Abstract
Ixodes ricinus and Dermacentor reticulatus ticks are important reservoirs and vectors of pathogens. The aim of the present study was to investigate the dynamic of the prevalence and genetic diversity of microorganisms detected in these tick species collected from two ecologically diverse biotopes undergoing disparate long-term climate condition. High-throughput real time PCR confirmed high prevalence of microorganisms detected in sympatrically occurring ticks species. D. reticulatus specimens were the most often infected with Francisella-like endosymbiont (FLE) (up to 100.0%) and Rickettsia spp. (up to 91.7%), while in case of I. ricinus the prevalence of Borreliaceae spirochetes reached up to 25.0%. Moreover, pathogens belonging to genera of Bartonella, Anaplasma, Ehrlichia and Babesia were detected in both tick species regardless the biotope. On the other hand, Neoehrlichia mikurensis was conformed only in I. ricinus in the forest biotope, while genetic material of Theileria spp. was found only in D. reticulatus collected from the meadow. Our study confirmed significant impact of biotope type on prevalence of representatives of Borreliaceae and Rickettsiaceae families. The most common co-infection detected in D. reticulatus was Rickettsia spp. + FLE, while Borreliaceae + R. helvetica was the most common in I. ricinus. Additionally, we found significant genetic diversity of R. raoultii gltA gene across studied years, however such relationship was not observed in ticks from studied biotopes. Our results suggest that ecological type of biotope undergoing disparate long-term climate conditions have an impact on prevalence of tick-borne pathogens in adult D. reticulatus and I. ricinus.
Similar content being viewed by others
Introduction
Ixodes ricinus and Dermacentor reticulatus are two of the most widely distributed representatives of over 60 species of ticks (Acari: Ixodida) occurring in the Western Palearctic1. In Europe, I. ricinus is common throughout the continent2, while the compact geographical range of D. reticulatus extends from the western parts of the British Isles and northern Italy through the countries of Central Europe to the Ural Mountains in the east3.
The habitats preferred by I. ricinus include deciduous and mixed forests, pastures, and moorlands. Specimens of this species are often found in city parks as well2, 4. D. reticulatus ticks are most frequently collected in meadow habitats and river valleys but less frequently in forest habitats5,6,7. Populations of D. reticulatus were found to be particularly abundant in habitats of agriculturally unused fields, especially those undergoing progressive ecological succession8, 9.
With their wide occurrence range, considerable population size, plasticity in relation to occupied habitats, and diverse range of host organisms, I. ricinus and D. reticulatus ticks play an important role in the transmission and maintenance of tick-borne pathogen foci in the environment10. In the case of pathogens transmitted by I. ricinus, the highest medical importance is ascribed to Borrelia burgdorferi sensu lato (s.l.) spirochetes, which cause Lyme borreliosis (LB) in humans, and tick-borne encephalitis virus (TBEV), i.e., the etiological agent of tick-borne encephalitis in humans (TBE)11. Besides mentioned pathogens I. ricinus ticks are reservoirs and vectors of e.g., Babesia sp. Bartonella sp., Neoehrlichia mikurensis and microorganisms representing Francisella sp. These pathogens are commonly detected in ticks from previous reports, affecting human and animal health and may cause diagnostic difficulties12,13,14.
D. reticulatus ticks attack humans sporadically but can transmit TBEV15, 16 as well as Rickettsia slovaca and R. raoultii, which are agents of tick-borne lymphadenopathy (TIBOLA)17. The ability of D. reticulatus to transmit B. burgdorferi s.l., and Francisella tularensis has not been fully elucidated12, 18. In veterinary practice, the most important pathogens transmitted by D. reticulatus are Babesia canis protozoa causing canine babesiosis and Anaplasma marginale rickettsiae responsible for the development of bovine anaplasmosis19,20,21.
According to predictions, progressive climate change will affect the distribution ranges of various tick species and their hosts, changing the incidence of tick-borne diseases and zoonotic disease risk22. Ecological networks analysis suggested that interactions between ticks and transmitted pathogens evolved to minimise interspecific competition while allowing ample but modular circulation of transmitted pathogens among vertebrates23. Similarly, ecological network interactions between I. ricinus ticks and their hosts evolved to maximize habitat overlap with some hosts that are super-spreaders of pathogens24. Despite tick density being highly influenced by the presence of suitable hosts22, immediate tick survival and questing activities are highly dependent on suitable and specific environmental conditions (temperatures between 8 and 24 °C and humidity of up to 80%). In addition to environmental variables and host availability, shifts in tick microbiota dynamics over time were also reported to influence the presence of tick-borne pathogens (TBPs) in ticks25. The complex interactions between abiotic and biotic factors can cause temporal variations in tick-borne pathogen prevalence26. For instance, significant variations in seasonal and/or inter-annual pathogen prevalence were observed for R. helvetica, B. burgdorferi s.l., B. miyamotoi and A. phagocytophilum in I. ricinus over a period of three years in a French peri-urban forest26. Understanding how variations in microclimate conditions impact tick-borne pathogen prevalence in questing ticks is important for predictions of zoonotic disease risk under global climatic fluctuations27, 28.
In this study we collected questing adult I. ricinus and D. reticulatus ticks over a period of three years in two biotopes (the forest and the meadow) with differences in climate condition and we assessed variations in prevalence of multiple tick-borne pathogens and endosymbiont (hereafter microorganisms) across time, as well as between biotopes and tick species. We additionally compared the occurrence of co-infections of TBPs and genetic diversity of pathogens of the same species between sites and tick species. Hence, we hypothesized possible differences in species diversity, prevalence and genetic diversity of detected TBPs in sympatric tick species collected from ecologically and climatically diverse habitats.
Results
Selecting biotope locations for assessing pathogen prevalence using long-term climatic data
The two regions within which study transects were established differed significantly in long-term climatic data, i.e., mean temperature (t = − 2.11, p = 0.02); total sum of precipitation (t = 3.20, p < 0.01); length of vegetation period (t = 18.39, p < 0.01), in opposite to total number of days with snow cover (t = 0.52, p = 0.30).
