Abstract
Aerobic rice cultivation progresses water productivity, and it can save almost 50% of irrigation water compared to lowland rice with the appropriate development of genotypes and management practices. Two field trials were conducted during 2020, and 2021 seasons to determine the validation of different rice varieties under aerobic cultivation based on their plant defense system, physio-morphological traits, stress indices, grain yield, and water productivity. The experiments were designed in a split-plot design with four replications. Two planting methods, transplanting and aerobic cultivation, were denoted as the main plots, and ten rice genotypes were distributed in the subplots. The results revealed that the planting method varied significantly in all measured parameters. The transplanting method with well watering had the highest value of all measured parameters except leaf rolling, membrane stability index, antioxidant, proline, and the number of unfilled grains. EHR1, Giza179 and GZ9399 as well as A22 genotypes a chief more antioxidant defense system that operated under aerobic conditions. Giza179, EHR1, GZ9399, and Giza178 showed high cell membrane stability and subsequently high validation under such conditions, and also showed efficiency in decreasing water consumption and improving water use efficiency. In conclusion, this study proves that Giza179, EHR1, GZ9399, Giza178, and A22 are valid genotypes for aerobic conditions.
Similar content being viewed by others
Introduction
Rice (Oryza sativa L.) is an essential staple crop that secures food for almost two-thirds of the world's population1. The cultivation of rice occupies about twenty percent of the agricultural land area planted for cereals2. In Egypt, rice production faces many grand challenges such as freshwater scarcity, salt stress, the high cost of inputs, and overpopulation. It is estimated that rice's annual production deficit will increase from 400,000 t in 2016 to 800,000 by 20303. Nowadays, the insufficient available freshwater for agriculture pose a challenge for big water consumer crops production (i.e., rice). The 79 million hectares of irrigated rice worldwide consume 34–43% of the irrigation water around the globe4.
Water resources management is one of the main factors affecting rice production globally5. Aerobic rice cultivation is a medium option to save water with an minimal yield reduction for irrigated rice ecotypes. Primarily, a water deficit may result in increasing spikelet sterility6. However, relative water content (RWC%) is easy to use for determining physiological status of plant under drought stress7. Also, RWC% is a vital guide to recognize the tolerant variety under various stresses such as aerobic cultivation with minimal water supply8,9,10,11,12,13 and salinity13,14,15,16,17,18. Decreasing freshwater resources are threatening agricultural production in many parts of the world19.
Plants suffer from various stresses, such as drought, low and high temperatures, salinity, etc.20. Among all, RWC%, leaf rolling, grain yield, leaf drying, root/shoot ratio, and root length offer high scope for rice development for aerobic cultivation7. There is an ever-increasing need to improve water productivity to stay commercially competitive. Abiotic stresses, namely, water deficit developed by aerobic cultivation or drought, are the most critical factors negatively influencing growth, and several physiological and biochemical processes21. Plants developed many adaptation mechanisms, such as antioxidant defense systems, i.e., Superoxide dismutase (SOD), Catalase (CAT), and Peroxidase (POD). These enzymatic components play a central role in the defense system. The SOD decomposes superoxide (O2) to H2O2 by catalyzing superoxide anion radical disputation. The POD and CAT decrease H2O2 in water using various substrates as electron donors to preserve the roots and help the plants endure stress22,23,24,25. Aerobic rice improves water productivity with the appropriate development of genotypes and management practices7. Aerobic rice is specially developed rice, combines drought tolerance of upland rice and yield potential in lowland rice6. Therefore, it can save almost 50% of irrigation water compared to lowland rice. Aerobic rice cultivars with high yield potential and moderate drought stress tolerance have been developed through traditional upland farming with improved irrigated cultivars6. Aerobic rice cultivars achieve high yields under appropriate management practices and deep root systems, as well as water stress tolerance in the vegetative and reproductive stages26.
