Abstract
The current work deals with the synthesis of a new glass series with a chemical formula of 5Al2O3–25PbO–10SiO2–(60-x) B2O3–xBaO; x was represented as 5, 10, 15, and 20 mol%. The FT-IR spectroscopy was used to present the structural modification by rising the BaO concentration within the synthesized glasses. Furthermore, the impacts of BaO substitution for B2O3 on the fabricated borosilicate glasses were investigated using the Makishima-Mackenzie model. Besides, the role of BaO in enhancing the gamma-ray shielding properties of the fabricated boro-silicate glasses was examined utilizing the Monte Carlo simulation. The mechanical properties evaluation depicts a reduction in the mechanical moduli (Young, bulk, shear, and longitudinal) by the rising of the Ba/B ratio in the fabricated glasses. Simultaneously, the micro-hardness boro-silicate glasses was reduced from 4.49 to 4.12 GPa by increasing the Ba2+/B3+ ratio from 0.58 to 3.18, respectively. In contrast, the increase in the Ba/B ratio increases the linear attenuation coefficient, where it is enhanced between 0.409 and 0.448 cm−1 by rising the Ba2+/B3+ ratio from 0.58 to 3.18, respectively. The enhancement in linear attenuation coefficient decreases the half-value thickness from 1.69 to 1.55 cm and the equivalent thickness of lead is also reduced from 3.04 to 2.78 cm, at a gamma-ray energy of 0.662 MeV. The study shows that the increase in the Ba2+/B3+ ratio enhances the radiation shielding capacity of the fabricated glasses however, it slightly degrades the mechanical properties of the fabricated glasses. Therefore, glasses with high ratios of Ba2+/B3+ have high gamma-ray shielding ability to be used in hospitals as a shielding material.
Similar content being viewed by others
Introduction
Radioactive materials are being extensively used in technologies of this century. Different medical, agricultural, and space research sectors are now relying on these materials. Depending on their application, various levels of radiation are being exploited; the amounts of energy emitted by these sources can be harmful to humankind. Acute exposure to high energy levels of ionizing radiation can cause major health issues, damaging the body cells, and leading in some cases to genetic disorders1,2,3,4,5. It is then crucial to manipulate radioactive materials with a high level of caution and to protect the working personnel from any radiation hazard. Therefore, shielding materials are being used as a barrier to these offensive radiations6,7,8,9. Research on radiation shielding and radiation attenuation has revealed a wide range of materials that are of high efficiency in reducing radiation levels10,11,12,13. The two classic materials commonly adopted as shielding materials are concrete and lead. Although they possess numerous advantages, these classical shielding materials suffer from many drawbacks. For instance, concrete is heavy and bulky, causing challenges in its transportation and installation14,15. Moreover, concrete can degrade over time when exposed to moisture, affecting its integrity, and thus endangering its shielding performance. On the other hand, lead-based shielding materials are highly toxic, they are poor in flexibility and chemical stability. Therefore, developing novel shielding materials, that hold great shielding properties, is highly necessary to replace conventional ones16.
Extensive research has been done on alternative radiation shielding materials, suggesting glass as an effective solution17,18,19. In fact, glass is highly abundant and of competitive price, resulting in being very accessible. Additionally, glass is easy to process and does not require extensive maintenance. Most importantly, glass is optically transparent, which makes its employment very convenient for numerous shielding applications20,21,22. The merge of different oxides in the glass system gives it diversity in characterization and enhancement in density, effectively improving the absorption of gamma rays.
Different types of glasses, such as silicate, borate, tellurite, phosphate, and antimonate, are available, but a few glasses were used in different applications due to their low structural stability. Soda-lime glass is a famous glass type widely used in different applications. Still, it has many drawbacks, such as limited thermal shock resistance, susceptibility to chemical attack, and relatively low mechanical strength23. At the same time, borate glass has lower chemical durability and a higher coefficient of thermal expansion, which leads to limited use. The mixing of borate and silicate gives a mixture of two glasses called borosilicate, which has desirable properties such as lower thermal expansion and high durability24. This type of glass in a radiation shielding field is a good idea due to its properties. The addition of oxides to the glass system changes the glass structure and enhance the mechanical and optical properties of the glass. Heavy oxides such as PbO and BaO can increase the glass density and improve the shielding properties, while Al2O3 can play two roles: glass modifier or glass former, according to the ratio in the glass system25,26.
In particular, it has been proven that borosilicate glasses, adopted in the vitrification of nuclear waste, are highly efficient in stabilizing the High-Level Liquid Waste through the suppression of its migration into the natural environment27,28. For example, borosilicate glass is known to be water and chemical-resistant, it possesses a lower melting point than classic silicate glass, which increases its lifetime29. Moreover, concerning their applications in radiation protection, oxide incorporation is found to improve the radiation-shielding abilities for glass materials in absorbing various types of ionizing radiation30.
Many studies have explored borosilicate glass, especially in the radiation shielding field31,32,33,34,35. Cheewasukhanont et al.31 studied the impact of particle size on radiation shielding properties for the (55-x)SiO2–xBi2O3–20B2O3–10CaO–15Na2O glass system where x = 0, 5, 10, 15, 20 and 25 mol%. The particle size did not affect glass density, while nano-sized particles showed better-shielding properties than microsized particles. Chanthima and Kaewkhao32 investigated the radiation shielding for 23Na2O–15B2O3–2Al2O3–10CaO–(50-x)SiO2–xBi2O3 where x = 0, 5, 10, 15, and 20 mol%. Adding Bi2O3 instead of SiO2 increased the radiation shielding ability of the glass system. Mhareb35 explored the structural, mechanical, optical, and radiation shielding properties for 10SiO2–10TiO2–30SrO–(49.5-x)B2O3–xGd2O3 glass and glass ceramics where x = 0.5, 1, 1.5, and 2 mol%. Adding Gd2O3 led to a slight variation in mechanical and optical properties. At the same time, the shielding properties showed gradual enhancement.
