Abstract
It is well known that vitamin D plays a pivotal role in immune system modulation; however, its role in liver transplantation (LT) has not yet been well elucidated. This study aimed to assess the association between vitamin D status and LT outcomes. This retrospective cohort study was conducted on 335 registered cirrhotic patients with end-stage liver disease (ESLD) who underwent LT during 2019–2021 and had measurement of serum vitamin D before LT. The association of vitamin D levels before LT with the odds of acute cellular rejection (ACR) and risk mortality was assessed by applying logistic and cox regression, respectively. The mean MELD-Na and serum level of vitamin D were 20.39 ± 9.36 and 21.52 ± 15.28 ng/ml, respectively. In the final adjusted model, there was a significant association between vitamin D deficiency in the pre-transplant period and odds of ACR (odds ratio [OR] 2.69; 95% confidence interval [CI] 1.50–4.68). Although in the crude model, vitamin D deficiency in the pre-transplant period was significantly associated with an increased risk of mortality after two years of follow-up (Hazard ratio (HR) = 2.64, 95% CI 1.42–4.33), after adjustment for potential confounders, the association of vitamin D status and mortality became non-significant (HR = 1.46, 95% CI 0.71–3.00). The present study provides evidence that pre-transplant serum vitamin D levels may be a predictor for ACR in patients with cirrhosis undergoing LT.
Similar content being viewed by others
Introduction
Cirrhosis is the terminal stage of chronic liver disease, characterized by chronic inflammation and progressive fibrosis. This condition is primarily caused by chronic alcoholism, metabolic-associated fatty liver disease, and viral hepatitis1. In the United States, cirrhosis is the ninth leading cause of death, accounting for 1.2% of all deaths2. Globally, the incidence of liver cirrhosis increased by 16.7% from 2009 to 2019, rising from 1.8 million to 2.1 million cases3. End-stage liver disease (ESLD) is a culmination of chronic liver disease progressing to decompensated cirrhosis and hepatocellular failure1. These patients may benefit from liver transplantation (LT), which can provide a cure and improved long-term survival outlook4. However, it is often accompanied by acute cellular rejection (ACR), which occurs in 30–70% of cases5,6,7,8. LT outcomes may be affected by a number of factors, such as liver enzymes, cytomegalovirus (CMV) infections, biochemical parameters such as serum vitamin D levels, and dietary factors9,10,11,12.
Vitamin D plays a crucial role in bone metabolism13, gene expression in various tissues14, and calcium absorption in the gut15. Aside from that, there is some evidence that vitamin D may benefit individuals suffering from liver diseases due to its anti-inflammatory and immunomodulatory properties16,17,18,19. Interestingly, the prevalence of vitamin D insufficiency is extremely high among patients with ESLD who are eligible for transplantation, with up to 93% of these patients suffering from some degree of insufficiency20,21.
Furthermore, several studies have shown that vitamin D can reduce the risk of ACR in solid organ transplants such as kidneys and lungs22,23. Consequently, vitamin D may contribute to improved outcomes in LT from a nutritional perspective11.
To the best of our knowledge, few studies have investigated the association between pre-transplant vitamin D and ACR or mortality in liver transplant recipients. In the present study, we aimed to examine the relationship between vitamin D status and ACR and mortality in liver transplant recipients. By examining this relationship, we hope to develop a better understanding of the role of vitamin D and its serum level correction in the successful management of LT.
Materials and methods
Study population
This retrospective cohort study examined the registration information of individuals with cirrhosis who underwent LT between 2019 and 2021 at Taleghani Hospital (Tehran) and Abu-Ali Sina Hospital (Shiraz). Patients with ESLD with complete lab results for vitamin D and other routine laboratory testing were eligible to enter the study. On the other hand, certain groups of patients were excluded from the study. These included individuals with acute liver failure, those who had undergone multiple organ transplants, and people who had experienced primary graft non-function (PNF). By setting these criteria, the study aimed to ensure that the results were obtained from a homogeneous group of individuals with similar characteristics to enhance the validity of the findings. Finally, three hundred thirty-five cases were considered for the follow-up in this study (Table 1). This study was conducted in accordance with the Declaration of Helsinki, and the ethics committee of National Nutrition and Food Technology Research Institute approved the protocol.
