Abstract
Three borate glasses of 50, 35, and 15 mol% PbO-doped Ce, Sb, or Mn ions were fabricated via the melting-annealing procedure. Their structural features were inspected before and after 250 kGy of gamma irradiation using FTIR and ESR techniques. The spectra of the ESR and FTIR vibrational bands remain constant, with a minor reduction in N4 and an enhancement in density values after irradiation, indicating the large structural stability and glass compactness. Many radiation shielding parameters were studied, such as gamma dose rate (µSv/h), dose transmission %, lifetime cancer risk %, macroscopic effective removal cross-section (∑R), mass stopping power, and projected range values were considered for protons particles by SRIM Monte Carlo simulation code and ESTAR program. The whole data reveals the high radiation shielding efficiency of the glasses compared to other standard shields to be used as glass immobilizers for radioactive wastes or storage containers, e.g., for nuclear medicine units in hospitals.
Similar content being viewed by others
Introduction
Although nuclear power has great importance in many industrial, medical, and environmental fields, its advanced generation and the convoyed nuclear fuel cycle yield a variety of radioactive wastes that have become increasingly hazardous. Due to the radioactive contamination and harmful effects of exterior exposure to radiation, handling radioactive wastes is a very hazardous procedure1. So, it is mandatory to follow some strict radiation protection rules, such as treating and conditioning nuclear waste containers in a way that keeps dose rates below certain limits. For example, in shielding nuclear medical facilities, this process can be done by covering radioactive materials in containers to minimize radiation contact with medical employees during radiation therapy. Transporting and storing radioactive materials also require safe separation, which includes the preventing of radiation waste leakages2.
Several safety and regulatory requirements must be met when disposing radioactive wastes, but it is a very expensive and difficult procedure because many activities must be performed in order to reduce the cost as much as possible1.
One of the most proficient and extremely desirable methods to deal with the problem of radioactive wastes is the fabrication of lightweight materials with radiation shielding efficiency, e.g., glass1,2,3. In recent years, glass has emerged as one of the most hopeful light materials to immobilize high-level radioactive wastes (HLWs) due to its complex structure. It has a stretchy structure for incorporating many types of waste elements by extensively atomic-bonding among the glassy network and radioactive wastes4,5,6. Borosilicate glasses, e.g., Pamela, SON68, and WAK7, are the most common glasses used as nuclear waste immobilizers.
The bezel proficiency of nuclear waste glass can be detected by its affinity to struggle with the released photons of ionizing radiation and stop their paths6. Subsequently, the chosen glass should have many properties, such as high structural stability against high radiation doses, mechanical steadfastness, thermal stability, and chemical durability in various leaching media6,8. The capability of a precise glass to inhibit radiation photons is controlled predominantly by its main host composition. The amorphous nature of the glassy structure assists in the housing of various metal ions, which afford the glass radiation shielding proficiency. So, the selection of each metal oxide and its concentration in the glassy network should be sensibly considered. Particularly, heavy metal ions afford the exceptional radiation shielding characteristics of their host glasses owing to their astounding mass attenuation coefficients related to their large atomic weights9. For instance, lead and bismuth oxides are the most common oxides used for developing radiation shielding properties in glass due to their heavy masses, small field strength, and high polarizability to capture radiation photons, e.g. Pb is the central component of radiation protection concrete. B2O3 glasses are also distinguishable in their properties due to their low cost, the simple way of preparation and shaping, and the tendency of boron to absorb radiation photons. The trigonal BO3 and tetrahedral BO4 are the central structural units in borate glasses. The accommodation of modifying oxides in the glassy network tends to transform BO3 to BO4 units by forming more non-bridging oxygens (NBO)10. While the incorporation of intermediate oxides like Pb2+ ions propose significant variations in the glass network correlated to its native environment and coordination’s by forming the expected resilient building units such as PbO3, PbO4, and PbO611, Lead-borate glasses can be broadly used for radiation protection purposes for observers and workers in healthcare, industrial environments, veterinary medicine, and dentistry9,12,13. Furthermore, rare earth ions and transition metal ions enhance the radiation protection proficiency of their host glasses14,15,16 because of their ability to change their coordination to deal with radiation photons and heal the expected defect centers17,18,19,20.
For instance, Ce ions have highly required features correlated to their nature as rare earth ions having an outer surface electronic configuration of 5s2, 5p6, and 4f. orbitals9. Cerium ions can present as Ce3+ ↔ Ce4+ states in an equilibrium reaction to balance the negative charges on the neighboring tetrahedrons, helping in the formation of more connected bonds and relaxed or rigid glassy network. On the other side, Sb3+ has a massive weight and can form stable SbO3 trigonal pyramids in borate network, giving a compacted glassy network. Moreover, Mn2+ ions can exist in many oxidation states and participate as octahedral and/or tetrahedral units in the glassy network. Such induced ions are able to alter their valence states to absorb defect centers that may be caused by the effect of irradiation and inhibit the passage of radiation photons9,21.
Numerous examinations should be conducted on each selected glass composition to evaluate its tendency to inhibit the activity of ionizing radiation photons to its minimum level, such as structural stability, formation of free radicals because of breaking bonds, density, chemical durability, and many other experimental and theoretical radiation shielding parameters, so that the glass's capability to contain different radioactive wastes can be evaluated.
Further work was published indicating the promising radiation shielding application of the investigated glasses21. The objective of the present work is to continue the work on the prepared lead borate glasses and make a comparison test on the effect of 250 kGy of gamma radiation on some of their structural and physical properties using FTIR and ESR techniques to estimate their possible usage as storage containers for radioactive wastes. Additionally, Phy-X/PSD software was used to determine the macroscopic effective range cross-section of the fast neutron, ∑R, and the simulation code MCNP5 has also been inspected, e.g., gamma dose rate (µSv/h) from exposure to a radioactive source, dose transmission %, lifetime cancer risk % compared to other standard shields, and macroscopic effective removal cross-section (∑R). In addition, the projected range (ΠP) and mass stopping power (ψp) of protons (H ions) were estimated using the SRIM Monte Carlo simulation system and its subroutine TRIM. The continuous slowing down approximation (CSDA) range and the electron mass stopping power (ψe) were both calculated using the ESTAR program. Then the best glass system for radiation shielding ability can be determined as promising storage containers for radioactive wastes.