The current work is innovative in that it examines the effects of increasing the Ba2+/B3+ ratio on the mechanical, structural, and gamma-ray shielding characteristics of a newly synthesized boro-silicate glass consisting of Al2O3-PbO-SiO2-B2O3-BaO.
Materials and methods
Glasses preparation
To prepare the investigated glasses, of general formula 5Al2O3–25PbO–10SiO2–(60-x) B2O3–xBaO, where x was varied between 15, 10, 15, and 20 mol%, the melt quenching method was employed. The following oxides: MgO (purity of 99.99%), BaO (purity of 99.99%), SiO2 (purity of 99.99%), B2O3 (purity of 99.99%), and PbO (purity of 99.99%) were supplied by Sigma Aldrich (USA) and used for fabrication of the glasses in the current work. The required amounts of each metal oxide were accurately weighed and mixed using agate mortar to ensure a homogeneous and uniform glass structure. The powders in high-purity alumina crucibles were put in an electric furnace at 1100 °C for two hours. The glass was carefully poured into a metal mold once it had totally liquefied. The glasses followed an annealing procedure in a different furnace, where they were heated to 400 °C for 4 h, in order to reduce their internal stress. Figure 1 represents a photo of the prepared glass samples. In the mentioned figure the Ba/B ratios are 0.58 (for S1 glass sample), 1.27 (for S2 glass sample), 2.12 (for S3 glass sample), and 3.18 (for S4 glass sample).
Using the method outlined by Archimedes in Eq. (1), the density of the synthesized S-S4 glasses was measured. The weights of the S1-S4 glasses in liquid and air are denoted by \(W_{a}\) and \(W_{L}\). Additionally, the density of the submerged liquid used in the current study is \(\rho_{L}\)≈1 g/cm3 for water36.
Furthermore, the characterization of the S1–S4 glasses was performed using a Shimadzu-IRSpirit Fourier transform infrared (FTIR). It is used to explore the functional groups and vibration bonds for glasses within the wavenumber range from 500 to 2000 cm−1.
Mechanical properties evaluations
The elastic moduli of Young (Y, GPa), bulk (K, GPa), shear (S, GPa), and longitudinal (L, GPa) as well as mechanical properties like Poisson ratio (σ), micro-hardness (H, GPa), and fractal bond conductivity (d) were estimated using the Makishima and Mackenzie theory31,32 based on the chemical composition and density of the fabricated lead borosilicate glasses, according to Eqs. (2–8)37,38,39.
The Vt, and Gt are the respective packing density and dissociation energy per unit volume of the utilized metal oxides.
Monte Carlo simulation investigation
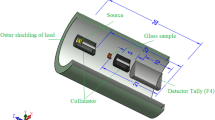
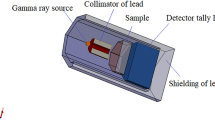
The radiation shielding parameters of the glasses under investigation were simulated and evaluated using the Monte Carlo N-particle transport code, 5th version (MCNP-5)40. During the simulation, the gamma-ray energy (Eγ, MeV) was chosen to vary over a wide range, from 0.033 to 2.506 MeV, in order to encompass nearly all of the actual γ-ray energies. The ENDF/B-V.8 nuclear database, which has the interaction cross-sections needed to assess the radiation shielding capabilities of the examined glasses, was linked to the MCNP-5 code. An input file should be created with all the details required to describe the simulation components (cell, surface, material, importance, source, and cutoff cards) in order to carry out such a simulation. The mentioned input file indicates that the geometry is encircled by a 5 cm-thick lead shielding cylinder. The dry air inside the outer shielding cylinder had a density of 0.001225 g/cm3. Subsequently, a radioactive disk source was positioned at the center of the outer shielding cylinder (POS = 0 0 0), measuring 2 cm in diameter and 0.5 cm in thickness. A flux of γ-ray photons (PAR = 2) is released by the source along a long + Z axis (AXS = 0 0 1). The source card of the input file also included the distribution and emission probability of the radioactive source. The released photon flux was guided towards the sample using a lead collimator that measures 7 cm in height and 2 cm in diameter. The material card was modified to include the chemical compositions and densities of the materials constituting the created geometry so that the collimated photon flux could interact with the examined glasses. The fabricated glasses, shaped in cylinders, measure 3 cm in diameter and 1 cm in height. The scattered photons were then directed toward the detector through a second collimator, with a diameter of 5 cm and a height of 3 cm, after the photon flux had interacted with the electrons and atoms in the glasses. To estimate the average flux per unit glass cell and the average track length (ATL) of γ photons within the glasses under investigation, the current work uses the “F4 tally” function embedded in MCNP-5 code. By using the cutoff card, which is set up to be 108, the photon-electron interaction is managed. Within the output file after the simulation runout, the simulated ATL of γ-photons was included. The mentioned output file indicates ± 1% for the relative error41,42,43,44. Using Eqs. (9 and 10), the linear attenuation coefficient (µ, cm−1) and mass attenuation coefficient (µm, cm2/g) of the fabricated composites were calculated based on the obtained ATL of γ-photons.
where Io and It values refer to the photon flux before and after interaction with the fabricated glasses.