Data collection
First, demographic and clinical pathophysiology data of the patients were obtained, including age, gender, body mass index (BMI), cause of cirrhosis, medical conditions at the time of LT, history of diabetes/hypertension, smoking, Alcohol consumption, and the waiting time it took for the liver transplant to occur.
Additionally, other variables, including lab results, such as alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), fasting blood sugar, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), white blood cells (WBC), and polymorphonuclear neutrophils (PMN) were collected based on the last laboratory result prior to LT. The final step was to collect data regarding ACR, mortality, and CMV infection after LT. Since this study was conducted at two centers, it is worth noting that the measurement methods were consistent in both centers. Moreover, after the transplant, the patients usually received a combination of antirejection medications, which include tacrolimus (0.02–0.03 mg/kg/day), mycophenolate mofetil (1–2 g/day, up to 3 g/day), and prednisolone (up to 20 mg/day).
Vitamin D measurement
25-hydroxyvitamin D levels (25(OH)D) were measured along with other laboratory tests before LT. In this study, serum levels of vitamin D under 20 ng/ml were considered deficient, and ≥ 20 ng/ml as sufficient. For vitamin D, we used the most recent lab results available before LT, from which we also obtained other lab results.
MELD and MELD-Na calculation
The Model for End-Stage Liver Disease (MELD) scores were calculated using the following formulas based on the last lab results before LT for bilirubin, creatinine, INR, and Na24. Serum sodium value was corrected in the range of 125–137 mEq/l, according to criteria determined by UNOS (United Network for Organ Sharing).
Outcomes identification
In clinical practice, ACR after LT is associated with allograft dysfunction, which is concurrently diagnosed by liver biopsy and histological analysis25. Accordingly, when there was suspicion that there may be an ACR reaction, a biopsy was performed in this study. Mortality was also monitored for two years following LT or until death occurred. In addition, CMV infection was detected and diagnosed by CMV antigen-positive peripheral leukocytes.
Statistical analysis
After providing the normality of the distribution of the studied variables by Kolmogorov–Smirnov test, independent sample T-Test used to compare quantitative variables between the two groups, as well as Chi-square or fisher exact statistical test was also used for qualitative variables. The baseline characteristics were reported as mean ± standard deviation (SD) for quantitative variables, and number and percentages for qualitative variables. Regression model was used to examine the correlation between variables. The association of vitamin D levels before LT with the odds of ACR and risk of mortality was assessed by applying logistic and cox regression, respectively. The analyses were adjusted for probable confounders, e.g., age, sex, BMI, waiting time for transplantation, medical condition at the time of LT, causes of cirrhosis, smoking, alcohol, hypertension, diabetes mellitus, infection of CMV, MELD-Na, AST, ALT, PMN, WBC, and lymphocytes. All analyses were performed by SPSS 25.0 statistical software, and P-value less than 0.05 was considered statistically significant.
Ethical approval
This study was approved by the research ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. We confirm that all methods were performed in accordance with the relevant guidelines and regulations. Also, informed consent was obtained and signed from all patients.
Results
The mean ± SD age of the study population was 40.22 ± 19.14 years. The mean ± SD BMI was 23.36 ± 4.84 kg/m2. About 62% of the participants were male, and the rest were female. The mean ± SD MELD-Na and serum vitamin D levels were 20.39 ± 9.36 and 21.52 ± 15.28 ng/ml, respectively. The cause of cirrhosis was mainly primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) (n = 88, 26.3%), nonalcoholic steatohepatitis (NASH) (n = 48, 14.3%), and viral or autoimmune hepatitis (AIH) (both causes: n = 46, 13.7%). The mean waiting time was 4.38 months. In addition, about 19% of the samples were infected with CMV after transplantation, and the prevalence of diabetes and hypertension in the study population was 22.7% and 7.5%, respectively (Table 1).
