Abstract
Diet modulates the genetic risk of obesity, but the modulation has been rarely studied using genetic risk scores (GRSs) in children. Our objectives were to identify single nucleotide polymorphisms (SNPs) that drive the interaction of specific foods with obesity and combine these into GRSs. Genetic and food frequency data from Finnish Health in Teens study was utilized. In total, 1142 11-year-old subjects were genotyped on the Metabochip array. BMI-GRS with 30 well-known SNPs was computed and the interaction of individual SNPs with food items and their summary dietary scores were examined in relation to age- and sex-specific BMI z-score (BMIz). The whole BMI-GRS interacted with several foods on BMIz. We identified 7–11 SNPs responsible for each interaction and these were combined into food-specific GRS. The most predominant interaction was witnessed for pizza (p < 0.001): the effect on BMIz was b − 0.130 (95% CI − 0.23; − 0.031) in those with low-risk, and 0.153 (95% CI 0.072; 0.234) in high-risk. Corresponding, but weaker interactions were verified for sweets and chocolate, sugary juice drink, and hamburger and hotdog. In total 5 SNPs close to genes NEGR1, SEC16B, TMEM18, GNPDA2, and FTO were shared between these interactions. Our results suggested that children genetically prone to obesity showed a stronger association of unhealthy foods with BMIz than those with lower genetic susceptibility. Shared SNPs of the interactions suggest common differences in metabolic gene-diet interactions, which warrants further investigation.
Similar content being viewed by others
Introduction
Obesity is a complex condition resulting from the influence of several common genetic factors in conjunction with various environmental and social factors1. Several candidate gene investigations1 as well as many recent genome-wide association studies (GWAS) have identified hundreds of single nucleotide polymorphisms (SNPs) associated with the susceptibility to obesity2,3,4,5. However, the genetic variants identified by GWAS with p < 10–8 explain less than 10% of the variance in body mass index (BMI)2. Among the genetic factors, SNPs within the FTO gene have been the most associated with obesity-related phenotypes in GWAS conducted in various populations3,4,5,6,7.
The role of diet in cardiometabolic diseases is widely recognized8,9,10. More specifically, unbalanced diets composed of processed, energy-dense foods, can promote weight gain in all ages11, 12. A genetic susceptibility to obesity appears stronger in an obesogenic environment, mainly due to an energy-dense diet, than in sparse ones13,14,15, pointing to an interaction between a person’s genotype and diet. Several studies have analyzed the interaction between SNPs in the FTO gene and dietary factors in determining obesity-related phenotypes16,17,18,19. However, the genetics of obesity is also complex20. Apart from the rare cases of monogenic obesity, common obesity is polygenic21, 22. In epidemiological studies, the combined polygenic risk of obesity has been computed using several approaches. Thus, so-called genetic risk scores (GRS) summarizing the additive effect of multiple, common SNPs have been proposed23, 24. An obesity-related GRS summarizes the estimated effect of common genetic variants on obesity phenotype25. Several GRSs have been constructed and validated for obesity phenotypes25,26,27,28,29. Although these prior studies did not analyze the interaction between the GRS for obesity and diet, subsequent studies that investigated such interactions, have mainly focused on macronutrients30, 31 or the overall quality of the diet32,33,34,35 and food groups36, 37.
Regarding food, the interaction between GRS for obesity and individual foods has been demonstrated previously for sugar-sweetened beverages38, 39 and fried foods40: these have been associated strongly with weight gain in those with a higher genetic predisposition to obesity. However, in most cases, GRSs were used as a total score, while specific SNPs that drive the interaction with foods have been poorly investigated.
Our working hypothesis is that despite diet modulating the genetic risk of obesity (assessed by a whole GRS), only specific SNPs in the GRS, as well as specific foods, are the main drivers of such modulation. Therefore, the identification of such specific interactions in specific populations will be of special relevance to provide a more focused recommendation to promote precision health41.
Compared to adults, research on the interaction between food consumption and genetic predisposition to obesity in children is very scarce, although understanding it could aid early risk detection and target preventive actions efficiently42. Therefore, we aimed at analyzing gene-diet interactions, considering not only the whole GRS but also identifying individual SNPs that drive the interaction of specific foods and combining these into GRSs. Finally, we illustrated the interactions and the shared SNP effects to gain a deeper insight into how individuals’ susceptibility to obesity modifies the effect of food consumption on BMI.