The objectives of the current study were (1) to determine the physiological, morphological, and stress indices of some Egyptian rice varieties under aerobic cultivation; (2) the possibility to explore such type of cultivation in Egypt, and (3) to nominate valid Egyptian rice varieties to lead cultivation under aerobic conditions.
Materials and methods
Experimental site
The experiment was conducted at the experimental farm of Sakha Agricultural Research Station, Kafr El-Sheikh, Egypt, during 2020 and 2021 seasons to compare the performance of some rice varieties under normal transplanting and aerobic cultivation conditions. All experiments were preceded by a barley crop (Hordeum spp.). The results of the chemical soil properties are presented in Table 1.
The experiment was performed in a split plot design with four replications. The main plots were devoted to two planting methods: normal transplanting and aerobic cultivation, and subplots were occupied by ten varieties. As for the aerobic cultivation pattern, the dry seeds were used in dry land on furrows. The furrow size was 70 cm from mid-bottom of each of the two furrows, with a depth of 30 cm. Rice irrigation during the first 25 days of rice growth was done as in drill seeded rice, and then watering was done each eight days to maturity by filling the bottom of furrow. The recommended dose of nitrogen fertilizer (i.e., 165 kg N ha−1) was applied in three equal doses (basal, top dressing at panicle initiation, and late booting stage). For the permanent field, phosphorus fertilizer at the rate of 35.5 kg P2O5 ha−1 was basally applied to the soil during the land preparation. The potassium fertilizer (57 kg K2O ha−1) was added as a basal dose and incorporated into dry soil, and zinc (Zn SO4) was applied at the rate of 23.9 kg ha−1 before continuous flood irrigation. For transplanting cultivation method, the seeds of different rice varieties were sown on May, 7th, and after 30 days, the seedlings were transplanted in the permanent field at 20 × 20 cm plant spacing in a plot area of 10 m2. As for aerobic cultivation, the 4 dry seeds/hill were sown at the space of 15 cm between each pair of hills and 30 cm between the two rows on the both sides of furrow. Origin, parentage, and variety group are presented in Table 2.
At heading stage, plants of five hills were randomly taken and pulled with their roots from each plot and transported to the lab to determine: leaf area index, flag leaf area using portable area meter membrane (Model LI-3000A), chlorophyll content (with SPAD meter M502), stability index, RWC%, length, and root volume. Also, some enzymatic antioxidants such as catalase, peroxidase, and super oxidase dismutase, and proline activity leaf content were assessed. In the field, leaf rolling was visually estimated according to the IRRI scale. The stomatal conductance was measured at head in the field. Root length and root volume was estimated as described by Bradford27. At harvest, plant height was estimated. The number of panicles on ten random hills was counted and then conformed to the number of panicles/hill. Ten random panicles were collected from each plot to estimate panicle length, number of filled grains/panicle, number of unfilled grains/panicles, panicle weight, 1000-grain weight and grain yield were randomly measured from an area of 1 m2 and adjusted to 14% moisture content.
Determination of enzymatic activities
Leaf samples (i.e., 200 mg) were soaked in liquid N2 and homogenized in 2.0 ml of extraction buffer: 100 mm potassium phosphate (pH 7.8), 0.1 mm ethylene diaminetetra acetic acid (EDTA) and 10 mm ascorbic acid. The homogenate was centrifuged at 13,000g for 15 min at 4 °C. CAT activity was assayed in the supernatant at 240 nm based on the consumption of H2O228. The activity of SOD was determined at 560 nm according to the method of Beauchamp and Fridovich29. Activity of POX was measured at 420 nm as described by Kar and Mishra30.
Proline content
Leaf sample (0.3 g) were placed in 3% sulphosalicylic acid and centrifuged for 20 min at 3000×g. From extract, 2 mL of supernatant was added to 2 mL of ninhydrin reagent and 2 mL of glacial acetic acid. Proline was determined as mg g−1 FW using a spectrophotometer.
