Abstract
Dermacentor reticulatus is tick species with an expanding geographical range in Europe, which creates the possibility of spreading microorganisms of significant veterinary and medical importance. The study aimed to investigate the prevalence and genetic diversity of Rickettsia spp., Babesia spp., Borrelia spp. and Anaplasma phagocytophilum in adult D. reticulatus ticks from the Eastern European population in the urban and the natural biotopes of north-eastern Poland. Microorganisms were detected by PCR and identified by DNA sequencing. The overall infection rate of at least one of the pathogens was 29.6%. The predominantly was Rickettsia spp. (27.1%) (with R. raoultii—9.1%) followed by Babesia spp. (2.4%) with B. canis (1.5%) as the most frequent. Based on 18S rRNA gene sequence, three B. canis genotypes were revealed. The prevalence of R. raoultii and B. canis was significantly higher in ticks from natural biotopes. The infection rates of B. afzelii and A. phagocytophilum were determined at 0.9% and 0.3%, respectively. Co-infections were detected in 3.8% of infected ticks. In diagnosing tick-borne diseases in humans, tick-borne lymphadenopathy should not be excluded. The prevalence of different genotypes of B. canis suggests differences in the clinical picture of canine babesiosis in the area.
Similar content being viewed by others
Introduction
Dermacentor reticulatus is the second most abundant tick species after Ixodes ricinus in central Europe1,2. Until the 1980s, the geographical range of D. reticulatus was clearly divided into two main zones: the Western European and the Eastern European populations. In between, there was a large area from the Baltic Sea coast through central Germany, western Poland to the southern border of Hungary where the meadow tick had never been observed3,4. Currently, those two populations of D. reticulatus (Western and Eastern) are among the most dynamic tick populations in Central Europe5. In several European countries (Slovakia, the Czech Republic, the United Kingdom, the Netherlands, and Germany), habitat expansion of D. reticulatus was noted6, and the northern distribution border in the Baltic countries (Lithuania, Latvia) moved further to the north7. On the Polish territory in “the gap” zone, new foci occurrences of this tick species have also been reported5,8,9,10 which leads to closer borders between the western and the eastern D. reticulatus populations.
With the expansion of D. reticulatus into new areas, the veterinary-medical importance of this species has increased due to the variety of transmitted pathogenic microorganisms11. D. reticulatus is considered the main vector of the protozoa Babesia canis (the etiological agent of canine babesiosis), as well bacteria of the genera Rickettsia, Anaplasma, Bartonella, Francisella, Coxiella burnetii2,12 and tick-borne encephalitis virus13. The significance of D. reticulatus in the transmission of Borrelia burgdorferi s.l. is still unclear2,12. Not without significance is also the contribution of D. reticulatus ticks to the maintenance of tick-borne pathogens in the environment through the transfer of pathogenic microorganisms between various vertebrate hosts, which are susceptible to infection and serve as efficient reservoirs12.
The eastern population of D. reticulatus ticks in Poland is considered a stable population and is the source of newly emerging foci of this species in the zone previously considered free (central and western Poland)5,9, and thus the spread of tick-borne microorganisms of significant veterinary and medical importance. The aim of the study was to assess the prevalence and diversity of tick-borne protozoa (Babesia spp.) and bacteria (Rickettsia spp., A. phagocytophilum, Borrelia spp.) in adult D. reticulatus ticks from the Eastern European population in the urban and the natural biotopes of north-eastern Poland.
Material and methods
Study area and tick collection
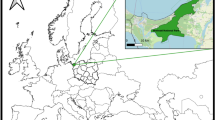
Questing adult D. reticulatus ticks (n = 886, 587 females, 299 males) were collected between March-June in 2016 and 2017. The collection sites were located in urbanized areas (within the administrative borders of the city of Olsztyn) (n = 395) and in natural biotopes of the central part of the Warmia and Mazury region (n = 87) as well as in the Biebrza National Park (n = 404) (Podlasie region) (Fig. 1) (Supplementary Table S1). Tick collections were performed twice per month in each year of the study during the daytime between 9 a.m. and 4 p.m. using the standard flagging method14. In the laboratory, specimens were morphological identified by species and sex using taxonomic key15 and were preserved individually in 70% ethanol at 4 °C.
Dermacentor reticulatus tick collection sites located in north-eastern Poland. The map was designed in CorelDRAWX5 based on Google Maps (https://www.google.pl/maps).
DNA extraction
The genomic DNA from D. reticulatus ticks was extracted using the ammonia method16. Before DNA extraction, ticks preserved in 70% ethanol were air-dried and then separately cut and crushed using a sterile mortar in 0.7 M ammonium hydroxide (NH4OH). The obtained DNA lysates were stored at − 20 °C for further molecular analysis.