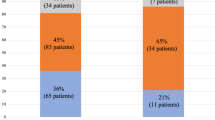
The baseline characteristics of the patients between two groups of subjects with sufficient and deficient levels of vitamin D in the pre-transplant stage are shown in Table 2. Compared to those with sufficient vitamin D levels, those with vitamin D deficiency had higher MELD-Na scores and waiting time for LT but lower albumin levels. In addition, the number of patients with CMV infection after LT was significantly higher in those with vitamin D deficiency. However, no significant differences were found for other characteristics between subjects with sufficient and deficient levels of vitamin D.
Characteristics of patients based on ACR and mortality status after liver transplantation are also indicated in Tables 3 and 4, respectively.
A significant difference was observed between the percentage of patients with hypertension and medical conditions at the time of LT between two groups of patients with and without ACR after LT. Furthermore, the findings of Table 4 showed that age, MELD-Na score, waiting time for LT, FBS, and WBC levels were significantly higher in patients with post-transplant mortality than the other group. However, albumin levels and the percentage of lymphocytes were lower.
The ORs (95% CIs) for ACR and HRs (95% CIs) for mortality after LT based on the pre-transplantation serum vitamin D level are reported in Tables 5 and 6, respectively. In the crude and first adjusted model (based on age, sex, and BMI), there was a significant association between vitamin D deficiency in the pre-transplant time and odds of ACR (odds ratio [OR] = 2.71, 95% confidence interval [CI] 1.61–4.29, P for trend < 0.001; OR = 2.68, 95% CI 1.63–4.39, P for trend < 0.001, respectively). Furthermore, in model 2, after adjusting for further confounders (waiting time for transplantation, medical condition at the time of LT, causes of cirrhosis, smoke, alcohol, hypertension, diabetes mellitus, and infection of CMV) and final model (further adjustment for MELD-Na, AST, ALT, PMN, WBC, and lymphocytes), vitamin D deficiency was associated with 2.68- (OR: 2.68; 95% CI 1.59–4.71, P for trend = 0.001) and 2.69-fold (OR: 2.69; 95% CI 1.50–4.68, P for trend = 0.001) increase in the odds of ACR, respectively.
Although in the crude and first adjusted model, vitamin D deficiency in the pre-transplant stage was significantly associated with an increase in the risk of mortality after two years follow-up in cirrhotic patients undergoing transplantation (HR = 2.64, 95% CI 1.42–4.33; P for trend = 0.022; HR = 2.02, 95% CI 1.04–3.93, P for trend = 0.038, respectively), in the second and final adjusted model, no significant relationship was observed (HR = 1.63, 95% CI 0.80–3.31, P for trend = 0.177; HR = 1.46, 95% CI 0.71–3.00; P for trend = 0.309, respectively).
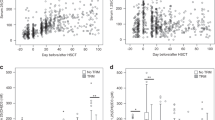
The relationship between the MELD-Na score and serum vitamin D levels in the pre-transplantation period is demonstrated in Fig. 1. The findings suggested an inverse relationship between these two. The correlation coefficient r was − 0.157 (P < 0.001; 95% CI − 0.210, − 0.104).
Discussion
The current retrospective cohort study investigated the association between vitamin D deficiency prior to LT with ACR and mortality in liver transplant recipients. The study's findings indicated a significant correlation between vitamin D deficiency and increased odds of ACR after LT. Our finding is consistent with the investigation conducted by Doi et al., which demonstrated an association between vitamin D deficiency and a greater incidence of ACR in the short-term following LT compared to individuals with adequate vitamin D levels11. Zhou et al. also reported that a high 25(OH)D level (> 25 ng/mL) in the pre-transplant period is significantly associated with a lower ACR within 30 days following LT17. Furthermore, another study by Bitetto et al. corroborated these findings, establishing an independent association between low serum 25-hydroxyvitamin D levels (< 5 ng/mL) and the occurrence of moderate to severe ACR episodes within two months following LT26. Generally, these findings suggest that maintaining adequate levels of vitamin D during the pre-transplantation period plays a pivotal role in minimizing the risk of ACR following transplantation. Aside from this, some research has shown that vitamin D supplementation is associated with a lower risk of ACR11,17,27. According to a study by Grant et al., supplementation with cholecalciferol (vitamin D3) for 12 weeks after LT increased vitamin D levels and reduced ACR and infection rates27. Hence, vitamin D deficiency can potentially affect ACR, and its correlation cannot only explained by deterioration in liver function.