Population dynamics of Dermacentor reticulatus and Ixodes ricinus in diverse habitats
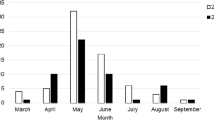
Two tick species D. reticulatus and I. ricinus were found at different frequencies in the two biotopes included in the study. In total, 1664 adults of D. reticulatus and 386 of I. ricinus ticks were collected in the forest biotope (D. reticulatus n = 432, I. ricinus n = 275) and meadow biotope (D. reticulatus n = 1,232; I. ricinus n = 111) over three years (2018–2020). D. reticulatus was dominant in both biotopes (Fig. 1). The abundance of D. reticulatus in the meadow biotope was significantly higher than in the forest biotope (Z = − 4.06, p < 0.01), while the abundance of I. ricinus was not statistically different between biotopes (Z = 1.72, p = 0.09). Two peaks of D. reticulatus activity were observed each year, in spring and in autumn, whereas I. ricinus population peaked only once a year, in spring (Fig. 1). The studied biotopes did not differ significantly in terms of values of temperature (H = 0.44, p = 0.66) and humidity (H = 1.80, p = 0.07) at time of tick collection. Moreover, no significant correlations were found between temperature or humidity and the number of active I. ricinus or D. reticulatus ticks (Supplementary Fig. S1).
Population dynamics of questing I. ricinus and D. reticulatus ticks in two ecological sites. (a) and (b) indicate the studied forest and meadow biotopes, respectively. Tick counts were differentiated between females and males from each tick species. The measurements were conducted for three consecutive years. The seasonal variation of environmental temperature and humidity were measured and displayed.
Prevalence of microorganisms in Ixodes ricinus and Dermacentor reticulatus ticks
High-throughput screening of TBPs and one endosymbiont (Francisella-like endosymbiont–FLE) DNA was carried out in questing ticks collected in the forest (D. reticulatus n = 81, I. ricinus n = 150; Table 1) and meadow (D. reticulatus n = 177, I. ricinus n = 35; Table 2) biotopes. The examined tick species differed in the number of infections by different tick-borne microorganisms. I. ricinus ticks had a greater variety of microorganisms (i.e., number of different genera and/or species detected by tick specimen) than D. reticulatus, however D. reticulatus ticks were more often infected (Tables 1 and 2). A higher diversity of microorganisms was found in ticks collected in the forest biotope (17 microorganisms species detected in I. ricinus and 6 species detected in D. reticulatus), while in the meadow biotope 14 microbial species were detected in I. ricinus and 11 in D. reticulatus (Tables 1 and 2). A strong association, that was found with multiple correspondence analysis (MCA), between biotopes, ticks and microorganisms was observed for D. reticulatus and B. canis, FLE and R. helvetica in the meadow biotope (Fig. 2). I. ricinus showed a strong association with R. helvetica and Bartonella henselae, but not with any habitat in particular (Fig. 2). Other detected TBPs were not associated with either ecological sites or particular tick species, but different prevalence of individual microorganisms was found between sites and tick species (Fig. 2, Tables 1 and 2).
Multiple correspondence analysis (MCA) plot showing associations between the tick-borne pathogens, tick species and sampling sites in Poland. The presence or absence of the pathogens is denoted by 1 and 0, respectively. The ellipsoid encompasses groups of pathogens associated with each sampling forest biotope end/or tick species. Node sizes are proportional to the pathogen prevalence in ticks. The fraction of each component is represented as a percentage for each dimension (axis).
Prevalence of Rickettsia spp
The presence of genetic material of Rickettsia spp. was confirmed in specimens from both tick species. In the forest biotope, R. helvetica and uncharacterized Rickettsia spp. were detected in D. reticulatus ticks, while I. ricinus ticks were infected by R. monacensis, R. helvetica and also by uncharacterized Rickettsia spp. (Table 1). In the meadow biotope, D. reticulatus ticks were infected by R. raoultii, R. aeschlimannii and uncharacterized Rickettsia spp.; while only uncharacterized Rickettsia spp. were found in I. ricinus (Table 2). There was a significant difference in Rickettsia prevalence between the sites regardless of the tick species (χ2 = 7.18, p = 0.02).
A higher prevalence of Rickettsia bacterial infection was found in D. reticulatus, compared to I. ricinus in both sites (χ2 = 9.32, p = 0.01). In the forest biotope, Rickettsia prevalence varied yearly from 68.2 to 91.7% in D. reticulatus depending on the year, but the differences in prevalence across time were not significant (χ2 = 4.81, p = 0.09). In the case of I. ricinus ticks from the forest biotope, the prevalence of Rickettsia ranged from 5.0 to 37.9%, and no significant differences were found between the consecutive years of the study (χ2 = 5.42, p = 0.06) (Table 1). In the meadow biotope, rickettsial infection was confirmed in up to 75.6% of D. reticulatus and in up to 13.3% of I. ricinus specimens (Table 2). The differences of prevalence of Rickettsia infection across the time was statistically significant for both tick species, i.e., χ2 = 13.70, p < 0.01; χ2 = 10.57, p < 0.01, respectively.
Prevalence of spirochetes of the Borreliaceae family
Genetic material of spirochetes of the Borreliaceae family (i.e., Borreliella garinii, B. spielmanii, B. afzelii, B. valaisiana, B. lusitaniae, as well as Borrelia miyamotoi) was detected mainly in I. ricinus collected in the forest biotope. B. afzelii infection was also found in one D. reticulatus individual from the meadow biotope (Table 2).
The prevalence of Borreliaceae spirochetes ranged from 1.5 to 25.0% in the forest biotope (Table 1), and from 2.1 to 20.0% in the meadow biotope (Table 2). The prevalence of Borreliaceae differed significantly in I. ricinus populations from the forest and the meadow biotopes (χ2 = 27.81, p < 0.01). The prevalence of Borreliaceae-infected I. ricinus ticks varied across time in the forest (χ2 = 79.04, p < 0.01), but not in the meadow biotope (χ2 = 3.31, p = 0.19).
Prevalence of Anaplasma phagocytophilum
In I. ricinus, the prevalence of A. phagocytophilum infections ranged from 4.5% in ticks collected from the forest biotope up to 26.7% in the meadow biotope (Tables 1 and 2). The presence of A. phagocytophilum in D. reticulatus inhabiting the forest biotope was confirmed only in 2019 year (8.6%), while in the meadow biotope infected specimens were collected in all studied years (up to 12.8%; Tables 1, 2). The prevalence of A. phagocytophilum infection varied significantly between tick species, regardless of biotope (ticks collected in the forest biotope χ2 = 20.39, p < 0.01; in the meadow biotope χ2 = 15.90, p = 0.03) (Tables 1, 2). No differences in the prevalence of this pathogen were noted between the biotopes (χ2 = 0.04, p = 0.94); however, temporal patterns of A. phagocytophilum infection were significantly different in the forest (χ2 = 21.03, p < 0.01) and the meadow biotope (χ2 = 12.50, p = 0.04) (Tables 1 and 2).
Prevalence of Babesia spp
In the forest biotope, genetic material of B. canis was detected in 12.5% of D. reticulatus ticks in 2018 and in 8.6% of I. ricinus ticks in 2020 (Table 1), while B. venatorum was confirmed in 3.1% of I. ricinus specimens in 2019 and 4.5% in 2020. In the meadow biotope, B. canis was found only in D. reticulatus ticks (up to 4.3% of infected specimens) (Table 2). There was no significant difference in the Babesia spp. prevalence between the biotopes (χ2 = 0.60, p = 0.74) and between studied years (χ2 = 3.26, p = 0.20).