Results
Participants
Background characteristics of the 1142 participants are described in Table 1 by groups with low and high genetic risk for obesity. The grouping was based on the median number of risk alleles (n = 27). The number of risk alleles varied between 17 and 27 in the low-risk group and between 28 and 39 in the high-risk group. There was a distinctive difference in BMIz and waist-to-height-ratio (WtHr) between the groups: higher BMIz and waist circumference, but not height, was observed in the high -risk group in comparison to the low-risk group. However, many demographic and lifestyle factors did not differ between the groups. Correspondingly, food consumption illustrated by three summary scores and 15 individual food items were similar between the groups. An exception was observed with the consumption of pizza, which was somewhat higher (0.63 vs. 0.53, p = 0.061) and had a double variation in the high compared with the low-risk group.
Foods with an interaction with the whole GRS
Interactions of dietary summary scores/individual food items with whole BMI-GRSs on BMIz are shown in Table 2. We witnessed interactions for five individual food items: dark bread, biscuits and cookies, sugary juice drink, sweets and chocolate, pizza, and milk and sour milk with at least one BMI-GRS p < 0.15. When using dichotomous BMI-GRS e.g., (low vs. high-risk group), an additional interaction was identified for hamburger and hotdog. Details of the interactions are shown in Supplementary Table 1. There was no interaction between any of the dietary summary scores and BMI-GRS on BMIz.
Specific SNP × food modulation: SNPs driving the interaction
The identified seven food items were further explored for interactions at individual SNP levels. SNPs with the same direction and p < 0.2 were included in the food-specific GRS (Supplementary Table 2). In total, dark bread had 10, biscuits and cookies 7, sugary juice drink 7, sweets and chocolate 10, pizza 11, hamburger and hotdog 7, and milk and sour milk 12 interacting SNPs.
Food-specific GRS and their interactions
The associations of food-specific GRS, food intake, and their interaction on BMIz were tested in two models (Table 3): model 1 was adjusted only for sex, while model 2 was additionally adjusted for physical activity and sleep duration (fully adjusted). The fully adjusted interactions were further illustrated in Fig. 1. The interactions were validated for pizza, sweets and chocolate, sugary juice drink, and hamburger and hotdog when the adjusted mean effect sizes differed between the low- and high-risk groups.
Food-specific GRS and their confirmed interactions. The results are divided into two panels for clarity and based on the food-specific GRS presented with b-coefficients with 95% CI. The most predominant interaction was marked for pizza. Other notable interactions were sugary juice drink, hamburger and hotdog, and sweets and chocolate but without formal significance. The figure was made with PRISM version 9.5.0 (https://www.graphpad.com/).
The most predominant interaction was marked for pizza: it associated inversely with b − 0.130 (95% CI − 0.23; − 0.031) with BMIz in those with low GRS, while positively with b 0.153 (95% CI 0.072; 0.234) with BMIz in those having high GRS. Sugary juice drink followed the same pattern with the exception that the association among the low GRS group did not reach formal significance. Significant interactions were noted for hamburger and hotdog (p = 0.027) and sweets and chocolate (p = 0.011): the verification followed the same pattern but without formal significance.
Shared SNPs
Figure 2 illustrates the shared SNPs between the four food items. In total, we identified 15 out of 30 SNPs presenting an interaction. Interestingly, 33% of the SNPs were shared between pizza, sweets and chocolate, sugary juice drink, and hamburger and hotdog. These SNPs are close to the following genes (expressed in high magnitudes in these tissues): NEGR1 (brain), SEC16B (liver/pancreas), TMEM18 (bone), GNPDA2 (non-specific), and FTO (non-specific). The description of the SNPs and genes is presented in Supplementary Table 3.
Venn diagram of shared SNPs by food items. Five (33%) SNPs interactions (in red intersection) were shared between pizza, sweets and chocolate, sugary juice drink, and hamburger and hotdog. The figure was made through R version 4.2.2 (https://posit.co/products/open-source/rstudio/).