The half-value thickness (Δ0.5, cm) describes the thickness of the material required to diminish the photon flux by 50%. It is inversely varied with the µ values according to Eq. (11).
Additionally, the transmission factor (TF, %) for the fabricated S1-S4 glasses was estimated according to Eq. 12, where the \(\frac{{I_{o} }}{{I_{t} }}\) represents the ratio of transmitted photons. On the contrast, the RPE (%) describes the amount of photons absorbed within the fabricated glasses thickness. It is calculated based on Eq. 13.
In addition to being evaluated through Monte Carlo simulation, the µm was theoretically calculated with XCOM software. The (µm)glass can be theoretically computed using Eq. (14)45.
ρ, ωi, and (\(\mu_{m}\))i describe the density of fabricated glasses, the weight fraction of ith element within the glass sample, and µm for the ith constituent element, respectively.
Results and discussion
The substitution of B2O3 by the BaO compound increases the Ba2+ ions while it decreases the B3+ ions in the boro-silicated glasses. This affects the color of the synthesized boro-silicate glasses. The transparent color is transferred to a light yellow and then to a dark yellow color by increasing the Ba2+/B3+ ratio between 0.58 (S1 glass sample) and 3.18 (S-4 glass sample), as illustrated in Fig. 1. The ratio of Ba/B plays an important role in controlling the density, molar weight, and molar volume of the fabricated glasses, as illustrated in Table 1 and Fig. 2. The increase in BaO concentration increases the Ba2+ ions, which in turn increases the Ba/B ratio due to the substitution of B3+ by Ba2+ ions. The increase in Ba2+/B3+ ratio is associated with an increase in the molar weight of the fabricated glasses, where the molar weight increases from 112.86 to 125.42 mol/g, when rising the Ba2+/B3+ ratio between 0.58 and 3.18, respectively. Moreover, the experimental measurements of the fabricated glass density show an increase from 4.48 to 4.97 g/cm3, when rising the Ba/B ratio between 0.58 and 3.18, respectively. The increase in the fabricated glasses density is attributed to the partial replacement of Ba (ρ = 3.34 g/cm3) for the B (ρ = 2.46 g/cm3) ions. The increase in the density and molar weight of the developed glasses is found to be accompanied by a negligible increase in the molar volume of the fabricated glasses, which varies from 25.17 to 25.21 cm3/mol. The aforementioned negligible increase in the Vm values is attributed to the close comparable Vm values for both B2O3 and BaO compounds, where the (Vm)B2O3 = 28.30 cm3/mol and (Vm)BaO = 26.805 cm3/mol.
Fourier transform infrared (FTIR) is an instrument used to analyze functional groups and provide information about chemical bonding and molecular structure for various materials. Table 2 and Fig. 3 illustrate the functional groups for the glass system. It can be noted in four bands. A small band is located at 550 and 560 cm−1, which is related to the O–Si–O bending vibration mode35, while the band appearance at 690 and 703 cm−1 can be assigned to bending vibration B–O–B46. The large band centered at 906 to 971 cm−1 and 1039 to 1076 cm−1 can correspond to the B–O stretching of the tetrahedral BO4 unit48 The last band at high wavenumbers from 1388 to 1453 cm−1 and 1202 and 1247 cm−1 is associated with B–O stretching of trigonal BO347. On the other hand, it can be noted that the change in band position for the BO3 band with adding a further amount of BaO is due to transforming BO3 to BO4 and forming nonbridging oxygen. Here, it can be concluded that the BaO plays a modifier in the glass system.
Table 3 depicts the variation in the mechanical properties of the fabricated glasses with the increasing of Ba2+/B3+ ratio. Rising the Ba2+/B3+ ratio between 0.58 (for S1) and 3.18 (for S4) is found to reduce the total dissociation energy Gt from 60.87 to 54.37 kcal/cm3 and the packing density Vt from 0.57 to 0.50 m3/mol. The reduction in the Gt and Vt values are attributed to the packing factor Vi and dissociation energy (Gi) for both B2O3 and BaO compounds, where the B2O3 compound has Gi = 82.8 kcal/cm3 and Vi = 20.8 m3/mol while for BaO compound Gi = 39.5 kcal/cm3 and Vi = 9 m3/mol48. The replacement of B2O3 (high Gt and Vi values) with BaO (low Gi and Vi values) is the main reason for the reduction in both Gt and Vt of the fabricated glasses. The reduction in the Gt and Vt for the fabricated glasses reflects on the mechanical moduli. The increase of substitution ratio Ba/B between 0.58 and 3.18 decreases the moduli from 69.35 to 54.21 GPa (for Y modulus), from 47.41 to 32.43 GPa (for K modulus), from 27.60 to 22.19 GPa (for S modulus), and from 84.22 to 62.02 GPa (for L modulus). Additionally, the Poisson ratio is slightly reduced from 0.26 to 0.22, associated with a similar reduction in the micro-hardness of the fabricated glasses from 4.49 to 4.12 GPa. After that, the increase in the fractal bond conductivity from 2.33 to 2.74 confirms a transformation of the glassy structure to a 3D network.