Pathogen DNA detection
Tick DNA samples were used to detect tick-borne microorganisms. For the detection of DNA of Babesia spp., a nested PCR (nPCR) reaction targeting the 18S rRNA gene using the primers CRYPTO F/CRYPTO R and Bab GF2/Bab GR217,18,19 was used (Table 1). The presence of Rickettsia spp. in tick genomic DNA samples was confirmed by using a set of primers (CS409/Rp1258)20 specific to the fragment of the citrate synthase (gltA) gene (Table 1). A. phagocytophilum DNA was detected using the nPCR with two sets of primers (ge3a/ge10r and ge9f/ge2) targeting fragments of the 16S rRNA gene21 (Table 1). Detection of B. burgdorferi s.l. DNA was performed via the nPCR-restriction fragment length polymorphism (RFLP) method using primers 132f/905r and 202f/832r specific to the flaB gene22,23 (Table 1). The identification of species belonging to the Borrelia burgdorferi s. l. complex was based on the restriction patterns obtained by digestion of the inner-PCR product (604 bp) by using the restriction enzyme HpyF3I (DdeI) (ThermoFisher Scientific, Waltham, MA, USA)22,23.
All amplifications were performed in a total volume of 25 µL of PCR mixture containing 12.5 µL of 2× PCR Master Mix Plus (0.1 U/µL of Taq polymerase supplied in a PCR buffer, 4 mM of MgCl2 and 0.5 mM of each dNTPs) (A&A Biotechnology, Gdynia, Poland), 0.5 µL of each primer (10 µM), 2–4 µL of template DNA (in nPCR—1 µL of template DNA or 1 µL of the outer-PCR product) and an appropriate amount of sterile nuclease-free water. DNA of B. canis isolated from the blood of an infected dog, Rickettsia spp., A. phagocytophilum and B. afzelii obtained from an infected I. ricinus tick (confirmed by sequencing in earlier studies) were used as a positive control. PCR amplicons were visualized on 1.5% agarose gels stained with Midori Green Stain (Nippon Genetics Europe, Düren, Germany) using GelDocXR (Bio-Rad, Hercules, California, USA).
Identification of pathogen species
To confirm the species of the detected microorganisms, selected PCR products (81 for Rickettsia spp., 21 for Babesia spp., three for A. phagocytophilum, five for Borrelia spp.) were purified using the CleanUp purification kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s protocol and sequenced bi-directionally (Macrogen Europe, Amsterdam, the Netherlands) with forward and reverse primers. The obtained nucleotide sequences were edited in BioEdit v. 7.2 software (https://bioedit.software.informer.com, accessed on February 2022) and compared with data registered in the GenBank database (http://www.ncbi.nih.gov/Genbank/index.html, accessed on May 2023) using the BLAST-NCBI program (http://www.ncbi.nlm.nih.gov/BLAST).
Consensus sequences were deposited in the GenBank database and registered under the accession numbers ON660868-870 for the gltA gene of Rickettsia spp., OR056353, OR064513-518 for the 18S rDNA of Babesia spp., OR096226-227 for the 16S rDNA of A. phagocytophilium and OR046059-060 for the flaB gene of Borrelia spp.
The phylogram was constructed using the Maximum Likelihood method based on the Kimura 2-parameter model. The topology of the phylogenetic tree was evaluated using the bootstrap method with 1000 replicates. The phylogenetic analysis was conducted using MEGA X software (https://www.megasoftware.net).
Statistical analysis
A chi-square test (with post-hoc Bonferroni test) and 95% confidence intervals (95% CI) were used to compare differences in the prevalence of detected microorganisms in the sex of ticks, regions, habitats and years of study. In all analyses, p-values below 0.05 were considered statistically significant. The analysis was conducted using the software package SPSS version 27.0 for Windows (SPSS Inc., Chicago, USA).
Results
Total prevalence of pathogens
In north-eastern Poland, the overall infection rate of D. reticulatus ticks for at least one of the examined pathogens was 29.6% (262/886) (Table 2). Females (30.2%) and males (28.4%) were infected in a similar proportion (χ21 = 0.283, p = 0.595). Significant differences in pathogen prevalence were noted between the years of the study (χ21 = 8.515, p = 0.004) (Table 2). The DNA of microorganisms was confirmed in 33.3% and 24.3% of the tested ticks in 2016 and 2017, respectively. The highest prevalence was recorded in D. reticulatus ticks collected in Biebrza National Park (39.4%) compared to the central part of the Warmia-Mazury region (28.7%) and the city of Olsztyn (19.7%) (χ22 = 36.909, p < 0.001) (Table 2). In the population of D. reticulatus from natural areas, the level of infection was almost twice as high as from urban areas, 37.5% and 19.7% (χ21 = 33.032, p < 0.001), respectively (Table 2).