Discussion
Initially, we observed interactions between the whole BMI-GRS and certain foods on BMIz in school-aged children from Finland. Further investigations demonstrated that each interaction was driven by 7–11 SNPs. When combining these SNPs into food-specific GRS we verified an interaction for pizza, sweets and chocolate, sugary juice drink, and hamburger and hotdog. Thus, children bearing more risk alleles for obesity showed a stronger association of weight-promoting foods on BMI than those with fewer obesity-risk alleles. Importantly, there were no differences in the consumption frequency of these foods between the groups with varying genetic susceptibility, suggesting that the effect originates from a different kind of response to food than from a difference in consumption pattern. No interactions were observed for dietary summary scores describing overall eating habits or summary food scores for sugary foods or fruits and vegetables.
The health profile of the interacting foods was considered weight-promoting based on earlier studies due to their energy-dense-nutrient-poor characteristics43. The most predominant interaction was observed with pizza on BMIz, e.g., a positive association with BMIz in the high-risk group while opposite in the low-risk group. Pizza consumption is the top contributor for intakes of total energy, saturated fat and sodium in US children and teens, with a daily consumption frequency of 20%44. In our study, once a week/in two weeks were the most common consumption patterns of pizza (> 70%). In a systematic review45, TMEM18 and FTO were linked with total energy and fat intakes, thus partly supporting our findings.
In total, we identified 15 out of 30 SNPs as being responsible for the observed interactions for pizza, sweets and chocolate, sugary juice drink, and hamburger and hotdog. Interestingly, 33% of the interacting SNPs were shared between the foods. These included SNPs in or near genes NEGR1 (rs2815752), SEC16B (rs543874), TMEM18 (rs2867125), GNPDA2 (rs10938397) and FTO (rs1421085). Except for TMEM18, the other SNPs were previously shown to drive the interaction of fried foods, e.g., any deep-fried foods enjoyed at home or away from home on BMI in three US cohorts40, while an independent effect was noted only for FTO. The FTO (rs1421085) gene has been associated repeatedly with various obesity phenotypes in different study designs and populations46 (Supplementary Table 3), and its expression is aggregated in primary adipocytes47. The variant rs1421085 in the first intron of the FTO gene regulates the adipocyte-thermogenesis pathway by interacting with other genes (ARID5B, IRX3, and IRX5)47. Previous reports have witnessed multiple interactions of FTO variant rs1421085 with the intake of fiber48, dietary variation, alcohol consumption, and sedentary behaviors on BMI among adults49.
Our unique finding concerned TMEM18 (rs2867125), which has been associated with pediatric BMI50, 51, but here it presented an interaction with several foods. The contribution of any GRS or SNP may vary with age and in different stages of life13. The total BMI-GRS used here was significantly associated with BMIz and explained 3.7% of the variance in children52, which is somewhat higher than reported in adults22. Studies looking at genetic interaction with diet on BMI in pediatric cohorts are scarce51 but informative, since food consumption is more naïve and less affected by social acceptance in children than in older age groups. Thus, our results on TMEM18 may imply that the BMI trajectory in childhood is modified by the food intake, e.g., most likely energy-rich foods provide more support for growth.
Although the weight and waist differed by the genetic susceptibility to obesity; other lifestyle factors including sleep duration, and leisure-time physical activity (LTPA) were similar between the groups. Furthermore, sleep duration and LTPA only marginally affected the results, suggesting that the interaction was independent of these factors. Certain risk variants of FTO (rs9939609), TMEM18 (rs4854344), and NRXN3 (rs10146997) have been reported to increase the vulnerability to metabolic conditions in children under sleep deprivation53, thus interacting with lifestyle factors, but that was not observed here.
Sugary juice drinks are widely consumed among young Finnish children and enjoyed with a snack, instead of milk or juice at meals54, 55. The healthiness of sugary juice drinks is frequently discussed as the dilute berry-derived squash contains mainly sugar and provides energy, but barely nutrients. Frequent sugary juice drinkers will likely evolve with time into consumers of carbonated sugary-sweated beverages (SSB), which are deemed as weight-promoting foods56. Additionally, two earlier studies have demonstrated SSB to interact with the obesity-prone genotype38, 39. Similar to our finding on sugary juice drink, the reported magnitude of association between SSB and BMI was greater among those genetically prone to obesity, implying that the downstream effects after consuming SSB differ between the individual, making obese-prone more vulnerable to weight gain. Furthermore, Brunkwall’s study39 highlighted that the SSB-BMI interaction was mainly driven by one SNP – rs1555543, close to gene PTBP2, among middle-aged Swedish individuals. The same SNP has demonstrated an interaction with smoking on BMI in the Pakistani population57. However, we did not observe any interaction of rs1555543 in our sample, possibly due to the young age of the participants.