The radiation shielding properties of the fabricated glasses were investigated utilizing the Monte Carlo simulation (MCNP-5) code and the XCOM theoretical program, as illustrated in Fig. 4 (a and b). The gamma-ray shielding evaluations show a dependence of the shielding ability on some parameters related to the γ-ray source and the examined materials. Regarding the γ-ray source, the source energy (Eγ, MeV) greatly affects the µ values of the fabricated glasses, where greeting the Eγ is associated with a reduction in the µ values. The reduction in the µ values is a result of the reduction in the interaction cross-section of γ-photons, where the cross-section varied with \(E_{\gamma }^{ - 3.5}\) and \(E_{\gamma }^{ - 1}\) for photoelectric (PE) and Compton scattering (CS) interactions49. Figure 4-a shows that the high µ values are 51.51 cm−1, 53.52 cm−1, 55.52 cm−1, and 57.52 cm−1 for samples S1, S2, S3, and S4, respectively, at 0.033 MeV. Then, the µ values obtained at 0.033 MeV were reduced by approximately 85.3% for all samples when the Eγ values are increased to 0.122 MeV. This high reduction is attributed to the PE cross-section. After that, when increasing the Eγ values above 0.122 MeV, the µ decreased by 88.8%, 89.6%, 88.8%, and 88.7%, respectively, S1, S2, S3, and S4. In particular, the reduction in the µ values, for Eγ interval between 0.244 and 2.506 MeV, is due to the CS interaction50.
Based on the measured µ, the µm values were calculated for the fabricated glasses, the results are grouped in Table 4. The µm values reduced in the interval between 11.488–0.041 cm2/g for sample S1, 11.516–0.040 cm2/g for sample S2, 11.542–0.040 cm2/g for sample S3, and 11.567–0.040 cm2/g for sample S4, all when rising the Eγ values from 0.033 to 2.506 MeV. Moreover, Table 4 shows an agreement between the simulated MCNP and XCOM calculated µm values with a difference of less than ± 2% in average.
The reduction in µ values due to the Eγ increase is followed by an increase in the Δ0.5 values, as illustrated in Fig. 5. The Δ0.5 values increased under the effect of PE and CS interactions between 0.0–3.81 cm for sample S1, 0.01–3.69 cm for sample S2, 0.01–3.57 cm for sample S3, and 0.01–3.46 cm for sample S4, with rising Eγ between 0.033–2.506 MeV. As illustrated earlier, the increase in the Eγ values reduces the PE and CS cross-sections, leading to a reduction in the photon-electron interactions51. Therefore, the transmitted photons It increased while the absorbed photons Ia increased, resulting in a reduction in the µ values and an increase in the Δ0.5 values of the fabricated glasses, where µ = 0.693/Δ0.5.
The mode of variation in the µ values for the fabricated samples affects their Δeq values, where the increase in Eγ values decreases the Δeq of the fabricated glasses, as presented in Fig. 6. The reduction in the Δeq values is attributed to the comparable reduction in both µ values for Pb and fabricated glasses. While increasing the Eγ values in the PE interval, the µ values of all tested samples were reduced by approximately 85.3%. Simultaneously, the increase in the Eγ values in the same PE energy interval (0.033 MeV ≤ E ≤ 0.122 MeV) decreases the µ values for lead by 85.8%. The comparable reduction in the µ values for both fabricated glasses and Pb is the main reason behind the exponential reduction in the Δeq values. Additionally, due to the K-absorption of Pb, the Δeq values increased around 0.081 MeV because of the high µ values of lead at this energy. In the PE interval, the Δeq values were reduced by 15.58%, 21.41%, 14.93%, and 13.78% for samples S1, S2, S3, and S4, respectively. Furthermore, the increase in Eγ values above 0.122 MeV (i.e., CS interval) leads to a moderate reduction in the Δeq values. This reduction was achieved due to the moderate reduction in the µ values for both fabricated samples and Pb, obtained by rising the Eγ between 0.244 and 2.506 MeV, where the µ value of lead was reduced by 93.1%, while the µ values were reduced by 88.8%, 89.6%, 88.8%, and 88.7% for samples S1, S2, S3, and S4, respectively. In the CS interaction interval, while rising the Eγ values between 0.244 and 2.506 MeV, the Δeq for S1, S2, S3, and S4 was reduced by 38.28%, 33.37%, 38.16%, and 38.72%, respectively.
The increase in Eγ values is accompanied by an increase in the It photons and a reduction in the Ia photons. Since the It/Io ratio determines the TF value and the Ia/Io determines the RPE values, the TF value increased while the RPE values decreased with rising the Eγ value, as presented in Fig. 7. Due to the PE interaction behavior at low energy, the photon energy was transferred to one electron, and the photon disappeared in the medium, leading to a reduction in the It photons and TF values52. The TF values in the interval between 0.033 and 0.122 MeV are less than 1% for a 1 cm thickness of the fabricated samples S1 and S4. In contrast, the Ia photon number increases and reaches its maximum, leading to an increase in the RPE values, where the RPE values are close to 100% for all samples. Increasing the Eγ values between 0.244 and 2.506 MeV is associated with a high increase in It photons and a reduction in the Ia values due to the CS behavior. Therefore, the TF was highly increased, while the RPE values were reduced with rising Eγ values. For example, increasing the Eγ values between 0.244 and 2.506 MeV increases the TF values of a 1 cm thickness of the fabricated glasses between 19.68–83.37% for sample S1 and 16.91–81.84% for sample S4. On the other hand, the RPE values were reduced by a factor ranging between 80.32–16.63% for sample S1 and 83.09–18.16% for sample S2, when rising the Eγ between 0.244 and 2.506 MeV.