Prevalence of other tick-borne pathogens
Depending on the year, up to 16.7% of the ticks were infected by Bartonella spp. The presence of Theileria spp. was confirmed in one D. reticulatus specimen (2.1%) in the meadow biotope, while N. mikurensis was detected only in I. ricinus specimens collected in the forest biotope (up to 5.0%). From 11.0 to 33.3% of the ticks in both sites were infected by Apicomplexa parasites, excluding Babesia spp. or Theileria spp. (Tables 1 and 2).
Prevalence of tick Francisella-like endosymbiont (FLE)
In both the forest and meadow biotopes high prevalence of FLE was found only in D. reticulatus, reaching from 95.4 to 100.0% and from 86.1 to 97.8%, respectively (Tables 1 and 2). Whereas in the case of I. ricinus only one specimen collected in the meadow biotope was found FLE-positive (Tables 1 and 2). Significant differences were observed in the prevalence of FLE between tick species at both the forest (p < 0.01) and the meadow biotope (χ2 = 12.49, p < 0.01), but there were no significant differences in FLE prevalence between the study years (χ2 = 0.62, p = 0.73).
Co-infections in Ixodes ricinus and Dermacentor reticulatus ticks
The analyzed populations of I. ricinus and D. reticulatus exhibited a high rate of co-infections by tick-borne microorganisms (Fig. 3; Supplementary Table S1). The coexistence of multiple microorganisms was observed more frequently in D. reticulatus. In the study years, 85.72–100.00% of all D. reticulatus ticks were infected by two or more pathogens (Fig. 3). The most common co-infections were Rickettsia spp. + FLE and Rickettsia spp. + FLE + B. canis (Supplementary Table S1). There was no significant difference in the prevalence of polymicrobial infections in D. reticulatus between habitats (χ2 = 0.47, p = 0.78).
In I. ricinus single infections were frequently observed (ranged from 20.00 to 45.45%) and 30.00–50.00% of ticks of this species were not infected by any of the microorganisms (Fig. 3). Additionally, co-infections by up to five microorganisms were detected in the forest biotope and infections by maximum of three microorganisms were found in the meadow biotope (Fig. 3). The most common co-infecting pathogens in I. ricinus were Borreliacea + R. helvetica (Supplementary Table S1). However, no statistically significant difference in the prevalence of co-infections in I. ricinus was found between biotopes (χ2 = 0.51, p = 0.77).
In the case of I. ricinus, a reduction in co-infections with two or more microorganisms was observed over the studied years. This relationship was observed regardless of the type of biotope, i.e., the forest biotope: χ2 = 30.89, p < 0.01; the meadow biotope χ2 = 20.07, p < 0.01. On the other hand, the level of multiple co-infections in D. reticulatus, remained stable across the study period, with no significant variations (χ2 = 0.46, p = 0.79) (Fig. 3).
Phylogenetic and genetic diversity of microorganisms detected in Ixodes ricinus and Dermacentor reticulatus
Phylogenetic analysis of the flaB gene sequences in B. burgdorferi sensu stricto (s.s.). (OQ254784) showed that these sequences clustered with another sequence from Japan (Supplementary Fig. S2) and were not significantly different compared to all other sequences included in the phylogenetic tree (Fig. 4). In the result of Maximum Likelihood analysis, B. afzelii flaB (OQ076388, OQ076395) clustered together with other sequences reported from previous studies in Poland (Supplementary Fig. S3); however, they differed significantly in case of the proportion of nucleotide sites changes (Fig. 4, Table 3). B. garinii flaB (OQ076404, OQ076403) were clustered with other sequences from Poland and Europe with no significant differences in p-distance (Fig. 4, Supplementary Fig. S4, Table 3).
R. monacensis ompB gene isolated from I. ricinus in present study (OQ076408) was clustered with other sequences from Europe and the proportion of nucleotide sites changes between them were not significant (Fig. 4, Supplementary Fig. S5, Table 3). Similarly, there was no significant differences in p-distance between current study and other sequences from Poland in case of R. helvetica gltA (OQ102267-71) (Fig. 4, Supplementary Fig. S6, Table 3). Statistically significant differences of the proportion of nucleotide sites changes between sequences obtained in present study and those reported from other countries were observed in case of R. aeschlimannii gltA (OQ102275) and R. raoultii gltA (OQ102285-88) isolated from D. reticulatus (Fig. 4, Supplementary Figs. S7, S8, Table 3).
Analysis of genetic diversity of R. raoultii gltA sequences (Supplementary Fig. S9) from our study showed statistically insignificant differences of p-distance between the forest and the meadow biotopes (p = 0.64); however significant differences were observed between studied years of 2018 and 2019 (p < 0.01), 2018 and 2020 (p < 0.01) (Fig. 4, Table 3).
Discussion
Our results confirmed the occurrence of ticks and the presence of a broad spectrum of microorganisms in adults of I. ricinus and D. reticulatus regardless habitat type. Similar observations were reported from other previous studies from eastern Poland29. Although, I. ricinus and D. reticulatus prefer various habitat types where their abundance is the highest, both species may be found in the same ecosystems. This observation was confirmed in our study (Fig. 1; Tables 1 and 2). We observed lack of significant differences of number of I. ricinus ticks collected from studied biotopes (Fig. 1). In our opinion it is caused by relatively low abundance of I. ricinus collected from both habitats. Also, I. ricinus can be found in meadow habitats too2, 4, 30. In case of our study, occurrence of I. ricinus in the meadow biotope can also be explained by close vicinity of forest and location of the transect used in the study in the river valley which favors the maintenance of humidity in environment known to affect water balance in ticks, which is critical for this tick species2, 30. Co-occurrence of ticks can also favor transmission of pathogens between habitats and may support pathogens’ maintenance in the environment31. Based on the obtained results, it was observed that the studied sites were situated in subregions that exhibited variations in long-term climatic data. These differences in climatic conditions could potentially influence the vegetation and the range of hosts available for ticks. However, it is important to note that there were no significant differences observed in terms of microclimatic conditions such as air temperature and relative humidity (Fig. 1). This finding suggests that the occurrence of the same tick species and pathogens in both biotopes can be attributed to the similarities in microclimatic conditions32, rather than aforementioned variations in long-term climatic conditions.