Materials and methods
Preparation of glass systems
Three systems of different lead-borate glass compositions were organized by the communal melting-annealing method. The compositions of the fabricated glasses are scheduled in Table 1, and chemicals from Sigma-Aldrich Company (purity 99.9%) were used to prepare the glasses: H3BO3, PbO, K2CO3, Na2CO3, Al2O3, CeO2, Sb2O3, and MnO2. According to each glass composition listed in Table 1, three batches were exactly weighed using a sensitive balance (four digits ± 0.0001). The batches were differentiated carefully and ground into sufficient powders to be melted in porcelain crucibles in an electric muffle furnace with a temperature range of 950–1100 °C for 90–130 min. To achieve homogeneity, the crucibles were carefully stirred at 30-min intervals. After the complete melting, the homogenized melts were cast on heated stainless-steel molds and immediately transmitted to an annealing furnace at a temperature range of 350–400 °C to exclude stress or thermal strain excesses in the prepared samples. Then the annealing furnace was turned off after 1 h, leaving samples inside until the gradual cooling to room temperature with a rate of 30 °C.h−1. After that, bulk glass samples with a thickness = 2.98 ± 0.03 mm, or powered samples, were intended for characterization processes.
Characterization techniques
The FTIR absorption spectra of the prepared glasses were inspected before and after exposing them to 250 kGy of gamma radiation in a wavenumber range of 400–4000 cm−1 using the VERTEX 70, FT/ IR-430 spectrometer type at room temperature. N4 values were obtained from FTIR spectra according to the following relation: N4 (FTIR) = BO4/(BO3 + BO4) depending on the area under peaks that are associated with the corresponding sections of BO3 and BO4 units. Deconvolution of FTIR spectra was carried out using the Peak Fit program in Gaussian Amp. Mode.
The Bruker EMX spectrometer (X-band, Germany) was used to perform ESR measurements with the following operating parameters: microwave power of 2 mW, modulation amplitude of 3 G, sweep width of 200 G, microwave frequency of 9.72 GHz, time constant of 81.92 ms, and sweep time of 20.48 s at room temperature of 25 ± 2 °C. The ESR measurement was completed before and after exposing glasses to 250 kGy of gamma radiation.
Density (ρ) of the glasses was calculated at room temperature using the suspended weight method based on Archimedes principle13,18 according to the following relation:
where, (ρ) is density of the glass sample, (a) and (b) are weights of the glass specimens in air and xylene, respectively and (0.86) is xylene density at 20 °C.
Irradiation was performed with a 60Co gamma cell (2000 Ci) with a dose rate = 0. 717 kGy/h at 25 ± 5 °C. The samples were placed in the gamma cell in such a way that each sample was exposed to the required identical dose.
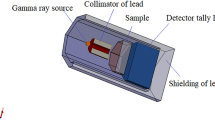
The MCNP5 simulation model was used to calculate the attenuation coefficient of gamma rays and radiation exposure doses behind each glass sample, as described in Fig. 1. The gamma radiation source was placed inside the glass container in the presence of a lead collimator between the glass container or source and detector in order to estimate the worker’s exposure doses. For capturing MCNP5 simulation data, Tally F4, F4 m, F5, and F5 m were utilized. Simulations were performed with 10,000,000 histories, and all results simulated by the MCNP-5 code were reported with less than 0.1% error. Additionally, mass stopping power and projected range values were estimated for protons using the SRIM Monte Carlo simulation code and the ESTAR program to calculate mass stopping power and the continuous slowing down approximation range for electrons. Also, many shielding parameters have been examined by Monte Carlo simulation code MCNP5 and Phy-X/PSD software to estimate radiation exposure doses at various distances, dose transmission%, and the excess lifetime cancer risk% for radiation workers behind the glasses.
Phy-X/PSD is online photon shielding and dosimetry (PSD) software available at https://phy-x.net/PSD that has been developed for the calculation of parameters relevant to shielding and dosimetry. Moreover, another parameter relevant to shielding, i.e., the fast neutron removal cross section (∑R), can be calculated for a compound or a mixture using this software22.
A Monte Carlo simulation computer model named Stopping and Range of Ions in Materials, or SRIM (formerly TRIM), is frequently used to calculate electronic and nuclear stopping powers and simulate the inelastic and elastic energy transfers from an energetic incident ion to target atoms. The SIRM software utilizes the Bethe-Bloch formula, which describes the energy loss of charged particles as they traverse through matter. This formula takes into account various factors, such as the density and composition of the material, as well as the energy and charge of the incident particles. By inputting these parameters, SIRM can provide accurate predictions of the material stopping power for protons with specific energies23.
The ESTAR program calculates stopping power, density effect parameters, radiation yield tables, and the CSDA range for electrons. The CSDA range is a very close approximation to the average path length traveled by a charged particle as it slows down to rest24.
The loss in proton or electron KE is generally characterized by the concept of stopping power, which is defined as the rate of energy loss by a charged particle per unit path length in an absorbing medium. The stopping power usually has units of MeV cm2/g when called the mass stopping power23 by selecting a material and entering the desired energies, or using the default energies. Energies are specified in MeV and must be in the range of 0.001 MeV to 10,000 MeV25.
Results and discussion
FTIR absorption spectra
FTIR spectroscopy is the most dependable technique for analyzing the structure of both amorphous and crystalline materials by identifying the several structural groups in the material's structure and explaining the location of various metal oxides and their interactions with adjacent ions through the glassy structure. So it can be used to detect the structural changes that could take place due to different effects, e.g., composition change, irradiation process, etc14. As known, the main structural building units of the borate network are the trigonal BO3 units, which are allied to form six-membered boroxol rings, and the tetrahedral BO4 groups. Hosting modifying ions in the glassy network, e.g., alkali ions, transition metal ions, and rare earth ions, causes the fracture of B-O-B bonds, producing more non-bridging oxygen (NBO), followed by the alteration of trigonal planar sp2 BO3 units into tetrahedral sp3 BO4 groups with penta, tetra, tri, and di borate groups. The type and concentration of the introduced modifier ions and their behaviors with surrounding metal ions control this process of forming NBOs. Lead borate glass has a complicated structure different from the traditional alkali borate glasses. Contrasting the traditional alkali oxides, the heavy metal lead oxide (PbO) works in a dual behavior in the glass network as both modifier and former11.
According to some authors11, the ratio (R) of PbO in the glass network controls its influence. They expected that PbO participates in the network initially as a modifier at 0 ≤ R ≤ 0.33 mol%, where each Pb2+ ion acts at double charge balance to deliver the positive charges required for forming two tetrahedral borate (BO4)− units. At R = 0.33, the role of cations starts to modify to provide PbO as a former oxide. Pb2+ ions can participate through strong covalent bonds to form compacted structural groups, e.g., PbO4 and/or PbO3 pyramidal units. Pb2+ cations and their connected oxygens in the pyramidal units work to diminish the ionic charge balance availability, lessening the rate of forming the four coordinated borons11.