Relative water content (RWC)
RWC was calculated according to González and González31 as the following:
where f. wt. is the fresh weight of leaves, d. wt is the dry weight of leaves, t. wt is the turgid weight of leaves.
Membrane stability index (MSI)
Youngest leaf tissues (0.2 g) were washed by deionized water, cut into sections (1 cm length), placed in 10 mL deionized water, and heated at 40 °C in a water bath for 30 min. Then the electrical conductivity (EC1) was measured by using a conductivity meter (ME977-C, Max Electronics). Subsequently, the content was boiled for 10 min in a boiling water bath (100 °C), and the conductivity (EC2) was measured. Finally, by using the formula described by Premachandra et al.32, the membrane stability index (MSI) was calculated as following equation
Stomatal conductance (GS)
The stomatal conductance (gs) (units; mol m−2 s−1) was measured at the heading stage via a portable photosynthesis measurement system (Li-Cor, Lincoln, NE, USA) according to Hubbard et al.33.
Statistical analysis
Data collected were statistically analyzed using the analysis of variance technique according to Gomez and Gomez34. Duncan's Multiple Range Test was used to compare the treatment means35. All statistical analyses were accomplished using the analysis of variance technique using the "COSTAT" statistical software package36.
Experimental research
The current experiment including all studied materials tightly comply with relevant plant guidelines and legislation at various levels.
Results
Antioxidant enzymes activity
The primary indicator of drought or low water supply for rice crop was releasing freer radical which could be treated by antioxidants formation. Notably, the activity of catalase, peroxidase, superoxide dismutase, and proline were significantly influenced by planting methods in both seasons (Table 3).
Aerobic cultivation conditions shows the highest values of catalase, peroxidase, superoxide dismutase, and proline compared with the transplanting method in both seasons. The tested rice varieties show remarkable variation in their antioxidant defense systems. EHR1 and GZ9399 rice genotypes were at the same level and recorded the highest value of catalase and peroxides, followed by Giza179 and Giza178 in both seasons. As for SOD and proline accumulation, EHR1 values were the highest, followed by GZ9399, Giza179 and Giza178. The interaction between planting methods and varieties effect significantly on catalase, peroxidase, proline, and superoxide dismutase (Figs. S1–S8). EHR1 surpassed in production of antioxidant system under aerobic cultivation method followed by GZ9399 and Giza179. Giza178, A22, Egyptian Yasmine and Sakha107 showed medium values of antioxidants and proline accumulation under aerobic cultivation (Figs. S1–S8). However, the lowest values of studied antioxidants and proline accumulation under aerobic cultivation were produced by Giza177, Sakha102 and Sakha106 varieties in both seasons.
Physio-morphological traits
Leaf area index, flag leaf area, and leaf rolling were significantly influenced by the cultivation methods in both seasons (Table 4). The two planting methods showed an apparent variation in both seasons. The transplanting method with well-watering treatment possessed the highest values of the leaf area index and flag leaf area, and the lowest values of leaf rolling. Therefore, aerobic cultivation showed a slight reduction in vegetative and physiological growth traits.
The tested genotypes significantly varied in their leaf area index, flag leaf area, and leaf rolling in both seasons. EHR1 had the largest leaf area index and flag leaf area, followed by GZ9399 in leaf area and Giza179 in flag leaf area. Giza178 and Egyptian Yasmine occupied the second rank regarding the above-mentioned traits. The known sensitive rice varieties to drought, such as Giza177, Sakha102, and Sakha106, showed the narrowest leaf area index and flag leaf area in both seasons. Leaf rolling peaked with Giza177 and Sakha106 rice varieties in the first and second seasons, respectively. In both seasons, EHR1 had the lowest means of leaf rolling in the two seasons, respectively. Results in Table 5 show that the interaction effect between planting methods and rice genotypes on leaf area index and flag leaf area was significant in both seasons.