Diversity and genotyping of pathogens
Rickettsia spp.
Rickettsia spp. DNA was the most frequently detected in questing D. reticulatus ticks (Table 3). The overall Rickettsia spp. infection rate was 27.1% (240/886). The prevalence of this bacteria did not differ between females (27.8%) and males (25.8%). A significant difference was noted between ticks collected in natural biotopes (35.6%) and in urban areas (16.5%) (χ21 = 40.797, p < 0.001). Rickettsia spp. DNA was the most frequently confirmed in ticks collected in the Biebrza National Park (38.1%), followed by the central part of the Warmia-Mazury region (24.1%) and the city of Olsztyn (16.5%) (χ22 = 47.882, p < 0.001). The percentage of Rickettsia-positive ticks was significantly higher in 2016 (32.0%) than in 2017 (20.2%) (χ21 = 15.210, p < 0.001).
Sequence analysis of the partial gltA gene indicated the presence of only R. raoultii in D. reticulatus. All obtained nucleotide sequences (n = 81) were similar and showed 100% identity to R. raoultii isolated from the blood of a patient (GenBank: KY474576) and dog (GenBank: MT019635) in China as well as ticks from Poland (Fig. 2).
Phylogenetic relationships between Rickettsia raoultii identified in the study and accessions from GenBank, based on the sequences of the gltA gene of Rickettsia spp. The phylogram was constructed conducted in MEGA X software (https://www.megasoftware.net) using the Maximum Likelihood method and the Kimura 2-parameter method as a distance method. The percentage of replicate trees in which the associated taxa are clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of base substitutions per site. The sequences obtained in this study are labelled with black symbols.
Babesia spp.
The total infection rate of Babesia spp. in D. reticulatus ticks was 2.4% (21/886) (Table 3). The infection rate did not significantly differ between females (2.6%) and males (2.0%) or the years of study (2016–2017) (1.5% and 3.5%, respectively). The highest prevalence of Babesia spp. was in the central part of the Warmia-Mazury region (9.8%) (χ22 = 23.946, p < 0.001). However, no significant differences between natural biotopes (2.0%) and urban areas (2.8%) (χ21 = 0.529, p = 0.467) (Table 3) were noted.
Among infected Babesia spp. ticks (n = 21), nucleotide sequences analysis of partial 18S rRNA gene revealed the presence of B. canis and B. microti. Statistically significant differences in the occurrence of both species of protozoa were determined in relation to the studied region (χ22 = 8.086, p = 0.018), biotope (χ21 = 6.390, p = 0.024) and sex of ticks (χ21 = 5.169, p = 0.046) (Table 3).
B. canis was identified in 1.5% of ticks (13/886), including females (1.2%, n = 7) and males (2.0%, n = 6) in all examined years (2016–2017). This species of protozoan was detected mainly in D. reticulatus ticks from the central part of the Warmia-Mazury region (9.2%, 8/87). Ticks from both natural and urban biotopes were B. canis-positive in similar proportions, 1.8% (n = 9) and 1.0% (n = 4), respectively. All obtained chromatograms of the partial 18S rRNA gene B. canis were checked manually. In analyzed sequences at the positions corresponding to the 609–610 nucleotides of the complete ssrRNA gene (GenBank: AY072926), two nucleotide substitutions (GA/AG) were observed (Fig. 3). In 69.2% (9/13) of B. canis isolates GA dinucleotide (genotype A) was detected. Those sequences were 100% identical to sequences detected in the blood of naturally B. canis-infected dogs from Poland (GenBank: EU622793) and from Croatia (GenBank: MK089785), as well as in questing D. reticulatus in Poland (GenBank: KT272401). The AG nucleotide combination (genotype B) (Fig. 3) was detected in one B. canis positive sample (7.7%, 1/13) and it showed 100% identity to sequences revealed in B. canis from the blood of a dog in Poland (GenBank: EU622792) as well D. reticulatus from Romania (GenBank: MK836022) and Kazakhstan (GenBank: MK070118). It was found that 23.1% of B. canis isolates (3/13) had the GA/AG nucleotide double peak at positions 609–610 of the complete ssrRNA gene (GenBank: AY072926) and belonged to genotype A/B (Fig. 3).
Babesia microti were found in 0.9% (8/889) of examined D. reticulatus ticks. B. microti were detected only in females, mostly collected from an urban area (Olsztyn city) (1.8%, n = 7). Only one tick infected by B. microti was from natural areas (Biebrza National Park). All isolates identified as B. microti showed 100% identity with the genetic variants included in nonpathogenic B. microti Munich type (GenBank: AB071177, AY789075).