The glass thickness also greatly affects the values of It and Ia which then affect the TF and RPE values. Rising the fabricated glass thickness reduces the TF, while increasing the RPE of the fabricated glasses, as shown in Fig. 8. In fact, the increase in glass thickness increases the pass length of γ-photons, which leads to an increase in the interaction probability between photons and surrounding electrons53. Therefore, the Ia photons increased and the It photons decreased, leading to an increase in the RPE and a reduction in the TF values. For example, increasing the glass thickness from 0.5 to 3 cm increases the RPE values at Eγ of 1.275 MeV between 11.64–52.41% for sample S1 and 12.61–55.47% for sample S4. On the other hand, for the same thickness variation, the TF values decreased by 46.14% and 49.04% for samples S1 and S4, respectively.
Increasing the substitution of B2O3 by BaO compounds increases the Ba/B ratio within the fabricated glasses, which affects the glass density and its molar weight, as presented earlier. The impact of the Ba2+/B3+ ratio on the µ values is illustrated in Fig. 9, where increasing the Ba/B ratio is found to slightly increase the µ values. Increasing the Ba2+/B3+ ratio also increases the electron density and Zeff of the fabricated glasses. Since the interaction cross-section proportion to Zeff increased, the µ values increased as the Ba2+/B3+ increased. Figure 9 shows that the increase in Ba/B ratio from 0.58 to 3.18 is associated with an increase in the µ values by 11.8%, 9.4%, and 8.94%, respectively, at Eγ of 0.122, 0.662, and 1.275 MeV. The impacts of Ba2+/B3+ on the Δ0.5 and Δeq values are opposite to those reported for the µ values. Figure 10 shows a reduction in the Δ0.5 values from 1.69 to 1.55 cm (at Eγ of 0.662 MeV) and from 3.81 to 3.46 cm (at Eγ of 2.506 MeV), when rising the Ba/B ratio between 0.58 and 3.18, respectively. The reduction in the Δ0.5 values is attributed to the reverse proportionality of µ and Δ0.5 values. Also, the Δeq values were reduced, while rising the Ba2+/B3+ ratio, where the Δeq values reduced from 3.04 to 2.78 cm at Eγ of 0.662 MeV and from 2.70 to 2.45 cm at Eγ of 2.506 MeV. The increase in the Ba2+/B3+ ratio increases the Ba2+ ions within the fabricated glasses, which increases the resistance of the material to the transposed photons. Therefore, the number of photon-electron interactions increased, It decreased, and Ia and µ values increased. The increase in µ values of the fabricated glasses compared to the µ values of lead is the main reason for the reduction of Δeq. Furthermore, the increase in Ia photons and the decrease in It photons affect the values of TF and RPE, as illustrated in Fig. 11. The increase in the Ba/B ratio from 0.58 to 3.18 decreases the TF values from 66.41% to 63.90% for Eγ of 0.662 MeV and from 83.37% to 81.84% for Eγ of 2.506 MeV. In comparison, the RPE values increase from 33.59% to 36.10% for Eγ of 0.662 MeV and from 16.63% to 18.16% for Eγ of 2.506 MeV.
To verify the capacity of the developed S1-S4 glasses to block the intermediate gamma rays energies, the µ values for the developed S1-S4 glasses at 0.662 MeV were compared to those reported commercial glasses (RS-520 and RS-360)54 and borate-based glasses for gamma ray shielding applications reported previously in various publications55,56,57,58,59,60, as illustrated in Fig. 12.
The developed glasses µ values at 0.662 MeV are 0.409 cm−1, 0.422 cm−1, 0.435 cm−1, and 0.448 cm−1, for glass samples S1, S2, S3, and S4, respectively. The aforementioned µ values for the developed glasses in the current study are lower than that reported for the commercial shielding glass RS-520 (µ = 0.50 cm−1) at 0.662 MeV. The high µ value for the RS-520 glass sample is attributed to its high content of PbO, which reaches 71 wt.% of its compositions54. Then, the fabricated glasses S1-S4 have µ values higher than that reported for commercial glass RS-360 (µ = 0.32 cm−1), which contains 45 wt.% of PbO in its composition54. Additionally, the developed S1-S4 glass samples have µ values higher than that reported for previously reported glasses SBNP-5 (µ = 0.227 cm−1), SBNP-10 (µ = 0.267 cm−1), SBNP-15 (µ = 0.307 cm−1), SBNP-20 (µ = 0.346 cm−1), SBNP-25 (µ = 0.386 cm−1), BTZ1 (µ = 0.192 cm−1), BTZ2 (µ = 0.191 cm−1), BTZ3 (µ = 0.195 cm−1), BTZ4 (µ = 0.226 cm−1), BTZ5 (µ = 0.302 cm−1), MTB1 (µ = 0.262 cm−1), MTB2 (µ = 0.299 cm−1), MTB3 (µ = 0.327 cm−1), MTB4 (µ = 0.350 cm−1), MoTeB0 (µ = 0.325 cm−1), MoTeB5 (µ = 0.339 cm−1), MoTeB10 (µ = 0.355 cm−1), MoTeB15 (µ = 0.376 cm−1), LiKBTe0 (µ = 0.166 cm−1), LiKBTe10 (µ = 0.192 cm−1), LiKBTe15 (µ = 0.206 cm−1), LiKBTe20 (µ = 0.219 cm−1), BTNKD-Li (µ = 0.228 cm−1), BTNKD-Ca (µ = 0.247 cm−1), BTNKD-Zn (µ = 0.299 cm−1), BTNKD-Sr (µ = 0.253 cm−1), and BTNKD-Ba (µ = 0.270 cm−1)55,56,57,58,59,60. The fabricated glass S1 with the lowest µ values in the current study is close to the µ values for the previously reported glass MTB5 (µ = 0.416 cm−1). The aforementioned MTB5 sample has high concentrations of dense metal oxides TeO2 and BaO, where their ratios reach 70 mol% and 20 mol%, respectively. The comparison shows high shielding capacity for the developed glasses compared to the commercial-based PbO compounds and those glasses reported recently for gamma ray shielding applications. Therefore, the fabricated glasses are suitable candidates for mid-energy gamma-ray shielding applications.