Additionally, we found that patients with vitamin D deficiency had higher scores on the MELD-Na than those with sufficient levels of vitamin D. This negative correlation has also been reported by Doi et al.11. MELD-Na/MELD scores are widely used to assess liver disease severity and predict mortality in individuals with cirrhosis28. Thus, vitamin D deficiency may be associated with the severity of cirrhosis and adversely affect LT outcomes. Nevertheless, it should be noted that malnutrition is common among patients with ESLD29, and it is well documented that malnourished ESLD patients exhibit a high prevalence of vitamin D deficiency20,21,30. Malnutrition per se can have a negative effect on survival31,32. Hence, serum 25-hydroxyvitamin D levels may function as a prognostic marker for morbidity and mortality.
Our retrospective cohort study also demonstrated a significant correlation between pre-transplantation vitamin D deficiency and an elevated mortality risk after transplantation in both the crude and first-adjusted models. Nevertheless, this association was not statistically significant in the second and final adjusted models, suggesting that other potential confounding variables may impact the relationship between vitamin D deficiency and mortality in cirrhotic patients undergoing transplantation. Similarly, Doi et al. reported no significant disparity in overall survival between individuals with vitamin D deficiency and those with sufficient vitamin D levels before LT. However, a notable discrepancy was observed in post-transplantation periods, suggesting that vitamin D supplementation or maintaining adequate vitamin D levels following transplantation may be of benefit11. In addition, it has been shown that vitamin D supplementation during the first month after LT is associated with a better survival rate than the control group17.
Several mechanisms may explain the protective effect of vitamin D, including decreased production of inflammatory mediators such as interleukin (IL)-2, IL-17, and interferon-gamma (IFN-γ)33,34,35, decreased dendritic cells (DCs) maturation36, and an increase in Treg cell immunoprotective activity37. Treg are immune-suppressive cells that suppress the immune system, maintain self-tolerance, and prevent autoimmune diseases38. Vitamin D has been shown to manipulate monocytes and dendritic cells at different levels, allowing these cells to exert tolerogenic effects39. Additionally, vitamin D can inhibit macrophage transition to the M1 phenotype and promote macrophages with M2 phenotypes40. M1 macrophages secrete inflammatory cytokines that hinder cell proliferation, potentially resulting in tissue damage, while M2 macrophages facilitate cell proliferation and tissue regeneration41. Furthermore, vitamin D is thought to inhibit the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway and cyclooxygenase (COX)-2 transcription42, leading to a reduction in reactive oxidative species (ROS) that can cause oxidative stress43, DNA damage, and cellular death.
Moreover, research has suggested that bacterial infections may serve as a possible trigger for transplant organ rejection44. Vitamin D is known to preserve the integrity of the intestinal barrier by upholding the expression of immune cells within the tight junction, which is crucial for impeding bacterial invasions45. Besides, vitamin D receptors are also present within the gut barrier, promoting the production of antimicrobial peptides (AMPs)46 and Claudin-5 expression45. The AMPs play a critical role in protecting against infection and innate immunity47. In addition to their broad-spectrum antimicrobial activity, these peptides possess diverse mechanisms of action and regulate the composition of the gut microbiome48. Claudin-5, a tight junction protein, plays a key role in maintaining the integrity of the bowel's physical barrier, and its disruption is directly related to intestinal inflammation49.
The present retrospective cohort study was conducted in two referral centers, which is a notable strength of the study. Additionally, the study had a relatively large sample size and included a comprehensive assessment of potential confounders while adjusting for multiple models, which further strengthens our findings. However, it is important to consider several limitations when interpreting the results. First, the study's retrospective design limits its ability to establish a causal relationship between vitamin D deficiency and ACR or mortality. Second, the study did not consider the effects of vitamin D supplementation, which may have affected the findings. Third, the characteristics of the donors were not included in the analysis, which could have impacted the results. Finally, we were not able to include immunosuppressant doses, which are used after transplantation, in our analysis.