Figure 2 depicts the FTIR absorption spectra of the three considered lead borate glasses before and after gamma irradiation with 250 kGy. Comparing the spectra of the three prepared glasses, there are many common structural units, as follows:
-
1.
A distinctive band in the far IR region (445–460–460 cm−1) at 445, 457, and 448 cm-1 for G1, G2, and G3, respectively, is ascribed to the vibration of building units of the glass network formed by heavy metal ions Pb2+9,26.
-
2.
A slight broad band in the region from 570 to 610 at 587, 608, and 577 cm−1 correlated directly to the vibrational motions of Ce, Sb, and Mn cations in their network positions, respectively14,15,27.
-
3.
A band centered at the medium area (~ 680–708 cm−1) at 687, 708, and 694 cm−1 is correlated to the bending vibrations of triangular B–O–B borate connections28.
-
4.
A clear, sharp band at 940, 960, and 950 cm−1 for G1, G2, and G3, respectively, is correlated to the asymmetric stretching vibrations of B-O bonds in tetrahedral BO4 units14.
-
5.
Quit broad bands at ~ 1200–1400 cm−1 assigned to stretching vibration of the trigonal BO3 units, such as the high peak at 1311 cm−1 for G1 and two high characteristic peaks at 1250–1380 cm−1 for G2 and 1370–1380 cm−1 for G3, attributed to the vibration of di-borate rings between B3O6-BO3 linkages and stretching vibration of tetraborate and/or penta-borate rings14,29.
-
6.
Bands in the region 1600–4000 cm−1 e.g. broad bands around 2000 and 2300 cm−1 correlated to vibrations of H-bonding, and bands at 2890 and 2990 cm-1 correlated to the vibrations of molecular H2O, OH and B–OH units14.
Figure 2 also displays the FTIR absorption spectra of the three investigated glasses after being exposed to 250 kGy of gamma rays. The spectra reveal almost no main changes in the situation or intensity of the detected vibrational bands, indicating the large stability of lead-borate structures and the strong compacted networks. The closed and joined structures are supported by the highly polarizable heavy lead ions (Pb2+) that can block the path of gamma photons through the network and the effective role of the induced different metal ions. So outstanding stability and radiation shielding behavior against ionizing gamma radiation are observed.
Comparative analysis of deconvoluted FTIR spectra
More details regarding the effect of the introduced metal ions and the influence of irradiation on the fabricated glasses can be interpreted simultaneously according to each glass composition in a comparative analysis using the deconvoluted spectra of the Gaussian model as shown in Figs. 3 and 4. The spectra of the fresh glasses before irradiation are displayed in Fig. 3. G1 (Ce glass) shows seven deconvoluted peaks at 440, 690, 865, 940, 1050, 1248, and 1353 cm−1. G2 (Sb glass) shows nine deconvoluted peaks at ~ 438, 580, 610, 702, 902, 966, 1054, 1250, and 1375 cm−1, while G3 (Mn glass) shows nine peaks at ~ 430, 577, 700, 868, 947, 1066, 1237, 1366, and 1480 cm−1. Assignments of the observed bands can be understood according to the following arguments:
-
(a)
Bands at 430, 435, 438, 440 and 473 cm−1 can be recognized as the vibration of intermediated heavy metal Pb2+ ions as essential building units in their interstitial sites as PbO4/PbO3 units9,18,27. In addition to the vibrational motions of alkali modifier cations in their residing sites (bridging or non-bridging), e.g., Na+ or Al3+ ions26, the vibration of double degenerate bending vibrations of the SbO3 structural unit in G220 and/or vibrational motions of the transition metal Mn2+ ions in their octahedral sites in G326.
-
(b)
Bands at 560 and 577, ~ 580–585 and 610, 650, 690 cm−1 ascribed to bending vibration of tetrahedral BO4 groups associated with modifier ions, e.g. Pb2+, Sb3+, K+, Na+, Al3+, and/or Mn2+/ Mn3+. Furthermore, the overlaid stretching vibration of Ce–O bonds in Ce3+/Ce4+ and ions in the four-fold coordination19 or asymmetric bending vibrations in the trigonal pyramids of SbO328.
-
(c)
Bands at ~ 689, 700, 702–704 cm−1 correlated to bridging oxygen vibration in BO3 units and bending vibration of B-O-B linkages, where oxygen is allied between BO3 and BO4 units26 e.g. bending vibrations of B-O-B linkages and B-O-Sb linkages in G228.
-
(d)
Bands at 865, 868, 870 and 873 cm−1 attributed to the triangle and tetragonal vibrations of Pb2+ ions and vibration of Pb–O-B linkages9. Furthermore, vibration of boroxol rings in G1, where the cerium ion can be associated with hydroxyl OH− groups and overlapped with B–O–B links of the main tetrahedral BO4 borate structure, produces a noticeable bending vibration band at ~ 800 cm−114,29.
-
(e)
Main bands at 931, 938, 940, 947, 966- 977, 1066, 1048, 1237, 1233, 1366, and 1350 cm-1 correlated to the asymmetric stretching vibration of the B–O bonds in BO4 units and antisymmetric stretching vibrations of B–O–B vibrations for various borate units; tri, penta, and di-borate groups or B–O–B linkages30 e.g. triangle and tetragonal vibrations of Pb–O–B or O–Sb links9.
-
(f)
Bands at 1050 or 1054 cm−1 related to the antisymmetric stretching vibrations of B–O–B bond stretching vibrations for numerous borate units; tri, penta and di-borate groups, e.g., B–O–B linkages30 or B–O–Sb linkages20.
-
(g)
Bands at 1237, 1233, 1246 and 1250 cm-1 attributed to the stretching modes of trigonal BO3, typically in boroxol rings.
-
(h)
Bands at 1390, 1375, 1362, 1412 and 1480 cm−1 ascribed to vibration of B-O symmetric stretching within BO2O− units and varied types of borate groups, as well as vibration approaches of boron-oxygen in BO3 triangular units9.
Figure 4 discloses the deconvoluted spectra of the three glasses after 250 kGy of gamma irradiation. The contrast between their spectra can be individually discussed as follows:
-
1.
The cerium (Ce) ion belongs to rare earth ions, which have necessary properties endorsed to their surface electronic configuration through separating electrons in the 4f orbital and in the exterior electrons orbital 5s2 5p614. Ce ions participate in the glass structure either as Ce4+ or Ce3+ according to their concentration, host glass composition, and melting temperature of their glasses14,31,32. Cerium ions inhabit the glassy network in more than one valence state through an equilibrium redox reaction among tri and tetravalent states (Ce3+ ↔ Ce4+) to balance the negative charges in the neighboring tetrahedrons, but the absolute ratio of Ce4+ can often be improved. Some authors14,19 stated that cerium ions participate in the glass structure in the interstitial sites by way of imperfections and differentiated them as fall or cracked bonds.