Bacteria representing the genus Rickettsia were the most common pathogens detected in D. reticulatus ticks in the study sites. The presence of the genetic material of pathogens from this genus (various species) was confirmed up to in 91.7% of D. reticulatus ticks collected from the forest biotope in 2018 and in up to 75.6% of ticks from the meadow biotope in 2020. For comparison, Rickettsia spp. infected 15.0% of I. ricinus ticks from the forest biotope in 2018 (Tables 1 and 2). The study results indicate that the prevalence of Rickettsia spp. in D. reticulatus in the studied sites is higher than in other regions of the country. Previous studies on TBPs in eastern Poland reported that 53.0% of D. reticulatus ticks were infected by Rickettsia spp.33. The rate of Rickettsia sp. infections in D. reticulatus ticks was estimated at 41.0% in northern Poland34 and 57.0% in the northeastern part of the country35. A high prevalence of Rickettsia spp. infections in D. reticulatus was also reported in other Central and Eastern European countries36, 37. In the UK, in turn, Tijsse-Klasen et al.38 reported only 5.0% of Rickettsia sp. infection in D. reticulatus ticks. The high prevalence of Rickettsia sp. infections in ticks may be related to the fact that these pathogens can be regarded as endosymbionts of ticks39. It is also supported by the possibility of transovarial and transstadial transmission of Rickettsia pathogens40.
The present results show a significant difference in the prevalence of Rickettsia sp. between I. ricinus and D. reticulatus; nevertheless, the prevalence of infection of I. ricinus ticks in the analyzed sites should be regarded as high, in comparison with the results reported by other researchers, with minimum infection rate (MIR) reaching 8.6% of nymphs and adults of I. ricinus ticks collected in 2013 in Białowieża Primeval Forest41 (Tables 1 and 2). The relatively high prevalence of Rickettsia spp. in I. ricinus may contribute to the risk of transmission of these pathogens to humans. Research carried out in northeastern Poland has indicated the presence of anti-Rickettsia IgG antibodies in 39.02% of professionals that are most exposed to the risk of tick attacks (foresters and farmers)42.
From an epidemiological point of view, Borreliella spp. infections are the most important threat posed by TBPs to public health43. In the analyzed study sites, we observed a high prevalence of Borreliella spp. infection in I. ricinus ticks (depending on the study year, 1.5–25.0%) (Tables 1 and 2). Reports from other regions of the country show Borrelia spp. infection in 0.25% of I. ricinus ticks on the Baltic coast and 4.5% in the Opole region13, 44. Results reported by other authors confirm the trends observed in other Central European countries, where B. afzelii and B. garinii are the dominant Borrelia genospecies, similarly to the area analyzed in the present study45.
The I. ricinus populations analyzed in the current study differed significantly in the prevalence of Borreliacea spirochetes infections. This is most probably related to the differences in the ecological structure of the habitats where the ticks were collected and the spectrum of small rodent species inhabiting these sites46.
Similarly, to the present results (2.1%) (Tables 1 and 2), a low prevalence of Borreliella spp. in D. reticulatus was observed in previous studies conducted in eastern Poland and Europe47, 48. It is assumed that Dermacentor ticks are not competent vectors of Borrelia spp., as the development of spirochetes is inhibited by midgut secretions and the immune system of these ticks49.
As indicated by the results of the present study, I. ricinus ticks and, to a lesser extent, D. reticulatus should be regarded as important vectors and reservoirs of A. phagocytophilum in eastern Poland (Tables 1 and 2). There was no significant difference in the prevalence of A. phagocytophilum between the forest and the meadow biotopes, but the difference in the prevalence between the subsequent years turned out to be significant. This probably results from the variability of the structure of hosts for juvenile ticks in the habitat (periodic flooding). Differences in the A. phagocytophilum prevalence depending on I. ricinus and D. reticulatus habitats were reported from western Ukraine50.
In Europe, D. reticulatus is the main reservoir and vector of B. canis17. However, I. ricinus ticks also play an important role in the maintenance and circulation of this pathogen in nature51. B. canis and Rickettsia spp. co-infections52 are frequently observed in D. reticulatus, as confirmed by the results of the present study (Tables 1 and 2; Supplementary Table S1). In the macroregional scale, a higher prevalence of B. canis infections in D. reticulatus ticks has been observed in eastern regions of Europe. For example, the protozoan was found to infect up to 3.14% of examined tick specimens in Ukraine53. In turn, a lower percentage (0.3% to 1.64%) was reported from Western European countries54.
The present study confirmed Bartonella spp. infections in the ticks (up to 16.7%) (Tables 1 and 2). This indicates that the prevalence of infections by this bacterium in the analyzed sites is higher than e.g., in Ukraine (2.7–8.1%)55. Eastern Poland is an area with numerous populations of elks (Alces alces) and other deer ungulates (Cervidae) which may explain the high prevalence of Bartonella sp. in ticks56. These animals are one of the main reservoir hosts of Bartonella. Taking into account high tick infestation in deer, Cervidae can play important role in maintenance and circulation of Bartonella in environment57, 58. Our study also showed the presence of N. mikurensis in I. ricinus ticks collected in the forest biotope (Table 1). This bacterium is most often detected in I. ricinus from forest habitats and recreational areas14, 59.
The analyzed I. ricinus and D. reticulatus populations were characterized by a high prevalence of microorganism co-infections. 85.72–100.00% of the D. reticulatus ticks were infected by at least two microorganisms. There was no significant difference in the occurrence of co-infections between the examined sites (Fig. 3, Supplementary Table S1). A co-infection rate of 8.5% was reported in previous studies conducted in eastern Poland60. Polymicrobial infections by up to five pathogens were detected in I. ricinus ticks collected in the forest biotope, and 30.0–50.0% of ticks were not infected by any studied pathogen (Fig. 3). Co-infections of I. ricinus with TBPs has been also reported from other countries in Europe61, 62. In our opinion high rate of co-infections in examined tick species in current study is caused by favorable environmental conditions for both, hosts and ticks.
Our results show high prevalence of FLE infections in D. reticulatus ticks; regardless of the type of habitat they occupy (Tables 1 and 2; Supplementary Table S1). According to certain studies, co-infections with this microorganism affects immune system of ticks and promotes or limit infection of ticks by other pathogens, also affecting tick vector competence63.
The current study shows the genetic diversity of the R. raoultii gltA gene isolated from D. reticulatus ticks compared to other studies (Fig. 4). In addition, genetic differentiation of this gene was confirmed throughout the study period. In our opinion this is influenced by the type of biotopes. The meadow biotope proximity to the river causes seasonal floods, which can affect the structure of the tick population and their potential hosts; although adult D. reticulatus can survive temporal water flooding64. Similarly, periodical snowmelt floodings, occurring in early spring, were also observed in the forest biotope. Moreover, mowing and burning of meadows are other factors that can alter the size of populations of D. reticulatus and their hosts inhabiting such ecosystems. Such phenomena have been proven to reduce the abundance of ticks and thus can contribute to changes in the spectrum of vectored pathogens and their prevalence in ticks65,66,67,68.