EHR1 gave the highest leaf area index and flag leaf values when it was transplanted with well-watering compared with the other treatments. Whereas, the lowest leaf area index values were produced by Sakha106 under the aerobic conditions without significant differences with Giza177 and Sakha102 under the same conditions. The interaction effect showed the ability of EHR1, GZ9399, Giza179 and Giza178 to grow well under aerobic conditions as compared to the rest of the rice genotypes considering leaf area index and flag leaf area. The interaction effect between planting methods and varieties on leaf rolling was significant in both seasons. The tested genotypes exhibited great and marked differences, particularly under aerobic cultivation. Under the transplanting method, the tested genotypes were at par regarding leaf rolling in both seasons of the study. EHR1 and Giza179 showed the lowest leaf rolling under aerobic cultivation in both seasons, followed by GZ9399 and then Giza178. Egyptian Yasmine, A22, and Sakha107 had medium leaf rolling when cultivated under aerobic conditions. Giza177, Sakha106, and Sakha102 exhibited the highest leaf rolling under aerobic conditions, supporting their sensitivity to water deficit in both seasons (Table 6).
As seen in Table 7, aerobic cultivation with less irrigation waterhave increased water loss through stomata and high transpiration rate, resulting in low leaf water content, low chlorophyll content, and the highest electrolyte leakage (EL%) in terms of cell membrane stability index values. On the other hand, the transplanting method with well watering had the highest mean of RWC and chlorophyll content and the lowest values of the membrane stability index in both seasons. GZ9399 gave the lowest values of EL% in the terms of cell membrane stability index without significant differences with Giza179 in both seasons and EHR1 in the second season. Sakha106 had a higher in cell membrane stability index and Giza177 in the second season, followed by Sakha102. However, Sakha107, A22, and Egyptian Jasmine showed a medium rate of cell membrane stability. It is mentioned here that the high stability index meant a higher EL%, indicating the sensitivity of variety.
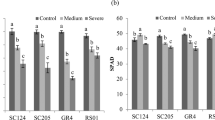
The low index of EL% is favorable because of low cell membrane damage. Giza179 rice variety had the maximum values of RWC%, followed by GZ9399 without significant differences in both study seasons. Giza177 rice variety gave the lowest values of RWC in the 2020 and 2021 seasons, followed by Sakha102 and Sakha106. The EHR1 came in advanced rank considering leaf RWC. Interestingly, Leaf chlorophyll content was high in EHR1 followed by GZ9399 and then Giza179 and Giza178. Whereas, the minimum averages of chlorophyll content were observed in Sakha106 followed by Giza177 and Sakha102 in both seasons (Table 5). The interaction effects between planting methods and rice genotypes on membrane stability index, RWC, and chlorophyll content were significant in both seasons of the study (Figs. S7, S8 and Table 6). The tested genotypes were apparently varied in both seasons under the transplanting method regarding to RWC and cell membrane stability index. Under aerobic cultivation, Sakha106 rice variety gave the highest values of membrane stability index followed, by Giza177 and Sakha102 under aerobic conditions indicating their unsuitability for that cultivation method. The previously mentioned varieties showed the same behavior with leaf RWC%. Giza177 gave the lowest values of RWC% and chlorophyll content followed by Sakha106, then Sakha107 under aerobic cultivation. GZ9399 gave the highest values of RWC% under aerobic, followed by Giza179 without significant differences in both seasons that was completely matching with their cell membrane stability (Figs. S7 and S8), EHR1 under both aerobic and transplanting methods gave the highest values of chlorophyll content in both seasons (Table 8).
Results in revealed that stomata conductance, root length, and root volume were significantly influenced by planting methods in both seasons (Table 9). The transplanting method with well-watering treatment possessed the highest stomata conductance, root length, and root volume values. In the tested rice genotypes, there were significant differences in stomata conductance, root length, and root volume in both seasons. Further, rice varieties significantly differed in their root length and root volume.