The ultimate strength of the study is that we used a cohort of school-aged children whose food consumption is likely less affected by social acceptance. Although mis- and underreporting are common challenges in dietary assessment, it is shown that amongst 11–12-year-old children that the FFQ is a valid method and independent of BMI, implying that social acceptance and desirability are less common in children than in older age groups58. Thus, we observed no differences in food consumption frequencies. However, we did not address portion sizes, which may differ by BMI59. The study was facilitated by a previously reported association of BMI-GRS and BMIz52, relying on 30 well-characterized SNPs. Our results may be generalized to a comparable European population with a similar socioeconomic background. Based on our earlier work52, using a GRS with more SNPs would most likely result in similar outcomes, as the GRSs present with corresponding associations with BMIz.
Due to the limited sample size and using the tailor-made Metabochip array only obesity SNPs were considered. Future studies with larger sample size and genome-wide coverage of SNPs are warranted for broader investigations of the interactions between genes and diet. The food frequency questionnaire (FFQ) covered 16 food items and was considered suitable for 11-year-old children to comprehend60, 61. However, it might have been too narrow to distinguish between foods with varying health profiles, e.g., all dairy products were considered together without considering differences in the nutrient content. Thus, we might have lost some of the information. Because power for detecting interactions is typically much lower than power for main effects, we raised the Type I error rate to 20% when assessing interactions as suggested62, 63. On the other hand, this might increase the chance of false positive results. However, we illustrated the association in subgroups as well.
In conclusion, the interacting foods with the genetic risk of obesity were mainly weight-promoting in Finnish children. Our results point out that children genetically prone to obesity showed a stronger association of unhealthy foods with BMIz than those with lower genetic susceptibility. Since a part of the SNPs driving the interactions were shared between the weight-promoting foods, this implies metabolic differences among genetically prone individuals, which warrants further studies in this and other geographically diverse populations.
Methods
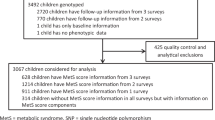
We have conducted a cross-sectional analysis of 1142 Finnish children. For this study, we utilized the background characteristics, genotype data, and anthropometric measurements from the Finnish Health in Teens cohort (Fin-HIT), launched in 2011 as a school-based cohort study, initially comprising 11,407 Finnish children aged between 9 and 12 years. The details of the Fin-HIT cohort are described elsewhere64. The Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa has approved the study protocol (169/13/03/00/10) and written informed consent was obtained from all participants and their parents. All study procedures adhered to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
DNA extraction, genotyping, quality control, and generation of genetic risk score
The participants provided saliva samples by using the Oragene® DNA (OG-500) Self-Collection Kit (DNA Genotek Inc., Ottawa, Ontario, Canada). DNA was extracted using an automated protocol with the chemagic DNA Saliva Kit (PerkinElmer, Wellesley, Massachusetts). DNA samples (n = 1368) were randomly selected from the Fin-HIT cohort and subjected to genotyping with the Cardio-Metabochip (Illumina, Inc., San Diego, California) at the Finnish Institute for Molecular Medicine Technology Centre (Helsinki, Finland) as explained elsewhere52. The number of individuals and SNPs included in the final analysis after QC was 1142 and 125,187 with a total genotyping rate of 99.9%. BMI-based genetic risk score25 was based on the results of Speliotes et al. 201021 but comprised 30 SNPs as rs4771122 and rs4836133 were not available and had no good proxies. Thus, within our BMI-GRS; PTBP2 rs11165643, TNNI3K rs1514175, NEGR1 rs2815752, SEC16B rs543874, RBJ rs11676272, LRP1B rs2121279, TMEM18 rs2867125, FANCL rs887912, CADM2 rs13078807, ETV5 rs7647305, GNPDA2 rs10938397, SLC39A8 rs13107325, FLJ35779 rs2112347, NUDT3 rs206936, TFAP2B rs987237, LRRN6C rs10968576, BDNF rs2030323, MTCH2 rs3817334, RPL27A rs7127684, FAIM2 rs7138803, PRKD1 rs10134820, NRXN3 rs17109256, MAP2K5 rs2241423, GPRC5B rs12444979, FTO rs1421085, SH2B1 rs7359397, MC4R rs571312, QPCTL rs2287019, KCTD15 rs29941, and TMEM160 rs3810291 were considered and each increased the risk of obesity.