Conclusions
In overall, our study confirmed high prevalence of TBPs detected in sympatrically occurring adults of I. ricinus and D. reticulatus ticks, regardless of biotope type and climatic conditions. Substantial differences in spectrum of detected pathogens between the forest and meadow biotopes were observed in case of N. mikurensis, Theileria spp., Borreliaceae and Rickettsiaceae families. Moreover, our study confirmed significant impact of biotope type on prevalence of representatives of these families. Additionally, Rickettsia and Anaplasma temporal prevalence varied significantly across years. Studied tick populations in both biotopes were characterized by high co-infections rate. Our results also confirm significant diversity of R. raoultii gltA and B. afzelii flaB genes isolated in current study compared to reports from other countries. However, no relationship was found between the genetic diversity of the R. raoultii gltA gene isolated from examined ticks collected from the forest and the meadow biotopes. Additionally, temporal genetic diversity of R. raoultii gltA gene over the studied years was observed. Finally, our study confirmed impact of ecological type of biotope on spectrum and dynamic of pathogens prevalence in sympatrically occurring adult I. ricinus and D. reticulatus ticks.
Materials and methods
Ecological characteristic of study area
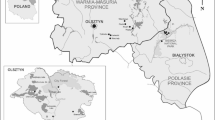
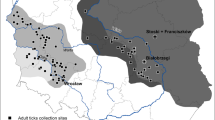
The field study was conducted in eastern Poland. Ticks were collected in two different types of ecological biotopes, forest (A) and meadow (B) (Fig. 5). Selection of biotope locations to assess pathogen prevalence was based on long-term climatic data of the 10 years previous to the study, (see below), altitude, and potential natural vegetation (PNV)69, 70.
The study site established in the forest biotope (51.48° N 23.16° E) was located in a sub-region within a temperate climate with features of continentalism with an average annual air temperature of 9.2 °C, low annual rainfall of 550 mm and a short growing season (less than 200 days), and altitude of 200 m a.s.l. A large part of the region is characterized by agricultural wasteland (about 40%). The PNV represents the Querco-Pinetum, Carici elongatae-Alnetum, Peucedano-Pinetum and Tilio-Carpinetum69,70,71. The tick collection site was established in a clearing surrounded by deciduous forest with the dominance of Querco-Fagetea vegetation (Fig. 5). Such type of biotope is preferred by small rodents, mainly of the genera Microtus and Apodemus, which are the main hosts of juvenile stages of ticks17, 72, 73.
The meadow study site (50.585° N, 23.07° E) was located within a sub-region within the highest altitudes in eastern Poland (above 300 m.a.s.l.), where the mean annual temperature is 9.3 °C. Total annual precipitation reaches over 700 mm and is the highest value in the entire Lublin province. The landscape is characterized by the mosaic type of small arable fields often separated by mid-field scrubs. The sub-region is covered in 40% with forests dominated by plant species such as Fagus sylvatica, Abies alba, and Picea abies. The PNV belongs to Leucobryo-Pinetum, Querco-Pinetum, and Dentario glandulosae-Fagetum types69,70,71. The tick collection site was established in a fragment of an agriculturally unused meadow, with dominant Molinio-Arrhenatheretea vegetation, located in the immediate vicinity of the Wieprz River (Fig. 5).
Tick collection and identification
The field study was carried out between 2018 and 2020 in eastern Poland. Collection was performed once per month, except in summer and winter months (July, August, November, December, January, and February) when behavioral diapause of D. reticulatus adults and substantial decrease of abundance of active adult stages of I. ricinus are observed7, 17, 30. To ensure an unbiased collection of ticks some criteria were followed: (i) questing ticks were collected systematically from the vegetation using the same method in all collections (i.e., flagging method74) following standard procedures (see below), (ii) ticks were invariably collected in the same transects of 900 m2 (30 × 30 m) in each site (i.e., one transect per site), (iii) tick collections were performed once per month in both sites, (iv) tick collections were synchronized (i.e., were carried out simultaneously at both study sites by different team members), and (v) ticks were collected only in days with similar weather conditions i.e., sunny, rainless and windless. The vegetation was swept with a 1-m2 white flannel cloth. After sweeping a transect of approximately 10-m, the cloth was turned over and attached ticks were transferred with metal tweezers into a 50-cm3 plastic container. This was repeated until the entire surface of the transects was covered. Grass blades were placed in the container to ensure the optimal level of humidity for the ticks. Additionally, each time when the ticks were collected, temperature and relative air humidity were measured at a level of 20 cm above the ground using a Data Loger device (R6030, Reed Instruments, Wilmington, NC, USA).
In the laboratory, the species, development stage, and sex of the tick specimens were identified using a Zeiss STEMI DV4 stereoscopic microscope (Carl Zeiss Light Microscopy, Göttingen, Germany). Taxonomically identification was based on morphological features and according to taxonomic key74. Next, the identified ticks were placed in an ULTF freezer (Arctico, Esbjerg, Denmark) at − 80 °C until DNA extraction.
Probably due to the ecological type of the studied habitats (high density of vegetation and tall grass), only a small number immature tick stages were collected. Therefore, only adult ticks of both species were included in further analyses.
Long-term climatic data collection and selection of biotopes
In order to select biotope locations for our study, we investigated the differences in climatic conditions between two regions in eastern Poland, within which the study sites were established. To this end, long-term climatic data, covering 10-year period prior the field study (2008–2017) was collected and analyzed. Such data included mean monthly values of temperature (calculated on the basis of 8 measurements taken each day) and total precipitation, total monthly number of days with snow cover and total length of the vegetation season. Data was obtained from meteorological stations located closest to tick collection sites (about 20 km in a straight line), i.e., Włodawa station for the study site established in forest biotope and Tomaszów Lubelski station for the meadow biotope71.
Molecular analyses
DNA extraction
Prior to the genomic DNA extraction procedure, the tick bodies of randomly chosen 258 D. reticulatus and 185 I. ricinus specimens were cut into smaller fragments using sterile scalpels. The DNA was extracted using a NucleoSpin® Tissue DNA extraction kit (Macherey–Nagel, Düren, Germany) following the manufacturer's instructions. Ticks were homogenized using a Precellys 24 lyser/homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) at 5500 rpm for 20 s using 2.8 mm stainless steel beads in 180 µL of lysis buffer and 25 µL of Proteinase K from the Nucleospin Tissue kit (Macherey–Nagel, Düren, Germany). Homogenates were incubated for 3 h at 56 °C and DNA was extracted according to the manufacturer’s instructions. The concentration of DNA was measured using a NanoDrop™ spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA) at a 260/280 nm wavelength. The samples were stored at − 20 °C until further processing.