Conclusion
The current study concludes with the efficiency of new BaO-doped lead borosilicate glasses adopted for gamma-ray shielding applications. The effects of partially replacing B3+ with Ba2+ ions on the developed glasses' mechanical, gamma-ray attenuation, and physical characteristics were assessed. The color of the fabricated glasses was turned to dark yellow with increasing the Ba2+/B3+ substitution ratio. Additionally, the density of the fabricated glasses enhanced by 11% from 4.48 to 4.97 g/cm3, increasing the Ba2+/B3+ substitution ratio from 0.58 to 3.18, respectively. The replacement of B3+ ions by Ba2+ ions reduces the mechanical moduli of the developed glasses, where they reduced by 21.84%, 31.61%, 19.61%, and 26.36% for Young, bulk, shear, and longitudinal moduli when the Ba2+/B3+ substitution ratio increased between 0.58 and 3.18. Also, the micro-hardness of the fabricated samples decreased by 8.12% (between 4.49 and 4.12 GPa). The reduction observed in the mechanical properties is attributed to the reduction in the packing density and dissociation energy due to the substitution of B by Ba ions. Additionally, the Monte Carlo simulation proves that the linear attenuation coefficient of the fabricated glasses was enhanced by 48.23%, 11.83%, 9.40%, and 10.13%, rising the Ba/B substitution ratio between 0.58 and 3.18, respectively. The enhancement in the linear attenuation coefficient reduces the half-value thickness and the equivalent thickness for lead. Compared to the shielding capacity of some commercial glasses and borate-based glasses, the developed glasses S1-S4 have suitable radiation shielding properties to be used in nuclear medicine applications at hospitals.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Liu, H., Shi, J., Qu, H. & Ding, D. An investigation on physical, mechanical, leaching and radiation shielding behaviors of barite concrete containing recycled cathode ray tube funnel glass aggregate. Constr. Build. Mater. 201, 818–827 (2019).
Hanfi, M. Y., Sayyed, M. I., Lacomme, E., Akkurt, I. & Mahmoud, K. A. The influence of MgO on the radiation protection and mechanical properties of tellurite glasses. Nucl. Eng. Technol. 53, 2000–2010 (2021).
Saca, N., Radu, L., Fugaru, V., Gheorghe, M. & Petre, I. Composite materials with primary lead slag content: Application in gamma radiation shielding and waste encapsulation fields. J. Clean. Prod. 179, 255–265 (2018).
Alasali, M. I. Sayyed. Studies of gamma radiation attenuation properties of silica based commercial glasses utilized in Jordanian dwellings. Opt. Quantum Electron. 56, 391 (2024).
Zughbi, A., Kharita, M. H. & Shehada, A. M. Determining optical and radiation characteristics of cathode ray tubes’ glass to be reused as radiation shielding glass. Radiat. Phys. Chem. 136, 71–74 (2017).
Elsafi, M. et al. Ecofriendly and radiation shielding properties of newly developed epoxy with waste marble and WO3 nanoparticles. J. Mater. Res. Technol. 22, 269–277 (2023).
Azman, M. N., Abualroos, N. J., Yaacob, K. A. & Zainon, R. Feasibility of nanomaterial tungsten carbide as lead-free nanomaterial-based radiation shielding. Radiat. Phys. Chem. 202, 110492 (2023).
Naseer, K. A. et al. Optical, elastic, and neutron shielding studies of Nb2O5 varied Dy3+ doped barium-borate glasses. Optik (Stuttg) 251, 168436 (2022).
Sopapan, P., Laopaiboon, J., Jaiboon, O., Yenchai, C. & Laopaiboon, R. Feasibility study of recycled CRT glass on elastic and radiation shielding properties used as x-ray and gamma-ray shielding materials. Progress Nucl. Energy 119, 103149 (2020).
Chen, Q. et al. Influence of modifier oxide on the structural and radiation shielding features of Sm3+-doped calcium telluro-fluoroborate glass systems. J. Aust. Ceram. Soc. 57, 275–286 (2021).
Choi, Y. S. & Lee, S. M. Fundamental properties and radioactivity shielding performance of concrete recycled cathode ray tube waste glasses and electric arc furnace slag as aggregates. Progress Nuclear Energy 133, 103649 (2021).
Agrawal, V. et al. Green conversion of hazardous red mud into diagnostic X-ray shielding tiles. J. Hazard. Mater. 424, 127507 (2022).
Sallam, O. I., Madbouly, A. M. & Ezz-Eldin, F. M. Impact of Nd3+ additive on the radiation shielding competence of borosilicate glasses fabricated from agro-waste materials. J. Non Cryst. Solids 590, 121691 (2022).
Kanagaraj, B., Anand, N., Diana Andrushia, A. & Naser, M. Z. Recent developments of radiation shielding concrete in nuclear and radioactive waste storage facilities: A state of the art review. Constr. Build. Mater. 404, 133260 (2023).