We summarized the number of risk alleles (unweighted) and created a weighted genetic risk score (BMI-GRS) using the score function in Plink version 1.09, which calculates an average score per non-missing SNP52. Besides using effect sizes of Speliotes et al. 201021 (BMI-GRSSpeliotes), also Fin-HIT effect sizes were used (BMI-GRSFin-HIT), and their ratio, e.g., Fin-HIT/Speliotes (BMI-GRSratio). Additionally, interactions between certain foods and individual SNPs were tested. The SNPs with the same direction of effect and p < 0.200 were incorporated into food-specific GRS.
Anthropometry measurements
Children’s anthropometry, including height, waist (centimeters, cm), and weight (kilograms, kg) were measured at baseline in a standardized way by trained field workers. Children’s body mass index (BMI) (kg/m2) was calculated, and age- and sex-specific z-scores (BMIz) were derived based on the International Obesity Task Force (IOTF) guidelines65 and used as continuous variables in the analysis.
Indicatory food items and their summary scores
Consumption frequencies of 16 food items were evaluated with a self-administered food frequency questionnaire (FFQ)66. For the food items, participants' ratings varied from 1; not at all, to 7; several times a day, which were recoded during analysis to scale from 0 to 14 times a week. In addition, two summary scores were created for the sweet treat index (STI) and plant consumption index (PCI) to indicate the weekly consumption frequencies of sweet treats67, and vegetables, fruits, and berries68, respectively. Our FFQ was adapted from the FFQ used in the World Health Organization’s International Health Behaviour in School-Aged Children study, which was validated and retested among school-age children in Europe60, 61.
Additionally, eating habits (healthy; fruit and vegetable avoider; unhealthy) were used to describe the whole diet. Those were derived with the hierarchical K-means method as explained elsewhere66, using the five factors obtained through factor analysis which represented 70% of the variability of the 10 selected food items.
Other background information
Leisure-time physical activity (LTPA) and sleep habits were self-reported in the baseline questionnaire as previously described67, 69. LTPA duration was reported for the whole week (h/week), while sleep habits, e.g., waking and bedtime hours, separately for school days and days off. Sleep durations (with 0.5-h accuracy) were calculated, and the weighted mean for sleep duration was used in the analysis. These were used as covariates in the statistical analyses.
The questionnaire included an evaluation of pubertal development based on the Tanner stage with a pictorial assessment of breast development and pubic hair for girls and only pubic hair for boys with a scale of 1–570. Due to several incomplete responses, the categorization was recoded into prepuberty (T1-2), puberty (T3-4), and postpuberty (T5) to describe the puberty phase.
Maternal occupational information at the time of the child’s birth was obtained from the Medical Birth Register maintained by the Finnish Institute for Health and Welfare and was used to describe the maternal socioeconomic status as previously described67. Mothers were categorized as upper-level employees, lower-level employees, manual workers, students, and others (including self-employed persons, stay-at-home mothers, unemployed persons, and pensioners). Additionally, the child’s age and sex were included.
Statistical analyses
Background characteristics and diet were compared between groups of low and high genetic susceptibility to obesity with independent samples t-test or Chi-Square, depending on a variable. Results are presented with the mean (SD) or with n and proportion (%).
Interactions between dietary factors and BMI-GRSs/individual SNPs were tested with a linear regression model, and p for claiming interaction was set to < 0.15 for BMI-GRSs and < 0.2 for individual SNPs62, 63. The linear modeling included adjustments for covariates: sex, LTPA, mean sleep duration and 1st and 2nd principal coordinates (PC). In the case of borderline significance, the interaction was further investigated with dichotomized BMI-GRS groups stratified by the median value.