As for the genotypes, EHR1 had much more profound and extensive root distributions than Giza177 in both seasons. The maximum values of stomata conductance was shown by EHR1 and Giza179 rice varieties without any significant differences among them, followed by GZ9399 in the two seasons. However, the known sensitive rice varieties, such as Giza177, Sakha102 showed the lowest values of stomata conductance in both seasons of study. Results in Table 10 shows that the interaction effect between planting methods and varieties on root length, root volume, and stomata conductance was significant in both study seasons. EHR1 gave the highest values of root length and volume under aerobic rice cultivation followed by Giza179 (Table 10).
The lowest mean value of the root length and root volume was produced by Giza177 under the aerobic conditions followed, by Sakha102 and Egyptian Yasmine in both seasons. Under the transplanting method, the majority of rice genotypes did not show remarkable differences regarding the studied root characteristics (Table 8). The interaction between rice genotypes and planting methods is significantly affected stomata conductance in both seasons.
Under the transplanting method, EHR1 gave the maximum values of stomata conductance without significant differences from those obtained by GZ9399, Giza179, Giza178, Sakha107, Sakha102 and Sakha106 in, while the lowest values were recorded by E. Yasmine followed by Giza177 and A22 in both season (Table 8). Under aerobic cultivation, Giza179 exerted the highest mean of stomata conductance, followed by EHR1 with the same level of significance.
Yield and yield components
The planting methods significantly influenced plant height, number of panicles/hills, and panicle length in both seasons as shown in Table 11. It was clear that the highest values of traits were recorded by the transplanting method compared to aerobic cultivation in both seasons. It was observed that rice genotypes significantly varied in their plant height, number of panicles/hills, and panicle length in both seasons.
Results revealed that theGZ9399and A22 produced the highest number of panicles/hills when compared at the same level of significance.. Meanwhile, the lowest values of the number of panicles/hills were recorded by Giza177 and Sakha102 in both seasons.
The interaction effects between planting method and rice genotype on plant height were significant in the 2020 and 2021seasons (Table 12).
The tested genotypes exhibited great and marked differences under two planting methods. Rice varieties A22 and E. Yasmine showed the tallest plant under transplanting method, whereas under aerobic cultivation, only A22 showed the tallest plant. E. Yasmine showed the longest panicle under the transplanting and aerobic cultivation methods in both seasons. EHR1 and GZ9399 had high values for the number of panicles/hills under the transplanting method in both seasons. The lowest values of traits were obtained by Giza177 under aerobic conditions in both seasons.
Results indicated that the planting methods significantly affected the number of filled grain/panicle and the number of unfilled grains/panicles in both seasons (Table 13). The transplanting method produced the maximum number of filled grain/panicle, except for the number of unfilled grains concerning the highest value with aerobic cultivation in both seasons.
The rice genotypes significantly differed in their number of filled grain/panicle and number of unfilled grains in both seasons. EHR1 gave the highest values of the number of filled grain/panicle. However, the lowest value was recorded by Sakha102 and Giza177 in the two seasons. The number of unfilled grains reached the lowest values by GZ9399 cultivar. Meanwhile, the highest values of the number of unfilled grains were obtained with Giza177 and Sakha102 in both seasons. The interaction between planting methods and varieties significantly affected the number of filled grains and the number of unfilled grains in the two seasons (Table 14).
