol. 3, 172 (2009).
Hemrungrojn, S. et al. Use of the montreal cognitive assessment Thai version to discriminate amnestic mild cognitive impairment from Alzheimer’s disease and healthy controls: Machine learning results. Dement. Geriatr. Cogn. Disord. 50, 183–194. https://doi.org/10.1159/000517822 (2021).
Vichitvejpaisal, P. et al. The montreal cognitive assessment as a screening tool for preoperative cognitive impairment in geriatric patients. J. Med. Assoc. Thai 98, 782–789 (2015).
Buawangpong, N. et al. Risk prediction performance of the Thai cardiovascular risk score for mild cognitive impairment in adults with metabolic risk factors in Thailand. Healthcare (Basel) 10, 2022. https://doi.org/10.3390/healthcare10101959 (1959).
Pinyopornpanish, K. et al. Circulating Lipocalin-2 level is positively associated with cognitive impairment in patients with metabolic syndrome. Sci. Rep. 12, 4635. https://doi.org/10.1038/s41598-022-08286-x (2022).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Demen. J. Alzheimer’s Assoc. 7, 270–279. https://doi.org/10.1016/j.jalz.2011.03.008 (2011).
Hänninen, T., Hallikainen, M., Tuomainen, S., Vanhanen, M. & Soininen, H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol. Scand. 106, 148–154. https://doi.org/10.1034/j.1600-0404.2002.01225.x (2002).
Tognoni, G. et al. From mild cognitive impairment to dementia: A prevalence study in a district of Tuscany, Italy. Acta Neurol. Scand. 112, 65–71. https://doi.org/10.1111/j.1600-0404.2005.00444.x (2005).
Juarez-Cedillo, T. et al. Prevalence of mild cognitive impairment and its subtypes in the Mexican population. Dement. Geriatr. Cogn. Disord. 34, 271–281. https://doi.org/10.1159/000345251 (2012).
Pais, R., Ruano, L., Moreira, C., Carvalho, O. P. & Barros, H. Prevalence and incidence of cognitive impairment in an elder Portuguese population (65–85 years old). BMC Geriatr. 20, 470. https://doi.org/10.1186/s12877-020-01863-7 (2020).
Bai, W. et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: A meta-analysis and systematic review of epidemiology studies. Age Ageing 51, afac173. https://doi.org/10.1093/ageing/afac173 (2022).
Xu, Z. et al. Incidence of and risk factors for mild cognitive impairment in chinese older adults with multimorbidity in Hong Kong. Sci. Rep. 10, 4137. https://doi.org/10.1038/s41598-020-60901-x (2020).
Jacob, L., Haro, J. M. & Koyanagi, A. Physical multimorbidity and subjective cognitive complaints among adults in the United Kingdom: A cross-sectional community-based study. Sci. Rep. 9, 12417. https://doi.org/10.1038/s41598-019-48894-8 (2019).
Murman, D. L. The impact of age on cognition. Semin. Hear 36, 111–121. https://doi.org/10.1055/s-0035-1555115 (2015).
Si, T., Xing, G. & Han, Y. Subjective cognitive decline and related cognitive deficits. Front. Neurol. 11, 493035. https://doi.org/10.3389/fneur.2020.00247 (2020).
Studart, A. N. & Nitrini, R. Subjective cognitive decline: The first clinical manifestation of Alzheimer’s disease?. Dement. Neuropsychol. 10, 170–177. https://doi.org/10.1590/s1980-5764-2016dn1003002 (2016).
Ramey, T. & Regier, P. S. Cognitive impairment in substance use disorders. CNS Spectr. 24, 102–113. https://doi.org/10.1017/S1092852918001426 (2019).
Stulemeijer, M., Vos, P. E., Bleijenberg, G. & van der Werf, S. P. Cognitive complaints after mild traumatic brain injury: Things are not always what they seem. J. Psychosom. Res. 63, 637–645. https://doi.org/10.1016/j.jpsychores.2007.06.023 (2007).
Roberto, N. et al. Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci. Rep. 11, 6448. https://doi.org/10.1038/s41598-021-83126-y (2021).
Banks, S. J. et al. The Alzheimer’s disease cooperative study prevention instrument project: Longitudinal outcome of behavioral measures as predictors of cognitive decline. Dement. Geriatr. Cogn. Dis. Extra 4, 509–516. https://doi.org/10.1159/000357775 (2014).
Chen, Y., Dang, M. & Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and–specific lesion patterns. Mol. Neurodegener. 16, 38. https://doi.org/10.1186/s13024-021-00456-1 (2021).
Kasper, S. et al. Management of mild cognitive impairment (MCI): The need for national and international guidelines. World J. Biol. Psychiatry 21, 579–594. https://doi.org/10.1080/15622975.2019.1696473 (2020).
Funding
The study was partially supported by Chiang Mai University.
Author information
Authors and Affiliations
Contributions
K.P., K.T., C.P., W.J., and C.A. were involved in the conception of the manuscript and the design. K.P., N.B., N.N., K.T., and W.J. collected the data. K.P., A.S., C.P., N.W., and C.A. analyzed and interpreted the data. K.P., N.B., N.W., and C.A. drafted the manuscript. All authors revised, read and then approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinyopornpanish, K., Buawangpong, N., Soontornpun, A. et al. A household survey of the prevalence of subjective cognitive decline and mild cognitive impairment among urban community-dwelling adults aged 30 to 65. Sci Rep 14, 7783 (2024). https://doi.org/10.1038/s41598-024-58150-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58150-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.