As is observed in G1, the obvious decline in the intensity afterward irradiation, specifically bands around 800 cm−1 designates more joining bonds and more stability in the glassy structure after irradiation. It is evident from Figs. 3 and 4 that the FTIR spectra of Ce lead borate glass before and after irradiation have similar vibrational manners with no changes in the position or intensity of bands, and the slight shift of the bands from 440, 865, 940 and 1353 cm−1 to 437, 864, 938 and 1350 cm−1, refers to more stability in the glass structure and provides the connection of the glassy network. This manner is also approved by the high stability of the band at 1250 cm−1 that indicates no alteration of di-borate rings into tetraborate or tri-borate rings, referring to the stability in the glass structure19. Additionally, the stable band at 690 cm−1 designates the absence of de-polymerization process by the insertion of Ce3+/Ce4+ ions, referring to the improvement of the glass structural stability where the central borate building units (BO4 and BO3) are kept constant in their positions. So, bond rearrangements and more relaxed, glassy network can be released. The relaxation process in the amorphous glass structure includes the liberation of the extra stored energy to give more stable, relaxed glass. So, an equilibrium between the two valence states of cerium ions can be expected, where no more NBO is obtained by irradiation. The presence of a high percentage of heavy Pb2+ (50 mol%) ions in G1 with their characteristics of high polarizability and the presence of cerium ions with their own behavior in captivating radiation photons and absorbing defect color centers (commercially, CeO2 is widely used as a decolorizing agent), yields an outstandingly stable glass structure of Ce-Pb-B2O3 with surprising gamma radiation shielding effect.
-
2.
Sb2O3 is a fragile glass network oxide; however, it can readily form glass by adding network modifier ions such as Li+, Na+, K+….etc. Some glass scientists assumed that enhancing the radiation shielding efficiency of Sb-glass could be carried out by the incorporation of WO3 and/or PbO as well as alkali ions to improve the glass shielding ability, particularly with the high PbO content ranging from 10 to 40 mol%33. Therefore, a strong gamma shielding performance can be expected for the prepared Sb-glass with 35 mol% PbO content. Sb2O3 and PbO are considered to be effective glass network intermediate oxides. Sb2O3 can modify the borate network (Sb2O3–B2O3 system), creating BO4 units at a slight level whenever the rest of Sb2O3 can form its particular matrix essentially as SbO3 trigonal pyramids. However, in the case of PbO–B2O3 glasses, the four-coordinated borons BO4 can exceed 0.5 in ~ 50 mol% PbO, where the former PbO4/2 pyramidal units contribute as glass formers in the glassy network28. So, the increase in Sb2O3 content at the expense of PbO causes a rise in BO3 content and a decline in BO4 content. This behavior is owing to the greater affinity of PbO to transform BO3 units into BO4 units than Sb2O3 in borate glasses.
After irradiation, G2 reveals peaks at ~ 435,585, 650, 704, 873, 977, 1246, 1362 and 1412 cm−1 as shown in Fig.4. According to the present composition of G2 glass listed in Table 1 (with 35 mol% PbO), a slight conversion of BO3 to BO4 groups would take place by irradiation, where only minor changes in absorption spectra can be observed, as shown by comparing Fig. 3 with Fig. 4. The deconvoluted bands are shifted lightly to longer wavenumbers, referring to the influence of gamma radiation in creating some atomic transpositions in the glassy structure or electronic deficiencies that include modifications in the valence states of lattice or impurity atoms, resulting in very slight deviations in the bond angles and/or bond lengths of the main structural borate groups16, thus a limited conversion between BO3 and BO4 units can be expected. Nevertheless, the stable performance of the central vibrational bands in the fabricated Sb-PbO-B2O3 glass system against such high dose of gamma radiation (250 kGy) reflects its particular shielding behavior as a result of the weighty massive polarizable Pb2+ and Sb3+ ions that deferment the allowed passage of liberated electrons from irradiation, inhibiting then the formation of more induced weaknesses, leaving the main structural building groups almost persistent14,15.
-
3.
Mn2+ as transition metal ions like Ti4+, V5+, Cr3+and Fe3+ ions are able to vary their valence states in the glassy structure reliant to their concentration and the main structure of the host glass. Mn2+ ion occupies the network as distorted octahedral or tetrahedral Mn3+ and can contribute in the glassy network in many coordination: Mn2+, Mn3+, Mn4+, MnO4- and MnO42- ions or a combination of them34. Mn ion participates in the glassy structure either as network modifier or network former according to its concentration in the glass structure and the possible redox equilibrium of Mn2+/Mn3+ in alkali lead borate glass system34. At lower deliberations of MnO2, most manganese ions are in the form of the Mn2+ state with five unpaired electrons in their valence shell dispersed in t2g and eg orbitals. The octahedral Mn2+ ions can control the electronegativity decrease of the present modifiers, Na+ and Al3+ ions, corresponding to the increase in basicity of the surrounding anions in alkali borate glasses34. Mn2+ ions are differently bonded in the form of higher octahedral coordination; however, a small fraction of the lower tetrahedral coordination Mn3+ may be also found at higher Mn levels35. According to the last assumption, it is expected that manganese ions participate mainly in their tetrahedral coordination’s as Mn3+ ions, due to their contribution in a relatively high percentages (5 mol. %), in addition to some octahedral coordination as Mn2+.
After the irradiation process, G3 reveals a quite shift to lower wavenumbers to 430, 560, 689, 873, 931, 1048, 1233 and 1350 cm-1. By comparing the detected vibrational bands in Figs. 3 and 4 for G3 before and after irradiation, an obvious stability in the glass structure can be noticed, where the main vibrational bands are kept in their sites with a slight shift to lower wavenumbers, referring to saturation and/or relaxation in the glass structure. Some factors provide the stability of such structure and its resistance to irradiation, e.g., the highly polarizable Pb ions (PbO3/PbO4), the compacted AlO4 groups that strengthen the glass firmness, and essentially the occurrence of Mn2+/Mn3+ ions as transition metal ions having the ability to alter their valence states and absorbing the predicted defect centers caused via irradiation (-ve electrons/+ve holes). Thus, the highly compacted and rigid structure of G3 is observed, providing the glass with the ability to shield gamma radiation at such high dose (250 kGy).