Anaplasma phagocytophilium and Borrelia spp.
Anaplasma phagocythophilum was identified in 0.3% (3/886) of tick DNA samples at the same proportion in females (0.3%) and males (0.3%) (Table. 3). DNA of B. burgdorferi s.l. was detected in 0.9% (8/886) of D. reticulatus of females (1%) and males (0.7%) (Table 3). There were no significant differences found in the infection rates of A. phagocythophilum or Borrelia spp. based on year, region or biotope.
All A. phagocythophilum-positive samples were confirmed by nucleotide sequencing. All of them were identical and showed 100% similarity to the sequence of A. phagocythophilum derived from the blood of red deer in Czechia (GenBank: EU839849) and of Merino sheep in Germany (GenBank: MZ348273). Borrelia spp. in D. reticulatus samples were identified as B. afzelii. The sequence showed 100% identity to the strains BO23 (GenBank: CP018262) and K78 (GenBank: CP009058) that were detected in symptomatic patients with borreliosis in Germany and Austria.
Co-infections of Rickettsia spp., Babesia spp., Anaplasma phagocytophilum and Borrelia spp.
Among 267 infected D. reticulatus ticks, co-infections of examined microorganisms were recognized in 3.8% (n = 10) of them (Table 4). All noted co-infections were double. Double infections of R. raoultii or Rickettsia spp./B. canis and R. rauoltii/B. afzelii were identified in equal proportions. Each of them accounted for 1.5% (n = 4) of the infected ticks (Table 4). Co-infections of R. raoultii/B. microti or B. afzelii/A. phagocytophilum were detected in only one infected D. reticulatus tick (0.4% each). Most co-infections (9/10; 90%) were detected in D. reticulatus females (Table 4).
Discussion
The authors’ previous monitoring of tick occurrence in north-eastern Poland demonstrated that D. reticulatus is permanently present in both natural biotopes as well in the green areas located in cities24,25,26,27. The present study focused on molecular evidence of veterinary-medical important pathogens transmitted by questing D. reticulatus ticks belonging to the Eastern European population from urban and natural biotopes of a region of north-eastern Poland. The presence of genetic material of all four studied groups of microorganisms (Rickettsia spp., Babesia spp., A. phagocytophilum, Borrelia spp.) was found in the study, and almost every third D. reticulatus tick (29.6%) was infected with at least one of them, regardless of sex.
From an epidemiological point of view, D. reticulatus ticks are less important than I. ricinus ticks. In veterinary practice, the most important pathogen transmitted by D. reticulatus is B. canis protozoa, which causes canine babesiosis28. In the current study, B. canis was identified in D. reticulatus ticks in similar proportions in both urban and natural biotopes. However, the overall level of infection was relatively low (1.4%) compared to ticks from other sites in the eastern macroregion. The prevalence of B. canis in ticks from the Eastern European population has been reported to vary from 0 to 6.8%19,25,29,30,31,32,33,34. It is worth underlining that in the current study, most of the D. reticulatus infected by B. canis were detected in an urban area of the city of Olsztyn in the central part of the Warmia and Mazury region, the oldest area known as endemic for D. reticulatus3,5. These results confirm the presence and relatively constant level of prevalence B. canis (2.5%) in this part of north-eastern Poland, previously established by other authors in the range of 2.3–6%5,19.
In spite of a low overall prevalence, the molecular analysis of a fragment of the 18S rRNA gene of B. canis indicates the presence of genetically heterogenic genotypes of B. canis in examined ticks. In Europe, based on two single nucleotide polymorphisms (SNPs), four B. canis genotypes related to GA → AG nucleotide substitutions are present at different rates of prevalence35,36,37. In the present study, among three revealed genotypes, the vast majority of B. canis isolates (69.2%) represented genotype A (GA nucleotides). However, the “mixed” A/B genotype (showing the presence of both G and A nucleotides—R/R) has also been recorded. The A/B genotype was the most frequently detected in naturally infected dogs in Poland and other countries from northern Europe37,38. The occurrence of genotype A and genotype B (AG nucleotides) of B. canis in D. reticulatus in north-eastern Poland may suggest differences in the clinical manifestation of canine babesiosis in this area. Adaszek39 revealed that the severity of thrombocytopenia in dogs infected with B. canis is related to different 18S rRNA genotypes of the pathogen. Genotype B of B. canis, also identified in the current study, was found to be more virulent in relation to thrombocytopenia than genotype A.