The statistical analyses were performed with IBM SPSS Statistics version 27. A significance level with 5% uncertainty was adopted.
Data availability
Due to ethical restrictions from the coordinating ethics committee of the hospital district of Helsinki and Uusimaa (Decision Number 169/13/03/00/10) and legal GDPR restrictions, genetic data that support our findings are available upon request from the data access committee of the Fin-HIT study by contacting Dr. Heli Viljakainen (heli.viljakainen@helsinki.fi). Processed data will be available on GitHub by request: (https://github.com/Fin-HIT/Heli-Viljakainen-et-al-2023-Genetic-susceptibility-to-obesity-and-food-intake-on-BMI-in-children).
References
Albuquerque, D. et al. The contribution of genetics and environment to obesity. Br. Med. Bull. 123(1), 159–173 (2017).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27(20), 3641–3649 (2018).
Speakman, J. R. et al. GWAS for BMI: A treasure trove of fundamental insights into the genetic basis of obesity. Int. J. Obes. 42(8), 1524–1531 (2018).
Huang, J. et al. Genomics and phenomics of body mass index reveals a complex disease network. Nat. Commun. 13(1), 7973 (2022).
Goodarzi, M. O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 6(3), 223–236 (2018).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316(5826), 889–894 (2007).
Loos, R. J. & Yeo, G. S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10(1), 51–61 (2014).
Srour, B. et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Sante). BMJ 365, l1451 (2019).
Alinia, S., Hels, O. & Tetens, I. The potential association between fruit intake and body weight—A review. Obes. Rev. 10(6), 639–647 (2009).
Eloranta, A. M. et al. Dietary factors associated with metabolic risk score in Finnish children aged 6–8 years: The PANIC study. Eur. J. Nutr. 53(6), 1431–1439 (2014).
Perez-Escamilla, R. et al. Dietary energy density and body weight in adults and children: A systematic review. J. Acad. Nutr. Diet. 112(5), 671–684 (2012).
Mendonca, R. D. et al. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra follow-up (SUN) cohort study. Am. J. Clin. Nutr. 104(5), 1433–1440 (2016).
Warrington, N. M. et al. A genome-wide association study of body mass index across early life and childhood. Int. J. Epidemiol. 44(2), 700–712 (2015).
Tyrrell, J. et al. Gene-obesogenic environment interactions in the UK Biobank study. Int. J. Epidemiol. 46(2), 559–575 (2017).
Walter, S. et al. Association of a genetic risk score with body mass index across different birth cohorts. JAMA 316(1), 63–69 (2016).
Sonestedt, E. et al. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 90(5), 1418–1425 (2009).
Corella, D. et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J. Nutr. 141(12), 2219–2225 (2011).
Phillips, C. M. et al. High dietary saturated fat intake accentuates obesity risk associated with the fat mass and obesity-associated gene in adults. J. Nutr. 142(5), 824–831 (2012).
Livingstone, K. M. et al. Associations between FTO genotype and total energy and macronutrient intake in adults: A systematic review and meta-analysis. Obes. Rev. 16(8), 666–678 (2015).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538), 197–206 (2015).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42(11), 937–948 (2010).
Hebebrand, J. et al. Chipping away the “missing heritability”: GIANT steps forward in the molecular elucidation of obesity—But still lots to go. Obes. Facts 3(5), 294–303 (2010).
Goldstein, B. A. et al. Contemporary considerations for constructing a genetic risk score: An empirical approach. Genet. Epidemiol. 39(6), 439–445 (2015).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 9(3), e1003348 (2013).
Belsky, D. W. et al. Development and evaluation of a genetic risk score for obesity. Biodemogr. Soc. Biol. 59(1), 85–100 (2013).
Seral-Cortes, M. et al. Development of a genetic risk score to predict the risk of overweight and obesity in European adolescents from the HELENA study. Sci. Rep. 11(1), 3067 (2021).
Peterson, R. E. et al. Genetic risk sum score comprised of common polygenic variation is associated with body mass index. Hum. Genet. 129(2), 221–230 (2011).