Fig. 5 displays the N4 values for each glass composition before and after gamma irradiation. N4 is a significant parameter that refers to a part of four coordinated boron atoms in the glass, determining the ratio of (BO4) groups and the possible conversion between the two main structural units of borate (BO3 and BO4). Thus, the glass's response to many external factors can be interpreted, such as variations in metal oxide content, the introduction of certain ions, heat treatment, irradiation process, etc. As obvious from Fig. 5, N4, for G1 with the higher PbO content (50 mol%) is decreased after irradiation, referring to more stable structure produced after the irradiation process. According to many authors, N4 is increased with PbO content up to 50 mol%, then further growth of PbO causes a drop in N4 value (ratio of BO4/BO3)11. This behavior approves the existence of more stable and relaxed structure of G1 glass (Ce–PbO–B2O3) without more NBO after irradiation. On the other hand, G2 and G3 obtain a slight increase in N4 values after irradiation, while the tendency of G3 (Mn–PbO–B2O3) to maintain a stable structure with lower N4 is greater than that of G2 (Sb–PbO–B2O3) because of the distinct impact of manganese ions in captivating radiation defect centers through changing their outermost configurations.
Density
Figure 6 displays the density of the three studied glasses before and after exposure to gamma radiation at a dose of 250 kGy. The density of glass is a very important physical factor used to estimate the glass's firmness and rigidity. It describes a race between masses and volumes of the glassy units and in what way influentially the ions packed in the glassy structure18. As shown from Fig. 6, the three prepared glasses have density values of 5.26, 4.25, and 3.34 g/cm3 for 50, 35, and 15 PbO-containing glasses, G1, G2, and G3, respectively. The values indcate a direct correlation with the concentration of heavy lead ions in each glass composition because density can be well-defined mainly as weight per unit volume, so it is directly interrelated to the molecular weights of the induced ions in the material structure. Therefore, a rise in density values is observed with Pb addition due to the replacement of lower atomic masses in the glass composition with the higher atomic mass of PbO (Pb207.2)18. After the irradiation process, the density of the glass reveals approximately the same values as those before irradiation, with slightly higher values after irradiation of 5.30, 4.26, and 3.38 for G1, G2, and G3, respectively. This observation indicates the positive effect of ionizing radiation, where more compactness in the glass network can be obtained, giving narrower interstices and more closed systems against the high dose of gamma radiation.
Electron spin resonance ESR
The non-destructive technique ESR is used for distinguishing the unpaired electrons made by exposing materials to external effects such as ionizing radiation. Intensity and signals on the ESR spectrum depend predominantly on the number of free radicals formed by irradiation influence, e.g., positive holes and/or negative electrons at diverse trapping positions on the glass structure. Figure 7 displays the impact of 250 kGy of gamma radiation on the ESR spectra of the three investigated lead borate glasses, and the spectra demonstrate that the spectra appear very identical for each glass before and after the irradiation process, in addition to the complete absence of real sharp ESR signals in the three spectra either before or after exposure to gamma rays. The asymmetry of the broadening features obtained between the three spectra can be attributed to the dissimilar compositions of the glasses and the different contributions of the induced ions in various valence states through the exchange coupling in the glass clusters19. The obvious, non-distinctive changes in the spectra positions and intensity for the three glasses after 250 kGy of gamma radiation indicate largely the insignificant impact of irradiation on the glass structures, where there are no free radicals formed by irradiation. Accordingly, the high stable structure or the resistance effect of the prepared glasses to ionizing irradiation at such high dose. The highly polarizable Pb2+ ions and the precious effect of each induced ion, Ce, Sb, and Mn, are the main reasons for forming the blocked glass system that can obstruct the way of radiation photons until they miss their energies through molecules, leaving the main structure without deviations and verifying the high tendency of the glasses to dodge or shield high doses of gamma radiation.
Shielding parameters
Figure 8 reveals the simulated dose rate (mSv/h) for each glass sample with thicknesses of 1 cm and 2 cm glass containers at distances (D) of 1 m, 1.5 m, 2 m, 2.5 m, and 3 m compared to the shield (N.S.) against exposure to radioactive sources (with photon energy of 511 keV (F-18) and 662 keV (Cs-137) and activity of 100 mCi, where the dose rate decreases with increasing either the distance or the thickness of the glass shield containers.
As obviously revealed from Fig. 8, the shielding efficiency order of the simulated glass containers is G3 < G2 < G1 depending on the order of decrease in dose rate (mSv/h). Furthermore, consistent with the ALARA concept of radiation shielding, the distance away from the source and thickness of the shield are very important factors to be detected. The results also show that the maximum dose rates appear at a distance of 1 m with a glass container of 1 cm thickness. While the minimum dose rates appear at a distance of 3 m with a glass container of 2 cm thickness, Additionally, the energy of the radioactive source plays a main role in determining the exposure dose rate. The dose rate from a radioactive source with a photon energy of 511 keV is less than that with a photon energy of 662 keV at the same distance and glass thickness, as shown in Fig. 8. It is noticeably that the dose rates of gamma radiation decrease as the density of the glassy containers rises as a result of the rise in absorption coefficient that leads to the absorption of a large proportion of photons, reducing the radiation source intensity. Also, the portion of the scattered photons would increase with increasing energy.
The rate at which the investigated glasses transmit gamma doses can be detected by the dose transmission percentage, which is another important shielding parameter used for detecting the fraction of the shielded gamma dose to the unshielded gamma dose36 according to the following equation:
By using the simulated MNCP5, the dose transmission% for each glass shield with 1 and 2 cm thickness and a distance of 2 m away from 511 and 662 keV energies is determined and epitomized in Fig. 9, where the dose transmission% decreases with growing PbO mol%. So, it appears smaller for G1 (with the highest PbO mol%) than G2 and G3 (with lower PbO mol%), indicating that G1 is a better shielding material for gamma sources than G2 and G3.
At gamma energy 662 keV, the dose transmission% reduces from 26.9 to 21.5% by increasing the thickness of G1 from 1 to 2 cm, while at 511 keV it decreases from 8.9 to 6.4%. Likewise, G2 decreases from 28.3 to 25.5% at 662 keV and from 9.7% to 8.3% at 511 keV, and G3 decreases from 32.1 to 29.7% at 662 keV and from 11.6 to 10.4% at 511 keV.
In order to reflect the potential health danger of radiation, one of the most vital studied parameters is the excess lifetime cancer risk % which evaluates the glass shields efficiency according to the point of view of health hazards. It can also differentiate between glass shields and other working shields in the radiological and nuclear fields.
The excess lifetime cancer risk due to gamma radiation, based on the effective dose using the simulated MCNP5 code values, is determined as follows37.