In 0.9% of examined D. reticulatus ticks, the DNA of B. microti, considered to be the most common causative agent for human babesiosis, was also detected40. Interestingly, it was detected only in females from urban areas. Although the occurrence of DNA of B. microti was previously reported in D. reticulatus19,25,30,41, a role for this tick species as a vector for B. microti has not been clearly confirmed. The presence of B. microti DNA in questing D. reticulatus ticks may be due to feeding on voles (Microtus spp.), which have been identified as the main reservoir of B. microti42.
“Contamination” with the blood of the host is probably also the cause of the detection of Borrelia spp. DNA in examined D. reticulatus ticks. Although the specific DNA of that bacteria has been detected in D. reticulatus in other regions of Poland19,26,27,30, their role as a vector was not confirmed. It has been proven that the salivary glands of Dermacentor spp. contain proteins (defensins), which are attributed to the role of specific antibiotics in eliminating spirochetes43.
The significance of D. reticulatus as a vector of A. phagocytophlium—the causative agent of human granulocytic anaplasmosis (HGA)44 also seems to be insignificant. A meta-analysis of the prevalence and distribution of A. phagocytophilum in tick vectors conducted by Karshim45 showed that the overall level of infection with this pathogen in questing D. reticulatus ticks is very low (0.42%). The current results (0.3%) correspond with the results of other studies on questing D. reticulatus ticks belonging to the East European population (0.7–3%)30,33,46,47,48 and confirmed that fact. However, it should be noted that the infection rate of A. phagocytophilum in D. reticulatus may vary significantly depending on the year of study, the local availability of hosts and the phase of the life cycle (non-feeding/feeding on the host)26,27,30,33,34,46,47,48,49,50,51.
From a medical point of view, D. reticulatus is of undeniable importance in the transmission of bacteria representing the genus Rickettsia12. Although D. reticulatus ticks attack humans sporadically52,53,54, it is a main vector of R. raoultii and R. slovaca. Tick-borne lymphadenopathy (TIBOLA)/Dermacentor-borne necrosis erythema-lymphadenopathy (DEBONEL) or scalp eschar and neck lymphadenopathy after a tick bite (SENLAT) are recently described infection syndromes in humans caused by R. raoultii and R. slovaca belonging to spotted fever group (SFG) rickettsiae55,56. To date, several cases of DEBONEL/TIBOLA have been described in Europe, including in Poland57,58,59,60,61. R. raoultii is the most commonly detected pathogen in both adult and juvenile D. reticulatus ticks in Poland62. R. raoultii was the only species identified by DNA sequencing in 33% of Rickettsia-positive ticks in this study. The frequency of this pathogen in the examined specimens ranged from 6.3 to 21.8% depending on the research region and was much higher in ticks collected from natural biotopes than in urban areas (the city of Olsztyn).The level of occurrence of R. raoultii in ticks in north-eastern Poland was comparable to that previously determined by Mierzejewska19 in this area (34.2%). A higher infection rate, ranging from 41 to 91.7%, was detected in adult D. reticulatus in populations in other parts of north-eastern and eastern Poland34,63,64,65. Mierzejewska19, comparing the prevalence of R. raouliti between two Polish tick populations (eastern and western) with an expansion zone between them, noted slight differences (42% in the East vs. 52% in the West), but with a clearly increasing gradient from east to west in Poland. A high prevalence of Rickettsia spp. in the population of D. reticulatus ticks may result from the possibility of transmission not only through the infected host—tick route, but also via the vertical transovarial, transstadial and, probably less frequently, transspermal transmission56.
In the case of tick-borne diseases in humans, tick co-infection with several species of pathogenic microorganisms and their co-transmission might have important relevance to public health66. In D. reticulatus from north-eastern Poland, co-infections were revealed in 3.8% of positive ticks. Considering that only co-infections with R. raoultii with B. canis were identified (which are not pathogenic for humans) and with B. afzelii (which are probably neutralized by defensins produced by D. reticulatus ticks), it seems that this tick species does not play an important role as a vector of mixed tick-borne infections in humans that could exacerbate the course of the disease severity and make it difficult to diagnose or treat.
Conclusion
The current study has confirmed the high prevalence of R. raoultii in adult ticks in the eastern population of D. reticulatus in north-eastern Poland. The presence of bacteria belonging to the rickettsiae from the spotted fever group indicates that tick-borne lymphadenopathy (DEBONEL/TIBOLA) should not be excluded in the diagnosis of tick-borne diseases. In turn, the relatively high prevalence of B. canis with different genotypes suggests differences in the clinical picture of canine babesiosis in this area. The risk of infection with these two pathogens is much higher in the natural biotopes of north-eastern Poland. D. reticulatus seems to play a minor role in the transmission of Borrelia burgdorferi s.l. and A. phagocytophilum, as well as a vector of mixed infections in humans.