The transplanting method gave the highest number of filled grains, which was obtained by EHR1 followed by Giza178. Meanwhile, the highest values of the number of unfilled grains were recorded by Giza177and Sakha102 followed by Sakha106 under aerobic cultivation (Table 14). Panicle weight, 1000-grain weight, and grain yield were greatly responded to planting methods (Table 15). Panicle weight, 1000-grain weight, and grain yield were significantly reduced as a result of low water supply under aerobic cultivation. The yield reduction under aerobic cultivation were 19.55 and 22.23% compared to transplanting method with sufficient water supply in the first and second seasons, respectively (Table 15). The heaviest panicle and 1000-grain, and the highest grain yield were produced by the transplanting method. Generally, the studied rice genotypes significantly differed in their panicle weight, 1000-grain weight, and grain yield in both seasons. The EHR1 rice variety gave the higher panicle weight and grain yield, followed by Giza179. At the same time, the lowest values were obtained by Giza177 and Sakha102 in both seasons. Meanwhile, the highest values of 1000 grain weight were produced by Sakha102 and Sakha106, and Sakha107 had the same statistical level.
However, the lowest values of 1000 grain weight were obtained by Egyptian Yasmine in both seasons. The lowest values of grain yield were obtained by Giza177. GZ9399 and Giza178 occupied the second order after EHR1 and Giza179 considering rice grain yield, while A22, Egyptian Yasmine, and Sakha107 came in the third-order concerning rice grain yield in both seasons. As mentioned in Table 14, the interaction between planting methods and rice genotypes significantly affected panicle weight, 1000-grain weight, and grain yield in both seasons. The EHR1 and Giza179 variety gave the heaviest panicle weight with transplanting planting method. Meanwhile, the highest value of 1000 grain weight was found in Sakha107, 106, 102, and Giza177 varieties under the transplanting method. On contrary, the lowest values of panicle weight were obtained by Sakha106 with aerobic cultivation. Furthermore, the lowest values of 1000 grain weight were found in E. Yasmine variety with aerobic cultivation. The interaction between planting methods and rice genotypes significantly affected grain yield in both seasons (Table 16). The maximum values of grain yield were noted in EHR1and Giza179 varieties with the transplanting method. Nevertheless, the lowest values of grain yield were obtained by Giza177. Under aerobic pital letterrice cultivation, Giza179 produced the maximum grain yield without significant variation with these brought by GZ9399 and EHR1 as well as Giza178. The worst varieties under aerobic were Giza177, Sakah102 and Sakha106 as drought sensitive ones. Furthermore, A22, E. Yasmine and Sakha107 showed a medium level of grain yield under aerobic cultivation as a medium drought tolerant variety considering grain yield.
Discussion
Aerobic rice cultivation is a medium option to save water with an insignificant yield reduction for irrigation rice ecotypes6.
Antioxidant enzymatic activity
From going findings, the tested rice genotypes were at par regarding the antioxidants plant content under the transplanting method (well watering) because gene expression less and low free radical formation. Under aerobic conditions, there are great variations among genotypes with respect to their ability to produce more antioxidant against free radicals development under such stress. Rice genotypes of EHR1, Giza179, GZ9399, Giza178 and A22 had high concentration of enzymatic antioxidants comparing others under the same conditions and particularly under aerobic condition in the terms of low water input. On contrary, Giza177, Sakha102 and Sakha106 showed weakness to release reasonable concentrations of antioxidants under aerobic conditions (stress case) even comparing to their concentration under transplanting. Oxidative damage by releasing reactive oxygen species (ROS) under abiotic stresses; high and low temperature, salt stress, water stress, and water dehydration represents a negative effect on plant growth and yield. One of the unavoidable consequences of drought stress is raising ROS production in the different cellular compartments, namely in the chloroplasts, the peroxisomes and the mitochondria. Drought caused greater inhibition of photosystem II quantum efficiency, carboxylation efficiency, and photosynthetic capacity parameters as a result of more accumulation of reactive oxygen species (ROS)37,38. The attach of ROS attack and react with the cell membrane's phospholipids, reducing its stability and causing a high EL% of the cell39. ROS in plants was removed by a variety of antioxidant enzymes and/or lipid-soluble and water-soluble scavenging molecules, the antioxidant enzymes being the most efficient mechanisms against oxidative stress40,41,42,43,44,45. Drought stress greatly influences physiological and biochemical functions that affect plant growth46,47,48,49. Catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD) increase in under drought conditions. These antioxidant enzymes can protect cells from oxidative damage. Similarly, proline synthesis increased under drought conditions, showing that proline could also act as part of a survival mechanism under drought50. Moreover, drought leads to an increase in proline accumulation, which proline helps to maintain tissue water status. Accordingly, rice plants adapted to drier environments have increased the activity of antioxidant enzymes to eliminate ROS13. Continuously, the varieties; EHR1, Giza179, GZ9399, Giza178, and A22 showed apparent capacity to exert an avoidant concentration of proline under aerobic cultivation (stress conditions), which acts as an osomo-protectant and water supplier under drought. The other genotypes including Giza177, Sakha102 and Sakha106 had low efficiency to accumulate sufficient level of proline to enable them to grow healthy under aerobic conditions, indicating their drought sensitivity. Like antioxidants, all tested rice entries were at the same level of significance regarding to the proline accumulation under the transplanting method (well watering). Therefore, the ability of rice genotype to accumulate continuously reasonable concentrations of both proline and antioxidants could be recognized as valid genotype under aerobic conditions.