Monnereau, C. et al. Associations of genetic risk scores based on adult adiposity pathways with childhood growth and adiposity measures. BMC Genet. 17(1), 120 (2016).
Damavandi, N. et al. Development of a genetic risk score for obesity predisposition evaluation. Mol. Genet. Genomics 297(6), 1495–1503 (2022).
Casas-Agustench, P. et al. Saturated fat intake modulates the association between an obesity genetic risk score and body mass index in two US populations. J. Acad. Nutr. Diet 114(12), 1954–1966 (2014).
Goni, L. et al. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 10(1), 445 (2015).
Heianza, Y. et al. Healthful plant-based dietary patterns, genetic risk of obesity, and cardiovascular risk in the UK biobank study. Clin. Nutr. 40(7), 4694–4701 (2021).
Nettleton, J. A. et al. Gene x dietary pattern interactions in obesity: Analysis of up to 68 317 adults of European ancestry. Hum. Mol. Genet. 24(16), 4728–4738 (2015).
Seral-Cortes, M. et al. Interaction effect of the Mediterranean diet and an obesity genetic risk score on adiposity and metabolic syndrome in adolescents: The HELENA study. Nutrients 12(12), 3841 (2020).
Wang, T. et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: Gene-diet interaction analysis in two prospective cohort studies. BMJ 360, j5644 (2018).
Wang, T. et al. Improving fruit and vegetable intake attenuates the genetic association with long-term weight gain. Am. J. Clin. Nutr. 110(3), 759–768 (2019).
Huls, A. et al. Polygenic risk for obesity and its interaction with lifestyle and sociodemographic factors in European children and adolescents. Int. J. Obes. 45(6), 1321–1330 (2021).
Qi, Q. et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 367(15), 1387–1396 (2012).
Brunkwall, L. et al. Sugar-sweetened beverage consumption and genetic predisposition to obesity in 2 Swedish cohorts. Am. J. Clin. Nutr. 104(3), 809–815 (2016).
Qi, Q. et al. Fried food consumption, genetic risk, and body mass index: Gene-diet interaction analysis in three US cohort studies. BMJ 348, g1610 (2014).
Khoury, M. J. et al. From public health genomics to precision public health: A 20-year journey. Genet. Med. 20(6), 574–582 (2018).
Torkamani, A. & Topol, E. Polygenic risk scores expand to obesity. Cell 177(3), 518–520 (2019).
Swinburn, B. A. et al. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 7(1A), 123–146 (2004).
Powell, L. M., Nguyen, B. T. & Dietz, W. H. Energy and nutrient intake from pizza in the United States. Pediatrics 135(2), 322–330 (2015).
Drabsch, T. et al. Associations between single nucleotide polymorphisms and total energy, carbohydrate, and fat intakes: A systematic review. Adv. Nutr. 9(4), 425–453 (2018).
Najd-Hassan-Bonab, L. et al. The role of FTO variant rs1421085 in the relationship with obesity: A systematic review and meta-analysis. Eat Weight Disord. 27(8), 3053–3062 (2022).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373(10), 895–907 (2015).
Hosseini-Esfahani, F. et al. The interaction of fat mass and obesity associated gene polymorphisms and dietary fiber intake in relation to obesity phenotypes. Sci. Rep. 7(1), 18057 (2017).
Young, A. I., Wauthier, F. & Donnelly, P. Multiple novel gene-by-environment interactions modify the effect of FTO variants on body mass index. Nat. Commun. 7, 12724 (2016).
Zhao, J. et al. The role of obesity-associated loci identified in genome-wide association studies in the determination of pediatric BMI. Obesity (Silver Spring) 17(12), 2254–2257 (2009).
Rask-Andersen, M. et al. Association of TMEM18 variants with BMI and waist circumference in children and correlation of mRNA expression in the PFC with body weight in rats. Eur. J. Hum. Genet. 20(2), 192–197 (2012).
Viljakainen, H. et al. Genetic risk score predicts risk for overweight and obesity in Finnish preadolescents. Clin Obes 9(6), e12342 (2019).
Prats-Puig, A. et al. Variations in the obesity genes FTO, TMEM18 and NRXN3 influence the vulnerability of children to weight gain induced by short sleep duration. Int. J. Obes. 37(2), 182–187 (2013).