Data availability
The datasets used and analyzed during this study are available from the corresponding author (K.K.) upon reasonable request. The nucleotide sequences have been deposited in the GenBank database under accession numbers: ON660868-870 (Rickettsia spp.), OR056353, OR064513-518 (Babesia spp.) OR096226-227 (A. phagocytophilium) and OR046059-060 (Borrelia spp.).
References
Noll, M., Wall, R., Makepeace, B. L. & Vineer, H. R. Distribution of ticks in the Western Palearctic: An updated systematic review (2015–2021). Parasit. Vectors 16, 1–15 (2023).
Rubel, F. et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick. Borne. Dis. 7, 224–233 (2016).
Mierzejewska, E. J., Estrada-Peña, A., Alsarraf, M., Kowalec, M. & Bajer, A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick. Borne. Dis. 7, 94–106 (2016).
Paulauskas, A. et al. Microsatellite-based genetic diversity of Dermacentor reticulatus in Europe. Infect. Genet. Evol. 66, 200–209 (2018).
Dwużnik-Szarek, D., Mierzejewska, E. J. & Bajer, A. Occurrence of juvenile Dermacentor reticulatus ticks in three regions in Poland: The final evidence of the conquest. Parasites Vectors 14, 1–8 (2021).
Springer, A. et al. Update and prognosis of Dermacentor distribution in Germany: Nationwide occurrence of Dermacentor reticulatus. Front. Vet. Sci. 9, 1044597 (2022).
Paulauskas, A. et al. New localities of Dermacentor reticulatus ticks in the Baltic countries. Ticks Tick. Borne. Dis. 6, 630–635 (2015).
Kiewra, D. & Czulowska, A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp. Appl. Acarol. 59, 501–506 (2013).
Kloch, A. et al. Origins of recently emerged foci of the tick Dermacentor reticulatus in central Europe inferred from molecular markers. Vet. Parasitol. 237, 63–69 (2017).
Kiewra, D., Szymanowski, M., Czułowska, A. & Kolanek, A. The local-scale expansion of Dermacentor reticulatus ticks in Lower Silesia, SW Poland. Ticks Tick. Borne. Dis. 12, 101599 (2021).
Bilbija, B. et al. Dermacentor reticulatus—A tick on its way from glacial refugia to a panmictic Eurasian population. Int. J. Parasitol. 53, 91–101 (2023).
Földvári, G., Široký, P., Szekeres, S., Majoros, G. & Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit. Vectors 9, 314 (2016).
Ličková, M. et al. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick. Borne. Dis. 11, 101414 (2020).
Petney T.N., Pfäffle M.P., Sprong H., Mihalca A.D. & A. Estrada-Peña of Referencing in Ticks of Europe and North Africa (ed. Estrada-Peña, A. et al.) 5–10 (Springer Nature, 2017).
Nowak-Chmura, M. Fauna kleszczy (Ixodida) Europy Środkowej (Wydawnictwo Naukowe Uniwersytetu Pedagogicznego, 2013).
Rijpkema, S., Golubić, D., Molkenboer, M., Verbeek-De Kruif, N. & Schellekens, J. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 20, 23–30 (1996).
Bonnet, S. et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology 134, 197–207 (2007).
Bonnet, S., Jouglin, M., Hostis, M. L. & Chauvin, A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 13, 1208–1211 (2007).
Mierzejewska, E. J., Pawełczyk, A., Radkowski, M., Welc-Falęciak, R. & Bajer, A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit. Vectors 8, 490 (2015).
Roux, V., Rydkina, E., Eremeeva, M. & Raoult, D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the Rickettsiae. Int. J. Syst. Bacteriol. 47, 252–261 (1997).
Massung, R. F. et al. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36, 1090–1095 (1998).
Wodecka, B. FlaB gene as a molecular marker for distinct identification of Borrelia species in environmental samples by the PCR-restriction fragment length polymorphism method. Appl. Environ. Microbiol. 77, 7088–7092 (2011).
Wodecka, B., Leońska, A. & Skotarczak, B. A comparative analysis of molecular markers for the detection and identification of Borrelia spirochaetes in Ixodes ricinus. J. Med. Microbiol. 59, 309–314 (2010).
Kubiak, K. et al. Dermacentor reticulatus ticks (Acari: Ixodidae) distribution in north-eastern Poland: An endemic area of tick-borne diseases. Exp. Appl. Acarol. 75, 289–298 (2018).
Kubiak, K., Dmitryjuk, M., Dziekońska-Rynko, J., Siejwa, P. & Dzika, E. The risk of exposure to ticks and tick-borne pathogens in a spa town in northern Poland. Pathogens 11, 542 (2022).