DNA pre-amplification
The PreAmp Master Mix kit (Fluidigm, San Francisco, CA, USA) was used for DNA preamplification. The procedure was carried out in accordance with the manufacturer's recommendations. All primer pairs targeting TBPs were pooled, combining equal volumes with a final concentration of 0.2 µM each. The reaction was performed in a final volume of 5 μL containing 1 μL Perfecta Preamp 5 ×, 1.25 μL pooled primer mix, 1.5 µL distilled water and 1.25 μL DNA. The thermocycling program consisted of one cycle at 95 °C for 2 min, 14 cycles at 95 °C for 15 s and 4 min at 60 °C. The products were diluted 1:10 in Milli-Q ultrapure water and stored at − 20 °C until further use.
Microfluidic real-time PCR for high-throughput microorganism detection
The BioMark™ real-time PCR system (Fluidigm, San Francisco, USA) was used for detection of the pathogens. Real-time PCR reactions were performed using 6-carboxyfluorescein (FAM)-labeled and black hole quencher (BHQ1)-labeled TaqMan probes with TaqMan Gene expression master mix according the manufacturer’s instructions (Applied Biosystems, France). The amplification process consisted of the following successive steps: 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles of two-step amplification of 15 s at 95 °C, and 1 min at 60 °C.
In the PCR reaction, 36 pathogen primers identical to those used by Boularias et al.75 were applied simultaneously. Additionally, primers targeting I. ricinus and D. reticulatus DNA were used to control for presence of tick species DNA. Escherichia coli DNA and Escherichia-specific primers were used as positive controls of the real-time PCR reactions. Ultra-pure water was used as a negative control. The real-time PCR results were analyzed using Fluidigm real-time PCR analysis software to obtain crossing threshold (Ct) values. Samples with a Ct value of < 25 was considered as positive.
DNA sequencing
Samples positive for Rickettsia spp. and Borreliella spp. were sequenced due to the epidemiological importance of these pathogens among the microorganisms detected in the current study. For the sequence analysis, 3–5 samples of extracted DNA were selected from the genetic material obtained from each of the studied sites in 2018–2020 to identify pathogens of the genus Rickettsia and Borreliella. The PCR products were sequenced by Eurofins Genomics (https://cochin.eurofins.com, accessed on 5 August 2021). The sequenced products were analyzed using BioEdit software (Ibis Biosciences, Carlsbad, CA, USA).
Phylogenetic analysis
To assess the genetic diversity of the microorganisms identified in this study, the obtained DNA sequences were analyzed using the Basic Local Alignment Search Tool (BLAST) with the NCBI database76 and similar sequences were searched in GenBank database77. Next, all sequences for specific pathogen and target genes available in BLAST were processed and redundant ones were deleted. Finally, phylogenetic trees were constructed with up to ten sequences obtained from other locations including Poland, Europe, Africa, Americas, Asia and Oceania, when available.
The evolutionary history was inferred by using the Maximum Likelihood method with complete deletion option and bootstrap set at 500 and analyzed in MEGA 1178. Depending on performed MEGA 11 analysis, different evolutionary models were used to construct the phylogenetic trees. Jukes-Cantor model was applied in case of R. helvetica gltA, R. raoultii gltA and R. monacensis ompB; while Kimura 2-parameter method was used in case of B. afzelii flaB and B. garinii flaB. Tamura 3-parameter model was chosen to construct B. burgdorferi flaB and R. aeschlimannii gltA phylogenetic tree. To determine genetic diversity of analyzed sequences, the proportion of nucleotide sites changes between them was calculated as p-distance in MEGA 1178.
Statistical analysis
The type of data distribution was checked using the Shapiro–Wilk test. Long-term climatic data was tested using parametric t-test. Non parametric tests were used to check differences in the number of collected ticks between studied sites (U-Mann Whitney test), while the statistical differences in the value of temperature and humidity measured at the time of tick collection between studied sites over the years were tested using Kruskal–Wallis test. Spearman’s correlation coefficient test was used to determine association of temperature and humidity with tick activity.
The chi-square test was used to analyze the prevalence of microorganisms detected in D. reticulatus and I. ricinus ticks, as well as the differences between subsequent study years and biotopes. The significance of differences in p-distance values were compared using U-Mann Whitney test.
The significance level was set at p < 0.05. Statistical calculations were performed using the STATISTICA 13.3 PL statistical package (StatSoft, TIBCO Software Inc, Palo Alto, CA, USA) and GraphPad 8.4 (GraphPad Software Inc., La Jolla, CA, USA).
Multiple correspondence analysis
Multiple correspondence analysis (MCA) was used to analyze the associations between the tick-borne pathogens, tick species and sampling sites (biotopes). The inertia values were calculated by the standard “Burt matrix” method. The analyses were performed using the statistical software package Statgraphics Centurion v. 16.1.03 (StatPoint Technologies Inc, Warrenton, VA, USA).
Data availability
All data generated in this study has been published in manuscript or Supplementary files.
References
Estrada-Peña, A., Mihalca, A. D. & Petney, T. N. Ticks of Europe and North Africa: A guide to species identification (Springer, 2018).
Medlock, J. M. et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 6, 1–11 (2013).
Karbowiak, G. The occurrence of the Dermacentor reticulatus tick-its expansion to new areas and possible causes. Ann. Parasitol. 60, 37–47 (2014).
Halos, L. et al. Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl. Environ. Microbiol. 76, 4413–4420 (2010).
Pfäffle, M., Littwin, N. & Petney, T. Host preferences of immature Dermacentor reticulatus (Acari: Ixodidae) in a forest habitat in Germany. Ticks Tick Borne Dis. 6, 508–515 (2015).
Rubel, F. et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 7, 224–233 (2016).
Zając, Z., Kulisz, J., Woźniak, A., Bartosik, K. & Khan, A. Seasonal activity of Dermacentor reticulatus ticks in the era of progressive climate change in eastern Poland. Sci. Rep. 11, 1–9 (2021).
Mierzejewska, E. J., Alsarraf, M., Behnke, J. M. & Bajer, A. The effect of changes in agricultural practices on the density of Dermacentor reticulatus ticks. Vet. Parasitol. 211, 259–265 (2015).
Zając, Z., Woźniak, A. & Kulisz, J. Density of Dermacentor reticulatus ticks in eastern Poland. Int. J. Environ. Res. Public Health 17, 2814 (2020).
Rubel, F. et al. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 82, 95–123 (2020).
Gustafson, R. Epidemiological studies of Lyme borreliosis and tick-borne encephalitis. Scand. J. Infect. Dis. 92, 1–63 (1994).