Excess lifetime cancer risk = Effective dose Mean duration of life Fatal cancer risk factor.
where mean duration of life and fatal cancer risk factor are the life duration (40 years) and fatal cancer risk factor for stochastic effect (0.04 Sv-1 for the workers) were used by the ICRP (international Commission on Radiological Protection) for stochastic effects, respectively37.
Figure 10 displays obviously the excess lifetime cancer risk % for workers with 2000 h per year for 40 years working38 from radiation exposure dose at 2 m far away the investigated glass shields and compares our studied samples to some of the glass shields from earlier study publications by employing the weight percentages of their elemental compositions in our simulated MCNP5 code to calculate the effective doses behind these glasses under identical conditions: A-glass 39, PbBaP540, ZBiB41 and Bi1042. The results reveal that G1 glass shield diminishes the lifetime cancer risk better than the other formerly studied shields, while G2 and G3 lessen the lifetime cancer risk better than ZBiB41, and Bi1042 biological shields. Lifetime cancer risk % indicates the effectual behavior of the studied glass shields in diminishing the lifetime cancer risks and their high importance in avoiding workers biological radiation dangers.
Recent research has shown that charged particles have radiobiological effects and result in defects in shield structures due to photon effects. Mass stopping power is a very important parameter used to ensure the security of radiation workers, who must have an adequate attenuator. Therefore, it's crucial to distinguish the mass-stopping power of the glass shields and their ranges in the shield.
An energetic ion misses energy nuclear reactions and bremsstrahlung, elastic energy transmission to atomic nuclei, and inelastic energy transmission to electrons (excitation and ionization of the target atoms and the ion itself). Stopping power, commonly identified as stopping force, is a unit of measurement for the rate of energy loss per unit route length (dE/dx). Ion energy loss can be divided into two categories: (1) energy transfer to target electrons, which causes ionization, and (2) energy transmission to target nuclei, which causes atomic dislocations or phonon energy debauchery for energy transmission overhead or under the threshold movement energy43.
In this part, it is recommended to examine how the charged particles (electrons) move through the materials by using the Esther code, which is based on Monte Carlo simulation. In reality, it can be noticed that the effective radiation quantity in a material, changes in response to the change in energy value, as per ψe calculations in (MeVcm2/g). The ψe results can be used to detect the material's radiation shielding capacity44. The software tool that accounts for ψe of the examined glasses for electron particles (Estar), refers to the decrease in electron kinetic energy as it travels through the glass samples with a certain density so ψe is regarded as a strong sheild factor.
For the samples under examination, Fig. 11(a, b) shows the fluctuation of ψe and CSDA values as a function of kinetic energy (0.010–10 MeV). It is observed that the, CSDA range increases with increasing kinetic energy, while ψe values decrease with energy. Moreover, the lowest ψe and largest CSDA range values are found for the highest Pb concentration (G1 glass). The CSDA lengthens to express the typical range of stopping any charged particles with kinetic energy (KE), and its values are relational to kinetic energy and rise very slightly with increasing Pb concentrations. This is because charged particles can penetrate the glass network more deeply where the interaction cross-sectional region of the particles decreases with the increase in KE.
Figure 12a,b shows how the proton mass stopping power (ψp) and projection range (ΠP) change with KE, where the greatest proton-protective material needs the least ΠP. As shown in Fig. 12b, G1 glass delivers complete proton protection as it has the lowest ΠP value compared to G2 and G3 samples.
Because proton particles have a higher mass (1.67262 × 10−27 kg) than electrons (9.1093837015 × 10−31 kg), which is only 1/1,836 the mass of the proton, they have a lower velocity, allowing protons to have smaller values of ψp than electrons ψe at the same energy.
The macroscopic effective range cross-section of the fast neutron, ∑R, is used to quantify the chance of a fission neutron to react in a medium that eliminates it from a neutron beam of fission energy (by decelerating through elastic scattering). It is desirable to use light and heavy elements in shield composites for optimal fast neutron attenuation.
∑R changes with the energy of fast (fission) neutrons, is thought to remain persistent for energies between 2 and 12 MeV for neutrons. Figure 13 displays the ∑R (cm-1) values of the examined glasses by Phy-X/PSD software. The graph demonstrates how ∑R steadily declines as PbO content decrease, where the sample G1 has a much higher ƩR than G2 and G3. This indicates a nominal improvement in the neutron-shielding performance for G2 and G3 than G1, which is a good behavior compared with some typical shielding materials, e.g. water = 0.102 cm−1, graphite = 0.077 cm−1, ordinary concrete = 0.094 cm−1 and hematite-serpentine concrete = 0.097 cm−145.
G1 glass sample compares favorably to these materials when the value of (ƩR cm-1) is exceeded, demonstrating that it is a more effective fast neutron attenuator.
Conclusion
The FTIR spectra of the three investigated Ce, Sb or Mn lead borate glasses reveal high structural steadiness against 250 kGy of gamma radiation. The spectra of Ce-glass display the same vibrational bands without changes in their position or intensity after irradiation, and the slight shift of the bands from 440, 865, 940 and 1353 cm−1 to 437, 864, 938 and 1350 cm−1, refers to stability and connectivity of the glass structure, since the stability of the band at 1250 cm−1 indicates no transformation of di-borate rings into tetraborate or tri-borate rings. The spectra of Sb-glass also reveal a stable performance after gamma irradiation thanks to the heavy, massively polarizable Pb2+ and Sb3+ ions that defer the free passage of radiation photons, leaving the structural building units almost constant. Mn ions containing glass reveal also a stable structure correlated to their nature as transition metal ions able to adjust their valence states (Mn2+/Mn3+) to captivate radiation defect centers (− ve electrons/ + ve holes), in addition to the compacted AlO4 groups that strengthen the glass rigidity. Attendance of the greatly polarizable Pb2+ ions in the three fabricated glasses is the main reason for such structural stability by forming strong covalent bonds, e.g., PbO4 and/or PbO3 pyramidal units that work to lessen the ionic charge balance accessibility. Accordingly, the higher the Pb content, the more stable and shielded the glass because PbO contributes to a dual behavior as a glass former and modifier, enhancing the glass structure's solidity. The decrease in N4 values after irradiation for Ce-glass with the higher PbO content (50 mol. % PbO), confirms its higher structural stability and relaxation than Sb-glass (35 mol. % PbO) and Mn-glass (15 mol. % PbO) with lower PbO content. The density of the glasses shows a direct relationship with the heavy PbO content, as it depends mainly on the molecular weights of the induced ions. So the replacement of lighter atomic masses in each glass composition by the heavier atomic mass of PbO (Pb207.2) causes a higher density for Ce glass than Sb and Mn glasses.