Kyttala, P. et al. Food consumption and nutrient intake in Finnish 1–6-year-old children. Public Health Nutr. 13(6A), 947–956 (2010).
Eloranta, A. M. et al. Dietary factors and their associations with socioeconomic background in Finnish girls and boys 6–8 years of age: the PANIC Study. Eur. J. Clin. Nutr. 65(11), 1211–1218 (2011).
de Ruyter, J. C. et al. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 367(15), 1397–1406 (2012).
Ahmad, S. et al. Physical activity, smoking, and genetic predisposition to obesity in people from Pakistan: The PROMIS study. BMC Med. Genet. 16, 114 (2015).
Saloheimo, T. et al. The reliability and validity of a short food frequency questionnaire among 9–11-year olds: A multinational study on three middle-income and high-income countries. Int. J. Obes. Suppl. 5(Suppl 2), S22–S28 (2015).
Burger, K. S., Kern, M. & Coleman, K. J. Characteristics of self-selected portion size in young adults. J. Am. Diet. Assoc. 107(4), 611–618 (2007).
Vereecken, C. A. et al. Comparison of a short food-frequency questionnaire and derived indices with a seven-day diet record in Belgian and Italian children. Int. J. Public Health 53(6), 297–305 (2008).
Vereecken, C. A. & Maes, L. A Belgian study on the reliability and relative validity of the Health Behaviour in School-Aged Children food-frequency questionnaire. Public Health Nutr. 6(6), 581–588 (2003).
Marshall, S. W. Power for tests of interaction: Effect of raising the Type I error rate. Epidemiol. Perspect. Innov. 4, 4 (2007).
Thiese, M. S., Ronna, B. & Ott, U. P value interpretations and considerations. J. Thorac. Dis. 8(9), E928–E931 (2016).
Figueiredo, R. A. O. et al. Cohort profile: The Finnish Health in Teens (Fin-HIT) study: A population-based study. Int. J. Epidemiol. 48(1), 23–24h (2019).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7(4), 284–294 (2012).
de Oliveira Figueiredo, R. A. et al. Identifying eating habits in Finnish children: A cross-sectional study. BMC Public Health 19(1), 312 (2019).
Lommi, S. et al. Frequent use of selected sugary products associates with thinness, but not overweight during preadolescence: A cross-sectional study. Br. J. Nutr. 124(6), 631–640 (2020).
Raisanen, L. et al. Central obesity in school-aged children increases the likelihood of developing paediatric autoimmune diseases. Pediatr. Obes. 17(3), e12857 (2022).
Engberg, E. et al. Physical activity among preadolescents modifies the long-term association between sedentary time spent using digital media and the increased risk of being overweight. J. Phys. Act Health 18(9), 1105–1112 (2021).
Tanner, J. M. Growth at Adolescence; with a General Consideration of the Effects of Hereditary and Environmental Factors upon Growth and Maturation from Birth to Maturity (Blackwell Scientific, 1962).
Acknowledgements
The Finnish Health in Teens (Fin-HIT) cohort study is funded by the Folkhälsan Research Foundation. We acknowledge the study participants and the fieldworkers for enabling the study. We appreciate Kris Elomaa for helping with data visualization. The study was supported by grants from the Medicinska Understödsföreningen Liv och Hälsa, and the Finnish Food Research Foundation Elintarvikkeiden for supporting the research visit at the Department of Preventive Medicine, University of Valencia, Spain.
Author information
Authors and Affiliations
Contributions
H.V. contributed to the study design, statistical analyses, interpretation of the results, drafting the tables and figures, and providing the first draft of the manuscript. J.V.S, N.A., O.P. contributed to the design of the article, interpretation of the results, and revising the manuscript. E.D. contributed by assisting with the genetic data, design of the article, interpretation of the results, and revising the manuscript. D.C. contributed to the study design and methodology, interpretation of the results, and critically revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viljakainen, H., Sorlí, J.V., Dahlström, E. et al. Interaction between genetic susceptibility to obesity and food intake on BMI in Finnish school-aged children. Sci Rep 13, 15265 (2023). https://doi.org/10.1038/s41598-023-42430-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42430-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.