Špitalská, E. et al. Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick Borne Dis. 9, 1207–1211 (2018).
Asman, M., Witecka, J., Solarz, K., Zwonik, A. & Szilman, P. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south–west Poland. Ann. Agric. Environ. Med. 26, 544–547 (2019).
Kjelland, V. et al. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick Borne Dis. 9, 1098–1102 (2018).
Hubálek, Z. & Rudolf, I. Tick-borne viruses in Europe. Parasitol. Res. 111, 9–36 (2012).
Ličková, M. et al. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick Borne Dis. 11, 101414 (2020).
Földvári, G., Široký, P., Szekeres, S., Majoros, G. & Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 9, 1–29 (2016).
Genchi, M. et al. Francisella tularensis: No evidence for transovarial transmission in the tularemia tick vectors Dermacentor reticulatus and Ixodes ricinus. PLoS ONE 10, e0133593 (2015).
Černý, J. et al. Management options for Ixodes ricinus-associated pathogens: A review of prevention strategies. Int. J. Environ. Res. Public Health 17, 1830 (2020).
Schaarschmidt, D. et al. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis. 4, 334–340 (2013).
Zivkovic, Z., Nijhof, A. M., De la Fuente, J., Kocan, K. M. & Jongejan, F. Experimental transmission of Anaplasma marginale by male Dermacentor reticulatus. BMC Vet. Res. 3, 1–6 (2007).
Estrada-Peña, A., Ostfeld, R. S., Peterson, A. T., Poulin, R. & de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 30, 205–214 (2014).
Estrada-Peña, A., de La Fuente, J., Ostfeld, R. S. & Cabezas-Cruz, A. Interactions between tick and transmitted pathogens evolved to minimise competition through nested and coherent networks. Sci. Rep. 5, 1–13 (2015).
Estrada-Peña, A., De La Fuente, J. & Cabezas-Cruz, A. Functional redundancy and ecological innovation shape the circulation of tick-transmitted pathogens. Front. Cell. Infect. Microbiol. 7, 234 (2017).
Lejal, E. et al. Temporal patterns in Ixodes ricinus microbial communities: An insight into tick-borne microbe interactions. Microbiome 9, 1–20 (2021).
Lejal, E. et al. A three-years assessment of Ixodes ricinus-borne pathogens in a French peri-urban forest. Parasit. Vectors 12, 551 (2019).
Glass, A., Springer, A. & Strube, C. A 15-year monitoring of Rickettsiales (Anaplasma phagocytophilum and Rickettsia spp.) in questing ticks in the city of Hanover, Germany. Ticks Tick Borne Dis. 13, 101975 (2022).
Zeman, P., Pazdiora, P. & Benes, C. Spatio-temporal variation of tick-borne encephalitis (TBE) incidence in the Czech Republic: Is the current explanation of the disease’s rise satisfactory?. Ticks Tick Borne Dis. 1, 129–140 (2010).
Wójcik-Fatla, A. et al. Occurrence of Francisella spp. in Dermacentor reticulatus and Ixodes ricinus ticks collected in eastern Poland. Ticks Tick Borne Dis. 6, 253–257 (2015).
Zając, Z. et al. Environmental determinants of the occurrence and activity of Ixodes ricinus ticks and the prevalence of tick-borne diseases in eastern Poland. Sci. Rep. 11, 15472 (2021).
Michelitsch, A., Wernike, K., Klaus, C., Dobler, G. & Beer, M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses 11, 669 (2019).
Randolph, S. E. Evidence that climate change has caused ‘emergence’of tick-borne diseases in Europe?. Int. J. Med. Microbiol. 293, 5–15 (2004).
Wójcik-Fatla, A. et al. Study on tick-borne rickettsiae in eastern Poland. I. Prevalence in Dermacentor reticulatus (Acari: Amblyommidae). Ann. Agric. Environ. Med. 20, 276–279 (2013).
Stańczak, J. Detection of spotted fever group (SFG) rickettsiae in Dermacentor reticulatus (Acari: Ixodidae) in Poland. Int. J. Med. Microbiol. 296, 144–148 (2006).
Chmielewski, T., Podsiadly, E., Karbowiak, G. & Tylewska-Wierzbanowska, S. Rickettsia spp. in ticks, Poland. Emerg. Infect. Dis. 15, 486 (2009).
Karbowiak, G. et al. Rickettsia raoultii in Dermacentor reticulatus ticks, Chernobyl exclusion zone, Ukraine, 2010. Emerg. Infect. Dis. 22, 2214 (2016).
Silaghi, C., Hamel, D., Thiel, C., Pfister, K. & Pfeffer, M. Spotted fever group rickettsiae in ticks, Germany. Emerg. Infect. Dis. 17, 890 (2011).
Tijsse-Klasen, E. et al. Spotted fever group rickettsiae in Dermacentor reticulatus and Haemaphysalis punctata ticks in the UK. Parasit. Vectors 6, 212 (2013).
Garcia-Vozmediano, A. et al. The genetic diversity of Rickettsiella symbionts in Ixodes ricinus throughout Europe. Microb. Ecol. 84, 613–626 (2022).
Eremeeva, M. E. & Dasch, G. A. Challenges posed by tick-borne rickettsiae: Eco-epidemiology and public health implications. Front. Public Health 3, 55 (2015).
Stańczak, J., Biernat, B., Racewicz, M., Zalewska, M. & Matyjasek, A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick Borne Dis. 9, 427–434 (2018).
Borawski, K. et al. Prevalence of spotted fever group Rickettsia in north-eastern Poland. Infect. Dis. 51, 810–814 (2019).
Banović, P. et al. Humans infested with Ixodes ricinus are exposed to a diverse array of tick-borne pathogens in Serbia. Ticks Tick Borne Dis. 12, 101609 (2021).
Asman, M., Witecka, J., Korbecki, J. & Solarz, K. The potential risk of exposure to Borrelia garinii, Anaplasma phagocytophilum and Babesia microti in the Wolinski National Park (north-western Poland). Sci. Rep. 11, 1–6 (2021).
Strnad, M., Hönig, V., Růžek, D., Grubhoffer, L. & Rego, R. O. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 83, e00609-e617 (2017).
Delattre, P., De Sousa, B., Fichet-Calvet, E., Quéré, J. P. & Giraudoux, P. Vole outbreaks in a landscape context: Evidence from a six year study of Microtus arvalis. Landsc. Ecol. 14, 401–412 (1999).
Bonnet, S. et al. Prevalence of tick-borne pathogens in adult Dermacentor spp. ticks from nine collection sites in France. Vector-Borne Zoonotic Dis. 13, 226–236 (2013).