Conclusion
The present study provides evidence that pre-transplant serum vitamin D levels may predict ACR in patients with cirrhosis undergoing LT. The findings of this study may have important implications for clinical practice, and further research is needed to fully understand the potential benefits and risks of vitamin D supplementation in these patients.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Philips, C. A. & Kedarisetty, C. K. Palliative care for patients with end-stage liver disease. J. Clin. Exp. Hepatol. 13(2), 319–328 (2023).
Swindell, W. R. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genom. 10(1), 585 (2009).
Lan, Y. et al. The burden of liver cirrhosis and underlying etiologies: Results from the Global Burden of Disease Study 2019. Hepatol. Commun. 7(2), e0026 (2023).
Duffy, J. P. et al. Long-term patient outcome and quality of life after liver transplantation: Analysis of 20-year survivors. Ann. Surg. 252(4), 652–661 (2010).
VanBuskirk, A. M., Pidwell, D. J., Adams, P. W. & Orosz, C. G. Transplantation immunology. Jama 278(22), 1993–1999 (1997).
Liu, L. U. et al. Marked Differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation 80(8), 1072–1080 (2005).
Ikegami, T. et al. A high MELD score, combined with the presence of hepatitis C, is associated with a poor prognosis in living donor liver transplantation. Surg. Today 44(2), 233–240 (2014).
Wiesner, R. H., Ludwig, J., Krom, R. A., Hay, J. E. & van Hoek, B. Hepatic allograft rejection: New developments in terminology, diagnosis, prevention, and treatment. Mayo Clin. Proc. 68(1), 69–79 (1993).
Lizaola-Mayo, B. C. & Rodriguez, E. A. Cytomegalovirus infection after liver transplantation. World J Transpl. 10(7), 183–190 (2020).
Chen, X. B. & Xu, M. Q. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreatic Dis. Int. 13(2), 125–137 (2014).
Doi, J. et al. Nutrition support in liver transplantation and postoperative recovery: The effects of vitamin D level and vitamin D supplementation in liver transplantation. Nutrients 12(12), 3677 (2020).
Rashidi-Alavijeh, J. et al. Primary sclerosing cholangitis as an independent risk factor for cytomegalovirus infection after liver transplant. Transpl. Infect. Dis. 23(3), e13553 (2021).
Holick, M. F. Vitamin D and bone health. J. Nutr. 126(4 Suppl), 1159S-S1164 (1996).
Dimitrov, V. et al. Vitamin D-regulated gene expression profiles: Species-specificity and cell-specific effects on metabolism and immunity. Endocrinology 162(2), bqaa218 (2021).
Christakos, S., Dhawan, P., Porta, A., Mady, L. J. & Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 347(1), 25–29 (2011).
Kubesch, A. et al. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PloS one 13(11), e0207162 (2018).
Zhou, Q. et al. Vitamin D supplementation could reduce the risk of acute cellular rejection and infection in vitamin D deficient liver allograft recipients. Int. Immunopharmacol. 75, 105811 (2019).
Lange, C. M. et al. Vitamin D receptor and Jak–STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-α. J. Immunol. 192(12), 6037–6044 (2014).
Saleh, M. et al. Differential modulation of hepatitis C virus replication and innate immune pathways by synthetic calcitriol-analogs. J. Steroid Biochem. Mol. Biol. 183, 142–151 (2018).
Arteh, J., Narra, S. & Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Dig. Dis. Sci. 55, 2624–2628 (2010).
Fisher, L. & Fisher, A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin. Gastroenterol. Hepatol. 5(4), 513–520 (2007).
Lee, J. R. et al. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation 98(3), 292 (2014).
Lowery, E. M. et al. Low vitamin D levels are associated with increased rejection and infections after lung transplantation. J. Heart Lung Transpl. 31(7), 700–707 (2012).