Gao, X. et al. Performance of fly ash-based geopolymer mortars with waste cathode ray tubes glass fine aggregate: A comparative study with cement mortars. Constr. Build. Mater. 344, 128243 (2022).
Saleh, A., Shalaby, R. M. & Abdelhakim, N. A. Comprehensive study on structure, mechanical and nuclear shielding properties of lead free Sn–Zn–Bi alloys as a powerful radiation and neutron shielding material. Radiat. Phys. Chem. 195, 110065 (2022).
Al-Buriahi, M. S., Rashad, M., Alalawi, A. & Sayyed, M. I. Effect of Bi2O3 on mechanical features and radiation shielding properties of boro-tellurite glass system. Ceram. Int. 46, 16452–16458 (2020).
Aşkın, A. et al. Investigation of the gamma ray shielding parameters of (100-x)[0.5 Li2O–0.1 B2O3–0.4 P2O5]-xTeO2 glasses using Geant4 and FLUKA codes. J. Non-Cryst. Solids 521, 119489 (2019).
Shahboub, A., El Damrawi, G. & Saleh, A. A new focus on the role of iron oxide in enhancing the structure and shielding properties of Ag2O–P2O5 glasses. Eur. Phys. J. Plus 136, 947 (2021).
Acikgoz, A. et al. Structural, mechanical, radiation shielding properties and albedo parameters of alumina borate glasses: Role of CeO2 and Er2O3. Mater. Sci. Eng. B 276, 115519 (2022).
Chaiphaksa, W., Borisut, P., Chanthima, N., Kaewkhao, J. & Sanwaranatee, N. W. Mathematical calculation of gamma rays interaction in bismuth gadolinium silicate glass using WinXCom program. Mater. Today Proc. 65, 2412–2415 (2022).
Adib, M. et al. Neutron characteristics of single-crystal magnesium fluoride. Ann. Nucl. Energy 60, 163–171 (2013).
Pawar, P., Ballav, R. & Kumar, A. Review on material removal technology of soda-lime glass material. Indian J. Sci. Technol. 10, 1–7 (2017).
Mhareb, M. H. A. et al. Morphological, optical, structural, mechanical, and radiation-shielding properties of borosilicate glass–ceramic system. Ceram. Int. 48, 35227–35236 (2022).
Day, D. E. & Rindone, G. E. Properties of soda aluminosilicate glasses: coordination of aluminum ions. J. Am. Ceram. Soc. 45, 579–581 (1962).
Day, D. E. & Rindone, G. E. Properties of soda aluminosilicate glasses: I, refractive index, density, molar refractivity, and infrared absorption spectra. J. Am. Ceram. Soc. 45, 489–496 (1962).
Saleh, A. Comparative shielding features for X/Gamma-rays, fast and thermal neutrons of some gadolinium silicoborate glasses. Progress Nucl. Energy 154, 104482 (2022).
Bagheri, R., Khorrami Moghaddam, A. & Yousefnia, H. Gamma ray shielding study of Barium–Bismuth–Borosilicate glasses as transparent shielding materials using MCNP-4C Code, XCOM program, and available experimental data. Nucl. Eng. Technol. 49, 216–223 (2017).
Wahab, E. A. A. et al. Novel borosilicate glass system: Na2B4O7-SiO2-MnO2: Synthesis, average electronics polarizability, optical basicity, and gamma-ray shielding features. J. Non Cryst. Solids 553, 120509 (2021).
Aktas, B. et al. The role of TeO2 insertion on the radiation shielding, structural and physical properties of borosilicate glasses. J. Nucl. Mater. 563, 153619 (2022).
Cheewasukhanont, W., Limkitjaroenporn, P., Kothan, S., Kedkaew, C. & Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 172, 108791 (2020).
Chanthima, N. & Kaewkhao, J. Annals of Nuclear Energy Investigation on radiation shielding parameters of bismuth borosilicate glass from 1 keV to 100 GeV. Ann. Nucl. Energy 55, 23–28 (2013).
Ruengsri, S., Insiripong, S., Sangwaranatee, N. & Kaewkhao, J. Development of barium borosilicate glasses for radiation shielding materials using rice husk ash as a silica source. Progress Nucl. Energy 83, 99–104 (2015).
Aktas, B., Yalcin, S., Dogru, K., Uzunoglu, Z. & Yilmaz, D. Structural and radiation shielding properties of chromium oxide doped borosilicate glass. Radiat. Phys. Chem. 156, 144–149 (2019).
Mhareb, M. H. A. Optical, structural, radiation shielding, and mechanical properties for borosilicate glass and glass ceramics doped with Gd2O3. Ceram. Int. 49, 36950–36961 (2023).
Bassam, S. A. et al. Physical, structural, elastic and optical investigations on Dy3+ ions doped boro-tellurite glasses for radiation attenuation application. Radiat. Phys. Chem. 206, 110798 (2023).
Makishima, A. & Mackenzie, J. D. Direct calculation of Young’s moidulus of glass. J. Non Cryst. Solids 12, 35–45 (1973).
Makishima, A. & Mackenzie, J. D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non Cryst. Solids 17, 147–157 (1975).
Abd El-Moneim, A. & Alfifi, H. Y. Approach to dissociation energy and elastic properties of vanadate and V2O5-contained glasses from single bond strength: Part I. Mater. Chem. Phys. 207, 271–281 (2018).
X-5 Monte Carlo Team. MCNP—A General Monte Carlo N-Particle Transport Code, Version 5. La-Ur-03-1987 II (2003).