Michalski, M. M., Kubiak, K., Szczotko, M., Chajęcka, M. & Dmitryjuk, M. Molecular detection of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in ticks collected from dogs in urban areas of north-eastern Poland. Pathogens 9, 1–11 (2020).
Michalski, M. M., Kubiak, K., Szczotko, M. & Dmitryjuk, M. Tick-borne pathogens in ticks collected from wild ungulates in north-eastern Poland. Pathogens 10, 587 (2021).
Bajer, A. et al. Babesiosis in southeastern, central and northeastern Europe: An emerging and re-emerging tick-borne disease of humans and animals. Microorganisms 10, 945 (2022).
Reye, A. L. et al. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS ONE 8, e54476 (2013).
Zając, V. et al. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann. Agric. Environ. Med. 24, 26–32 (2017).
Radzijevskaja, J., Mardosaite-Busaitiene, D., Aleksandravičiene, A. & Paulauskas, A. Investigation of Babesia spp. in sympatric populations of Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania and Latvia. Ticks Tick. Borne. Dis. 9, 270–274 (2019).
Rogovskyy, A. et al. Diversity of Borrelia spirochetes and other zoonotic agents in ticks from Kyiv, Ukraine. Ticks Tick. Borne. Dis. 9, 404–409 (2018).
Grochowska, A. et al. Detection of Borrelia burgdorferi s.l., Anaplasma phagocytophilum and Babesia spp. in Dermacentor reticulatus ticks found within the city of Białystok, Poland-first data. Exp. Appl. Acarol. 85, 63–73 (2021).
Zając, Z. et al. Disparate dynamics of pathogen prevalence in Ixodes ricinus and Dermacentor reticulatus ticks occurring sympatrically in diverse habitats. Sci. Rep. 13, 1–15 (2023).
Beck, R. et al. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 39, 843–848 (2009).
Schaarschmidt, D. et al. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick. Borne. Dis. 4, 334–340 (2013).
Radzijevskaja, J. et al. Genetic diversity of Babesia canis strains in dogs in Lithuania. Microorganisms 10, 1446 (2022).
Łyp, P., Adaszek, Ł, Furmaga, B. & Winiarczyk, S. Identification of new 18S rRNA strains of Babesia canis isolated from dogs with subclinical babesiosis. Pol. J. Vet. Sci. 18, 573–577 (2015).
Adaszek, L. & Winiarczyk, S. Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 152, 235–241 (2008).
Vannier, E. G., Diuk-Wasser, M. A., Ben Mamoun, C. & Krause, P. J. Babesiosis. Infect. Dis. Clin. North Am. 29, 357–370 (2015).
Wójcik-Fatla, A., Zając, V., Sawczyn, A., Cisak, E. & Dutkiewicz, J. Babesia spp. in questing ticks from eastern Poland: Prevalence and species diversity. Parasitol. Res. 114, 3111–3116 (2015).
Dwużnik, D. et al. The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of ‘meal contamination’. Exp. Appl. Acarol. 78, 181–202 (2019).
Wu, J. et al. Defensins as a promising class of tick antimicrobial peptides: A scoping review. Przegl. Epidemiol. 66, 347–350 (2012).
Bakken, J. S. & Dumler, J. S. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 29, 341–355 (2015).
Karshima, S. N., Ahmed, M. I., Kogi, C. A. & Iliya, P. S. Anaplasma phagocytophilum infection rates in questing and host-attached ticks: A global systematic review and meta-analysis. Acta Trop. 228, 106299 (2022).
Grochowska, A. et al. Prevalence of tick-borne pathogens in questing Ixodes ricinus and Dermacentor reticulatus ticks collected from recreational areas in northeastern Poland with analysis of environmental factors. Pathogens 11, 468 (2022).
Dunaj, J., Trzeszczkowski, A., Moniuszko-Malinowska, A., Rutkowski, K. & Pancewicz, S. Assessment of tick-borne pathogens presence in Dermacentor reticulatus ticks in north-eastern Poland. Adv. Med. Sci. 66, 113–118 (2021).
Levytska, V. A. et al. Detection of pathogens in ixodid ticks collected from animals and vegetation in five regions of Ukraine. Ticks Tick. Borne. Dis. 12, 101586 (2021).
Karbowiak, G. The occurrence of the Dermacentor reticulatus tick—Its expansion to new areas and possible causes. Ann. Parasitol. 60, 37–47 (2014).
Pańczuk, A. & Patrycja, M. T. Tick-borne pathogens in Dermacentor reticulatus collected from dogs in eastern Poland. Exp. Appl. Acarol. 86, 419–429 (2022).