Freitas, A. C. T., Rampim, A. T., Nunes, C. P. & Coelho, J. C. U. Impact of meld sodium on liver transplantation waiting list. Arquivos brasileiros de cirurgia digestive. ABCD 32(3), e1460 (2019).
Krenzien, F. et al. Diagnostic biomarkers to diagnose acute allograft rejection after liver transplantation: Systematic review and meta-analysis of diagnostic accuracy studies. Front. Immunol. 10, 758 (2019).
Bitetto, D. et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int 30(3), 417–444 (2010).
Grant, C. A vitamin D protocol post-liver transplantation. J. Am. Assoc. Nurse Pract. 29(11), 658–666 (2017).
Kamath, P. S. et al. A model to predict survival in patients with end–stage liver disease. Hepatology 33(2), 464–470 (2001).
Traub, J., Reiss, L., Aliwa, B. & Stadlbauer, V. Malnutrition in patients with liver cirrhosis. Nutrients 13(2), 540 (2021).
Lange, C. M. et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 54(5), 887–893 (2011).
Mazurak, V. C., Tandon, P. & Montano-Loza, A. J. Nutrition and the transplant candidate. Liver Transpl. 23(11), 1451–1464 (2017).
Englesbe, M. J. et al. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg. 211, 271–278 (2010).
Palmer, M. T. et al. Lineage-specific effects of 1, 25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 286, 997–1004 (2011).
Reichel, H., Koeffler, H. P., Tobler, A. & Norman, A. W. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 84(10), 3385–3389 (1987).
Colin, E. M. et al. 1, 25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 62, 132–142 (2010).
Ferreira, G. B. et al. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 27(8), 933–941 (2011).
Fisher, S.A.-O. et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PloS one. 14, e0222313 (2019).
Sakaguchi, S. Taking regulatory T cells into medicine. J. Exp. Med. 218(6), e20210831 (2021).
Adorini, L. & Penna, G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb. Exp. Pharmacol. 188, 251–73 (2009).
Zhang, X.A.-O. et al. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J. Cell. Physiol. 234, 6917–6926 (2019).
Arora, S., Dev, K., Agarwal, B., Das, P. & Syed, M. A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 223(4–5), 383–396 (2018).
Wang, Q. et al. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 289, 11681–11694 (2014).
Fantini, C., Corinaldesi, C., Lenzi, A., Migliaccio, S. & Crescioli, C. Vitamin D as a shield against aging. Int. J. Mol. Sci. 24(5), 4546 (2023).
Mori, D. N., Kreisel, D., Fullerton, J. N., Gilroy, D. W. & Goldstein, D. R. Inflammatory triggers of acute rejection of organ allografts. Immunol. Rev. 258, 132–144 (2014).
Zhang, Y., Garrett, S., Carroll, R. E., Xia, Y. & Sun, J. Vitamin D receptor upregulates tight junction protein claudin-5 against colitis-associated tumorigenesis. Mucosal immunol. 15, 683–697 (2022).
White, J. H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 14(2), 284 (2022).
Zhang, Q.-Y. et al. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 8(1), 48 (2021).
Gubatan, J. et al. Antimicrobial peptides and the gut microbiome in inflammatory bowel disease. World J. Gastroenterol. 27, 7402–7422 (2021).
Lu, Z., Ding, L., Lu, Q. & Chen, Y. H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 1, e24978 (2013).
Author information
Authors and Affiliations
Contributions
D.F., A.H., and MH.S., contributed to the conception and design and wrote the manuscript. D.F., H.S., M.S., H.N., MA.J., K.J., SS.A., SA.F., M.A., H.E., AH.R., N.B., A.J., and A.H. were responsible for the literature search and data collection. M.HS. was responsible for data analysis and interpretation of data. D.F, Z.Y., and G.MM. contributed to revision. A.H. supervised the study and contributed to the conception, design, statistical analyses, data interpretation, and drafting of the article. Final approval of the article before submission was performed by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fotros, D., Sohouli, M., Yari, Z. et al. Vitamin D status as a predictor for liver transplant outcomes. Sci Rep 13, 21018 (2023). https://doi.org/10.1038/s41598-023-48496-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48496-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.