Ekinci, N. et al. Synthesis, physical properties, and gamma–ray shielding capacity of different Ni-based super alloys. Radiat. Phys. Chem. 186, 109483 (2021).
Hannachi, E., Sayyed, M. I., Slimani, Y. & Mahmoud, K. G. Synthesis of pristine CaZrO3 and CaZrO3/Pr6O11 ceramic samples and assessment of their radiation protection features. J. Phys. Chem. Solids 181, 111498 (2023).
Alhindawy, I. G., Gamal, H., Almuqrin, A. H., Sayyed, M. I. & Mahmoud, K. A. Impacts of the calcination temperature on the structural and radiation shielding properties of the NASICON compound synthesized from zircon minerals. Nucl. Eng. Technol. 55, 1885–1891 (2023).
Mahmoud, K. A., Sayyed, M. I., Almuqrin, A. H., Elhelaly, M. A. & Alhindawy, I. G. Synthesis of glass powders for radiation shielding applications based on zirconium minerals’ leach liquor. Radiat. Phys. Chem. 207, 110867 (2023).
Berger, M.J., Hubbell, J.H., Seltzer, S.M., Chang, J., Coursey, J.S., Sukumar, R., Z. & D.S., K. O. XCOM:Photon Cross Section Database. Natl. Inst. Stand. Technol., Gaithersburg, MD. https://doi.org/10.18434/T48G6X (2010).
Hashim, S. et al. Luminescence features of dysprosium and phosphorus oxide co-doped lithium magnesium borate glass. Radiat. Phys. Chem. 137, 45–48 (2017).
Razak, N. A. et al. Impact of Eu3+ ions on physical and optical properties of Li2O-Na2O-B2O3 glass. Chin. J. Chem. Phys. 29, 395–400 (2016).
Al-Saeedi, F. H. F. et al. A novel barium oxide-based Iraqi sand glass to attenuate the low gamma-ray energies: Fabrication, mechanical, and radiation protection capacity evaluation. Nucl. Eng. Technol. 54, 3051–3058 (2022).
Fornalski, K. W. Simple empirical correction functions to cross sections of the photoelectric effect, Compton scattering, pair and triplet production for carbon radiation shields for intermediate and high photon energies. J. Phys. Commun. 2, 035038 (2018).
Tashlykov, O. L. et al. Tailor made barium borate doped Bi2O3 glass system for radiological protection. Radiat. Phys. Chem. 187, 109558 (2021).
Abdelghany, A. M., Diab, H. M., Madbouly, A. M. & Ezz-ElDin, F. M. Inspection of radiation shielding proficiency and effect of gamma-ray on ESR and thermal characteristics of copper oxide modified borate bioglasses. J. Inorg. Organomet. Polym. Mater. 32, 3204–3219 (2022).
Marashdeh, M. W. & Mahmoud, K. A. CaO-enhanced polyester for safety: experimental study on fabrication, characterization, and gamma-ray attenuation. Radiochim. Acta 0 (2024).
Al-Hadeethi, Y., Sayyed, M. I., Barasheed, A. Z., Ahmed, M. & Elsafi, M. Fabrication of lead free borate glasses modified by bismuth oxide for gamma ray protection applications. Materials 15, 789 (2022).
SCHOTT AG. Radiation Shielding Glasses. www.schott.com/advanced_optics (2013).
Sayyed, M. I., Al-Ma’abreh, A. M., Imheidat, M. A., Aldajah, S. O. & Mahmoud, K. A. Enhancement of borosilicate glass’s radiation shielding properties: Impacts of PbO substitution for SiO2. Silicon https://doi.org/10.1007/s12633-023-02766-z (2023).
Abdelghany, Y. A., Kassab, M. M., Radwan, M. M. & Abdel-Latif, M. A. Borotellurite glass system doped with ZrO2, potential use for radiation shielding. Progress Nucl. Energy 149, 104256 (2022).
Al-Ghamdi, H., Almuqrin, A. H., Sayyed, M. I. & Kumar, A. The physical, structural and the gamma ray shielding effectiveness of the novel Li2O–K2O–B2O3–TeO2 glasses. Results Phys. 29, 104726 (2021).
Evangelin Teresa, P., Naseer, K. A., Marimuthu, K., Alavian, H. & Sayyed, M. I. Influence of modifiers on the physical, structural, elastic and radiation shielding competence of Dy3+ ions doped Alkali boro-tellurite glasses. Radiat. Phys. Chem. 189, 109741 (2021).
Sayyed, M. I. et al. Effect of TeO2 addition on the gamma radiation shielding competence and mechanical properties of boro-tellurite glass: An experimental approach. J. Mater. Res. Technol. 18, 1017–1027 (2022).
Sayyed, M. I. et al. Gamma radiation shielding and structural features for barium strontium boro-tellurite glass modified with various concentrations of molybdenum oxide. J. Non Cryst. Solids 559, 120658 (2021).
Author information
Authors and Affiliations
Contributions
Formal analysis and software; K.A.M., M.I.S., Analysis review; Y.M.; K.A.M.; J.A.; M.I.S.; Supervision; M.I.S., Writing—original draft; K.A.M.; Writing—review and editing; M.H.A.M., J.A., Y.M., K.A.M. All Authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayyed, M.I., Mahmoud, K.A., Arayro, J. et al. An extensive assessment of the impacts of BaO on the mechanical and gamma-ray attenuation properties of lead borosilicate glass. Sci Rep 14, 5429 (2024). https://doi.org/10.1038/s41598-024-56040-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56040-2
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.