After irradiation, the values of density reveal a slight increase, which designates the positive influence of ionizing radiation on the glass compactness by narrowing the glassy network interstices, giving more closed systems. ESR spectra exhibit very identical behavior before and after 250 kGy of gamma radiation, and the complete absence of real sharp ESR signals indicates the absence of free radicals formed by irradiation, and the high resistance of the glass structure to such high gamma rays. Many radiation shielding parameters were evaluated, e.g., mass stopping power and projected range values for proton particles, using the SRIM Monte Carlo simulation code and the ESTAR program to calculate mass stopping power and the progressive slowing down approximation range for electrons.
For the three glasses, G1, G2, and G3, the maximum values of ψp are 0.415, 0.415 and 0.541 MeV cm2/g at energy of 0.09 MeV, respectively. While ψe values at 10 MeV are 2.073, 2.069 and 1.92 MeV cm2/g, and the electron CSDA range upsurges with electron KE, at KEs of 10 MeV, the CSDA values are 5.968, 5.930 and 5.892 g/cm2 and ΠP for proton 310.1, 387.5 and 435.8 μm for G1, G2, and G3 samples, respectively. The simulation of radiation dose rates (511 keV or 662 keV) at different distances for each glass (1 and 2 cm thickness) by MCNP5 code and Phy-X/PSD software follows the order of shielding efficiency as (Ce-glass) G3 < (Sb-glass) G2 < (Mn-glass) G1. The dose rate% decreases as the density of the glass containers increases due to the increase in absorption coefficient and thus the absorption of large proportions of photons, reducing the intensity of the radiation source and giving the best behavior at a distance of 2 m in the 2 cm thickness of the glass container. On the other side, the dose transmission % decreases with increasing PbO mol. %, so it appears smaller for G1 (with the highest PbO mol. %) than G2 and G3. Comparing the excess lifetime cancer risk % for workers (working 2000 h per year for 40 years due to radiation exposure at 2 m away from the glass containers of a radioactive source) with other shielded materials, reveal the ability of Ce- glass shield (G1) to reduce lifetime cancer risk better than many other shields. Additionally, the lowest ψe and largest CSDA range values are found for the highest Pb concentration (G1 glass). Macroscopic effective range cross-section of the fast neutron ∑R (cm−1) from Phy-X/PSD software displays a steady increase with PbO content, giving the order of increase: G3 < G2 < G1.
The closed and associated structures are supported by the highly polarizable heavy lead ions (Pb2+) that can block the path of gamma photons through the network and the effective role of the induced different metal ions. So outstanding stability and radiation shielding behavior against high ionizing radiation up to 250 kGy are obtained, specifically for the glass with the maximum PbO content G1 (Ce-glass). The whole results recommend the promising usage of the prepared glasses as storage containers for radioactive wastes, especially for nuclear medicine entities in hospitals or other amenities.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Saleh, H. M., Bondouk, I. I., Salama, E. & Esawii, H. A. Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy 137, 764 (2021).
Craeye, B., De Schutter, G., Vuye, C. & Gerardy, I. Cement-waste interactions: Hardening self-compacting mortar exposed to gamma radiation. Prog. Nucl. Energy 83, 212–219 (2015).
Beden, S. J. et al. Hypothetical method for gamma dose rate assessment to conditioned radioactive waste container. Iraqi J. Sci. 59, 476–481 (2018).
Donald, I. W., Metcalfe, B. L. & Taylor, R. N. J. Review: The immobilization of high level radioactive waste using ceramics and glasses. Mater. Sci. 32, 5851–5887 (1997).
Compton, K. L., Bennert, D. M. & Bickford, D. F. Regulatory issues in vitrification research: A case study of circuit board reclamation. Am. Ceram. Soc. 39, 3–12 (1993).
Abou Hussein, E. M. Vitrified municipal waste for the immobilization of radioactive waste: Preparation and characterization of borosilicate glasses modified with metal oxides. Silicon 11(6), 2675–2688 (2019).
Roth, G. & Weisenburger, S. Vitrification of high-level waste: Glass chemistry, process chemistry and process technology. Nucl. Eng. Des. 202, 197–207 (2000).
El-Alaily, N. A., Abou-Hussein, E. M., Abdel-Monem, Y. K., Abd Elaziz, T. D. & Ezz-Eldin, F. M. Vitrified municipal waste as a host form for high-level nuclear waste. J. Radioanal. Nucl. Chem. 299, 65–73 (2014).
Marzouk, M. A. & Abou Hussein, E. M. Induced defects by gamma irradiation doses on the structure and optical behavior of undoped and TiO2-, Cr2O3, or MnO-doped heavy metal borate glasses. Appl. Phys. A 125, 140 (2019).
Abouhaswa, A. S., Rammah, Y. S., Ibrahim, S. E. & El Mallawany, R. Optical and electrical properties of lead borate glasses. J. Elec. Mat. 48, 5624–5631 (2019).
ElBatal, H. A., Abdelghany, A. M. & Ali, I. S. Optical and FTIR studies of CuO-doped lead borate glasses and effect of gamma irradiation. J. Non-Crys. Solids 358, 820–825 (2012).
Schwarz, C. M. et al. Processing and properties of novel ZnO-Bi2O3-B2O3 glass-ceramic nanocomposites. J. Alloys Compd. 820, 153173 (2020).
Abou Hussein, E. M., Madbouly, A. M., Ezz Eldin, F. M. & El Alaily, N. A. Evaluation of physical and radiation shielding properties of Bi2O3–B2O3 glass doped transition metals ions. Mater. Chem. Phys. 261, 124212 (2021).
Abou Hussein, E. M., Abdel Maksoud, M. I. A., Fahim, R. A. & Awed, A. S. Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt. Mater. 114, 111007 (2021).
El Batal, H. A., Abou Hussein, E. M., El Alaily, N. A. & EzzEldin, F. M. Effect of different 3d transition metal oxides on some physical properties of γ-Irradiated Bi2O3- B2O3 glasses: A comparative study. J. Non-Cryst. Solids 528, 119733 (2020).
Abou Hussein, E. M., Madbouly, A. M. & El Alaily, N. A. Gamma ray interaction of optical, chemical, physical behavior of bismuth silicate glasses and their radiation shielding proficiency using Phy-X / PSD program. Non-Cryst. Solids 570, 121021 (2021).
Sayyed, M. I. et al. Physical, optical and gamma radiation shielding competence of newly boro-tellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Inter. 47, 611–618 (2021).
Abou Hussein, E. M. & Barakat, M. A. Y. Structural, physical and ultrasonic studies on bismuth borate glasses modified with Fe2O3 as promising radiation shielding materials. Mater. Chem. Phys. 290, 126606 (2022).