Dzięgiel, B. et al. Prevalence of Babesia canis, Borrelia burgdorferi sensu lato, and Anaplasma phagocytophilum in hard ticks collected from meadows of Lubelskie Voivodship (eastern Poland). B. Vet. I. Pulawy 58, 29–33 (2014).
Rudolf, I. & Hubálek, Z. Effect of the salivary gland and midgut extracts from Ixodes ricinus and Dermacentor reticulatus (Acari: Ixodidae) on the growth of Borrelia garinii in vitro. Folia Parasitol. 50, 159 (2003).
Ben, I. & Lozynskyi, I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and co-infection with Borrelia burgdorferi and tick-borne encephalitis virus in western Ukraine. Vector-Borne Zoonotic 19, 793–801 (2019).
Azagi, T. et al. Circulation of Babesia species and their exposure to humans through Ixodes ricinus. Pathogens 10, 386 (2021).
Grochowska, A. et al. Pathogens carried by Ixodes ricinus and Dermacentor reticulatus ticks including co-infections. Przegląd Epidemiol. 74, 466–474 (2020).
Karbowiak, G. et al. The infection of questing Dermacentor reticulatus ticks with Babesia canis and Anaplasma phagocytophilum in the Chernobyl exclusion zone. Vet. Parasitol. 204, 372–375 (2014).
Silaghi, C., Weis, L. & Pfister, K. Dermacentor reticulatus and Babesia canis in Bavaria (Germany)—A georeferenced field study with digital habitat characterization. Pathogens 9, 541 (2020).
Rogovskyy, A. S. et al. Borrelia and other zoonotic pathogens in Ixodes ricinus and Dermacentor reticulatus ticks collected from the chernobyl exclusion zone on the 30th anniversary of the nuclear disaster. Vector-Borne Zoonotic Dis. 19, 466–473 (2019).
Sacristán, C. et al. Bartonella spp. detection in ticks, Culicoides biting midges and wild cervids from Norway. Transbound. Emerg. Dis. 68, 941–951 (2021).
Dehio, C. et al. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deerInt. J. Syst. Evol. Microbiol. 51, 1557–1565 (2001).
Bermond, D. et al. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. J. Syst. Evol. Microbiol. 52, 383–390 (2002).
Derdáková, M. et al. Candidatus Neoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of Central Europe. Parasit. Vectors 7, 1–4 (2014).
Zając, V. et al. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann. Agric. Environ. Med. 24, 26–32 (2017).
Lommano, E., Bertaiola, L., Dupasquier, C. & Gern, L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl. Environ. Microbiol. 78, 4606–4612 (2012).
Milutinović, M. et al. Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and their co-infections in host-seeking Ixodes ricinus ticks collected in Serbia. Exp. Appl. Acarol. 45, 171–183 (2008).
Hussain, S. et al. The symbiotic continuum within ticks: Opportunities for disease control?. Front. Microbiol. 13, 854803 (2022).
Weiler, M., Duscher, G. G., Wetscher, M. & Walochnik, J. Tick abundance: A one year study on the impact of flood events along the banks of the river Danube, Austria. Exp. App. Acarol. 71, 151–157 (2017).
Bajer, A. et al. Abundance of the tick Dermacentor reticulatus in an ecosystem of abandoned meadows: Experimental intervention and the critical importance of mowing. Vet. Parasitol. 246, 70–75 (2017).
Goodenough, A. E. et al. Managing grassland for wildlife: The effects of rotational burning on tick presence and abundance in African savannah habitat. Wildl. Biol. 1, 1–8 (2017).
MacDonald, A. J. et al. Risk of vector tick exposure initially increases, then declines through time in response to wildfire in California. Ecosphere 9, e02227 (2018).
Gleim, E. R. et al. Frequent prescribed fires can reduce risk of tick-borne diseases. Sci. Rep. 9, 1–10 (2019).
Kaszewski, B. M. Climatic Conditions of the Lublin Region 1–42 (Maria Curie-Skłodowska University Publishing House, 2008).
Matuszkiewicz, J. M. Plant landscapes and geobotanical regions 1: 2,500,000. Plant landscapes and geobotanical regions. In Atlas of the Republic of Poland (IGiPZ PAN, Chief National Surveyor, 1994).
World Weather–Local Weather Forecast, https://en.tutiempo.net/climate/poland (Accessed on 20 December 2022).
Hofmeester, T. R. et al. Few vertebrate species dominate the Borrelia burgdorferi sl life cycle. Environ. Res. Lett. 11, 043001 (2016).
Fabri, N. D. et al. The circulation of Anaplasma phagocytophilum ecotypes is associated with community composition of vertebrate hosts. Ecosphere 13, e4243 (2022).
Nowak-Chmura, M. Fauna of Ticks (Ixodida) of Central Europe (Scientific Publishing House of the Pedagogical University, Cracov, 2013) ((In Polish)).
Boularias, G. et al. High-throughput microfluidic real-time PCR for the detection of multiple microorganisms in Ixodid cattle ticks in northeast Algeria. Pathogens 10, 362 (2021).
Basic Local Alignment Search Tool (BLAST), https://blast.ncbi.nlm.nih.gov/Blast.cgi (Accessed on 20 December 2022).
National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/ (Accessed on 20 December 2022).
Tamura, K., Stecher, G. & Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. https://doi.org/10.1093/molbev/msab120 (2021).
Funding
UMR BIPAR is supported by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (Grant No. ANR-10-LABX-62-IBEID). AWC was supported by Programa Nacional de Becas de Postgrado en el Exterior “Don Carlos Antonio López” (Grant No. 205/2018). AM is supported by the ‘Collectivité de Corse’, grant: ‘Formations superieures’ (SGCE-RAPPORT No. 0300).
Author information
Authors and Affiliations
Contributions
Z.Z. conceptualization, methodology, field work, molecular analysis, writing original draft, writing - review and editing, visualization; D.O. writing original draft, methodology, writing - review and editing, visualization; A.F.S. molecular analysis, writing original draft, writing - review and editing; A.W.C. writing original draft, molecular analysis, writing - review and editing, S.M. writing original draft, methodology, writing - review and editing; C.G. molecular analysis, writing - review and editing; J.K. field work, writing original draft, writing - review and editing, visualization; A.W. field work, writing original draft, writing - review and editing, visualization; K.B. writing - review and editing; A.C.C. conceptualization, methodology, writing original draft, writing - review and editing, visualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zając, Z., Obregon, D., Foucault-Simonin, A. et al. Disparate dynamics of pathogen prevalence in Ixodes ricinus and Dermacentor reticulatus ticks occurring sympatrically in diverse habitats. Sci Rep 13, 10645 (2023). https://doi.org/10.1038/s41598-023-37748-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37748-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.