Physio-morphological traits
Water deficit causes negative effects on the enzymes activities resulting in many changes in plant tissues, cell damage, nutrient uptake and photosynthesis rate51. Aerobic cultivation significantly decreased the measured physio-morphological such as leaf area index, flag leaf area, and chlorophyll content, but increased leaf rolling. The ten rice genotypes differed regarding the change rate under aerobic conditions owing to their adaptation capabilities. Like the antioxidant concept, Giza179, EHR1, GZ9399, Giza178 and A22 as one resilience category, showed higher leaf area index, flag leaf area, chlorophyll content and low leaf rolling combined with high stomata conductance under aerobic conditions irrespective of transplanting method. The high physio-morphological concept possessed by the resilience group might be reflected on raising its adaption, growth, photosynthesis, development and finally sink. On contrast, the worse category varieties including Giza177, Sakha102 and Sakha107 brought severe reduction in its physio-morphological traits along with high leaf rolling indicating its invalidation under aerobic conditions. It is important to address that high leaf roiling is a sensitivity indicator, not tolerance, which was matched with poor growth. Furthermore, the reduction in chlorophyll of sensitive varieties might affect the growth and physiological events such as photosynthesis and energy synthesis. Generally, the tolerant group keeps reasonable root length with proper volume under aerobic conditions comparing to other group which failed to give a good root system. It became clear that good root system is an essential option for validation under aerobic conditions. High leaf rolling for sensitive group might be affected the stomatal conductance and subsequently reducing gas exchange and restricting photosynthesis. One of the essential defense mechanisms in plants is avoiding drought, by adjusting some plant attributes, such as the reduction of leaf rolling or leaf size.
Cell membrane stability
It is well known that the conservation of integrity and stability of membranes under water stress is a major component of drought tolerance in plants52. Cell membrane stability diminished rapidly in Kentucky bluegrass exposed to drought and heat stress simultaneously53. The decrease in chlorophyll and increase in EL% may be due to the role of free radicals in damaging the membrane phospholipids of the cell wall and plant pigments. The antioxidant enzymes are the most efficient mechanisms against oxidative stress. Apart from catalase, various peroxidases and peroxiredoxins, four enzymes are involved in the ascorbate–glutathione cycle, a pathway that allows the scavenging of superoxide radicals and H2O227. The cell membrane stability was reduced under aerobic conditions, but the genotypes that possessed high concentrations of measured antioxidants under the current study kept high capability of cell membrane without damage such as GZ9399, Giza179 and EHR1. The previous varieties had low stability index, since a low index is favorable that indicating a low EL%. The low EL% provided the ability of these varieties to create satisfied defense system like antioxidants. The antioxidant could eliminate the hazard free radical developed under drought stress, which damaged the cell membrane54,55. The results pertaining to sensitive varieties such as Giza177, Sakha102 and Sakha106 confirmed the previous concept, which had a high stability index that matched the low concentration of antioxidants indicated apparent cell membrane damage. The damage of cell membrane induced more EL% of plant cell that was hold true with drought sensitive varieties such Giza177. Proline is acting as antioxidant and as osmo-protectant material under abiotic stresses, particularly water deficit and salt stresses11. Improved water status may be achieved through osmotic adjustment and/or changes in cell wall elasticity that results in maintaining physiological activity for extended periods of drought. Giza179, GZ9399 and EHR1 showed high proline accumulation that was completely matched with a high RWC under aerobic conditions.