Ben, I. & Lozynskyi, I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and coinfection with Borrelia burgdorferi and Tick-Borne Encephalitis Virus in western Ukraine. Vector-Borne Zoonotic Dis. 19, 793–801 (2019).
Pawełczyk, A. et al. Long-term study of Borrelia and Babesia prevalence and co-infection in Ixodes ricinus and Dermacentor recticulatus ticks removed from humans in Poland, 2016–2019. Parasites Vectors 14, 348 (2021).
Springer, A., Raulf, M. K., Fingerle, V. & Strube, C. Borrelia prevalence and species distribution in ticks removed from humans in Germany, 2013–2017. Ticks Tick. Borne. Dis. 11, 101363 (2020).
Koczwarska, J., Pawełczyk, A., Dunaj-Małyszko, J., Polaczyk, J. & Welc-Falęciak, R. Rickettsia species in Dermacentor reticulatus ticks feeding on human skin and clinical manifestations of tick-borne infections after tick bite. Sci. Rep. 13, 1–8 (2023).
Portillo, A., Santibáñez, S., García-Álvarez, L., Palomar, A. M. & Oteo, J. A. Rickettsioses in Europe. Microbes Infect. 17, 834–838 (2015).
Buczek, W. et al. Spotted fever group rickettsiae transmitted by Dermacentor ticks and determinants of their spread in Europe. Ann. Agric. Environ. Med. 27, 505–511 (2020).
Chmielewski, T., Rudzka, D., Fiecek, B., Maczka, I. & Tylewska-Wierzbanowska, S. Case of TIBOLA/DEBONEL (tick—borne lymphadenopathy/Dermacentor spp.—borne necrosis—erythema—lymphadenopathy) in Poland. Przegl. Epidemiol. 65, 583–586 (2011).
Switaj, K., Chmielewski, T., Borkowski, P., Tylewska-Wierzbanowska, S. & Olszynska-Krowicka, M. Spotted fever rickettsiosis caused by Rickettsia raoultii-case report. Przegl. Epidemiol. 66, 37–39 (2012).
Pietzsch, M. E. et al. Detection of Dermacentor marginatus and a possible Rickettsia slovaca case in the United Kingdom—The risk of the visiting traveller. Travel Med. Infect. Dis. 13, 200–201 (2015).
Silva, J. T. et al. Tickborne lymphadenopathy complicated by acute myopericarditis, Spain. Emerg. Infect. Dis. 21, 2240–2242 (2015).
Barlozzari, G. et al. Scalp eschar and neck lymphadenopathy by Rickettsia slovaca after Dermacentor marginatus tick bite case report: Multidisciplinary approach to a tick-borne disease. BMC Infect. Dis. 21, 1–4 (2021).
Dwużnik-Szarek, D. et al. Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018). Sci. Rep. 12, 1–9 (2022).
Chmielewski, T., Podsiadly, E., Karbowiak, G. & Tylewska-Wierzbanowska, S. Rickettsia spp. in ticks, Poland. Emerg. Infect. Dis. 15, 486–488 (2009).
Wójcik-Fatla, A. et al. Study on tick-borne rickettsiae in eastern Poland. I. Prevalence in Dermacentor reticulatus (Acari: Amblyommidae). Ann. Agric. Environ. Med. 20, 276–279 (2013).
Stańczak, J., Biernat, B., Racewicz, M., Zalewska, M. & Matyjasek, A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick. Borne. Dis. 9, 427–434 (2018).
Boyer, P. H., Lenormand, C., Jaulhac, B. & Talagrand-Reboul, E. Other Ixodes-borne microorganisms: A systematic review. Pathogens 11, 282 (2022).
Acknowledgements
Permission of the Director of the Biebrza National Park (Poland) to tick collection (Decision: DOP-WPN.286.31.2017.DW). The authors would like to thank Tański A. (Department of Medical Biology, UWM in Olsztyn) for drawing the map (Fig. 1).
Author information
Authors and Affiliations
Contributions
Conceptualization: K.K., methodology: K.K. and H.S.; formal analysis: K.K. and M.D., investigation: K.K., H.S., A.T. and J.D.R; writing—original draft preparation: K.K.; writing—review and editing: K.K., H.S., M.D., A.T., E.D., J.D.R.; visualization: K.K.; funding acquisition: J.D.R. and E.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubiak, K., Szymańska, H., Dziekońska-Rynko, J. et al. Tick-borne pathogens in questing adults Dermacentor reticulatus from the Eastern European population (north-eastern Poland). Sci Rep 14, 698 (2024). https://doi.org/10.1038/s41598-024-51299-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51299-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.