Abou Hussein, E. M. & Abdel-Galil, A. Synthesis, optical, chemical and electrical characterizations of γ-irradiated transition metal ions reinforced borate glasses. J. Non-Crys. Solids 610, 122302 (2023).
Abou Hussein, E. M., El-Agawany, F. I. & Rammah, Y. S. CuO reinforced lithium-borate glasses: Fabrication, structure, physical properties, and ionizing radiation shielding competence. J. Aust. Ceram. Soc. 58, 157–169 (2022).
Abou Hussein, E. M. & Madbouly, A. M. Chemical and radiation shielding effectiveness of some heavy metal oxide glasses for immobilizing radioactive wastes. J. Aust. Ceram Soc. https://doi.org/10.1007/s41779-023-00951-2 (2023).
Şakar, E., Özpolat, Ö. F., Alım, B., Sayyed, M. I. & Kurudirek, M. Phy-X/PSD: Development of a user-friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 166, 108496 (2020).
Ziegler, J. F., Ziegler, M. D. & Biersack, J. P. SRIM—The Stopping and Range of Ions in Matter (SRIM Co., 2008).
ESTAR. Stopping power and range tables for electron. Data available. NIST (national institute of standards and technology) http://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html.
Berger M.J., Coursey J.S., Zucker M.A., Chang J., Estar PSTAR, ASTAR. Stopping- power & range Tables for electrons, protons, and helium Ions. Updated 2017. NIST; 2017. https://doi.org/10.18434/T4NC7P. https://www.nist.gov/pml/stopping-power-range-tables-electrons-protons-andhelium-ions.
Wen, H., Tanner, P. A. & Cheng, B. Optical properties of 3dN transition metal ion-doped lead borate glasses. Mat. Sci. Mat. Res. Bull. https://doi.org/10.1016/J.MATERRESBULL.2016.06.032 (2016).
Abou Hussein, E. M. The impact of electron beam irradiation on some novel borate glasses doped V2O5; Optical, physical and spectral investigation. Inorg. Chem. Commun. 147, 110232 (2023).
Gao, G. et al. Effect of Bi2O3 on physical, optical and structural properties of boron silicon bismuthate glasses. Opt. Mater. 32, 159–163 (2009).
Ahmad, Z. et al. Radio-optical response of cerium-doped lithium gadolinium bismuth borate glasses. J. Lumin. 224, 117341 (2020).
Wen, H. & Tanner, P. A. Optical properties of 3d transition metal ion-doped sodium borosilicate glass. J. Alloys Compd. https://doi.org/10.1016/j.jallcom.2014.11.094 (2014).
De Echave, T. et al. Effect of clayey groundwater on the dissolution rate of SON68 simulated nuclear waste glass at 70 °C. Nucl. Mater. 503, 279–289 (2018).
Clark, D. E., Pantano, C. G. & Hench, L. L. Corrosion of Glass (Books for Industry, 1979).
Doweidar, H., El-Egili, K., Ramadan, R. & Al-Zaibani, M. Structural investigation and properties of Sb2O3–PbO–B2O3 glasses. J. Non-Cryst. Solids https://doi.org/10.1016/j.jnoncrysol.2018.01.025 (2018).
Abou-Laila, M. T., El-Zayat, M. M., Madbouly, A. M. & Abdel-Hakim, A. Gamma irradiation effects on styrene butadiene rubber/Pb3O4: Mechanical, thermal, electrical investigations and shielding parameter measurements. Rad. Phys. Chem. 192, 109897 (2022).
Sathish, K. V. et al. Specific absorbed fraction of energy of silicon-boron alloys. Indian J. Pure Appl. Phys. 58, 213–217 (2020).
Igwesi, D. I. & Thomas, O. S. A Comparative study of Dose transmission factor of polythene and borated polythene for high neutron source shielding. Int. J. Sci. Tech. Res. 2, 149–153 (2013).
Zarghani, H. & Jafari, R. Assessment of outdoor and indoor background gamma radiation, the annual effective dose and excess lifetime cancer risk in Birjand, Iran, Jundishapur. J. Health Sci. 9(3), e40791. https://doi.org/10.5812/jjhs.40791 (2017).
International Commission on Radiological Protection . ICRP publication 60: 1990 recommendations of the international commission on radiological protection. 60. Elsevier Health Sciences; 1991.
Tekin, H. O. et al. Heavy Metal Oxide (HMO) glasses as an effective member of glass shield family: A comprehensive characterization on gamma ray shielding properties of various structures. J. Mater. Res. Tech. https://doi.org/10.1016/j.jmrt.2022.02.074 (2022).
Kaur, K., Singh, K. J. & Anand, V. Correlation of gamma ray shielding and structural properties of PbO-BaO-P2O5 glass systems. Nucl. Eng. Des. 285, 31–38 (2015).
D’Souza, A. N. et al. Gamma ray shielding and thermoluminescence investigation of bismuth added heavy metal oxide glasses. Rad. Phy. Chem. 188, 109598 (2021).
Kulwinder Kaur, K.J. Singh, Vikas Anand, Correlation of gamma ray shielding and structural properties of PbO–BaO–P2O5 glass system. Nucl. Eng. Des.285 (2015) 31–38
Amable, A. K. S., Godsway, B. K., Nyaaba, R. A. & Eric, M. N. A theoretical study of stopping power and range for low energy (3.0mev) protons in aluminium, germanium, lead, gold and copper solid materials. Open Sci. J. https://doi.org/10.13140/RG.2.2.13069.18404 (2017).
Singh, V. P. & Badiger, N. M. Shielding efficiency of lead borate and nickel borate glasses for gamma rays and neutrons. Glass Phys. Chem. 41, 276–283 (2015).
Madbouly, A. M., Alazab, H. A., Borham, E. & Ezz-ElDin, F. M. Study of gamma radiation dosimeter and radiation shielding parameters of commercial window glass. Appl. Phys. A 127, 761. https://doi.org/10.1007/s00339-021-04889-9 (2021).
Acknowledgements
We are gratefully acknowledging the National Center for Radiation Research and Technology (NCRRT) and Nuclear and Radiological Safety Research Center (NRSRC) at Egyptian Atomic Energy Authority (EAEA) for the fruitful assistance in completing this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
E.M.A. Hussein and A.M.M.: contributed in this study: conceptualization, methodology, Methodology, formal analysis, Investigation, Resources, Data curation, Writing: original draft Writing: original draft, review, and editing Writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou Hussein, E.M., Madbouly, A.M. Fabrication and characterization of different PbO borate glass systems as radiation-shielding containers. Sci Rep 14, 2638 (2024). https://doi.org/10.1038/s41598-024-52071-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52071-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.