Stomatal conductivity
The fast and first response of plants to water deficit is the closure of their stomata to minimize the transpiration of water. Recently, stomatal closure was generally accepted to be the main determinant for decreased photosynthesis under mild to moderate drought56. Again, EHR1, GZ9399 and Giza179 might have a high affinity to regulate stomata conductance under aerobic conditions with high efficiency that might be due to their ability to do balance between the K and ABA concentration in the guard cells. Furthermore, The mentioned genotypes as resilience one based on previous findings showed less leaf rolling under aerobic conditions, reflecting on optimum regulating of stomata conductance and subsequently CO2 sufficient supply needed for photosynthesis. The group including Giza177, Sakha102 and Sakha106 exhibited low stomata conductance owing to sharp leaf rolling for a long time under aerobic conditions and poor adaptation. Meanwhile, aerobic cultivation showed a reduction in such traits with various levels according to the adaptation abilities of varying genotypes. Less water input might have increased water loss through stomata, and increased respiration rates resulted in a reduction of the most of the physiological processes and photosynthetic rate57. Rice plants adapted to dry environments develop fewer stomata on the surface to avoid losing more water and growth limiting, also, decreased transpiration rate as a function enhanced the drought tolerance. The management of stomata conductance is one of the most important parameters for plant resistance under stress.
Yield and yield components
Aerobic conditions with low water input might reduce cell division, elongation, and plant height as well as panicle length; also, low water input resulted in a low number of panicle/hill32. The previous concept was found under aerobic conditions comparing to transplanting method (well water supply). This may be attributed to the role of water and nutrients for producing new tillers and total number of grains/panicle6. Aerobic cultivation significantly reduced the number of filled grains combined with increasing unfilled grain, but the tolerant varieties; Giza79, EHR1, GZ9399, A22, and Giza178 were less affected comparing to another opposite group involving Giza177, Sakha102 and Sakha106. Low water supply under aerobic conditions might affect metabolism, development, photosynthesis assimilates and filled grains. Giza179, EHR1, GZ9399, A22 and Giza178 were found effective to developed different drought mechanisms, which reflected on their healthy growth and contentment sources that ensure proper sink along with heavy grain (panicle). Well sources and sink of tolerant rice genotype brought acceptable grain yield under aerobic conditions. The opposites was corrected with well watering under transplanting regarding plant development, physiological processes such as photosynthesis, enzyme activity, which assimilates transportation from source to sink, resulting in improved yield and yield components of rice58. Grain yield was reduced as a result of lower amounts of water supply. The reduction in grain yield might be attributed to declining in 1000 grain weight and spikelets/panicle51. Water deficit during early flowering reduced the number of fertile spikelets and resulted in decreased final grain yield production, this might also be associated with the reduction in leaf area, lower photosynthetic rates, and assimilate transportation from source to sink and dry matter partitioning59.
Conclusions
It is concluded that water deficit stress greatly influenced physiological functions and biochemical activities that negatively affect plant growth. Superoxide dismutase and catalase as well as, proline and RWC increased under drought stress condition, particularly with tolerant varieties, indicating that the tolerance to aerobic stress is not imputable to a single component, but it is a multifactorial response. Thereby, based on the performance of growth, biochemical, yield, and yield attributes, Giza179, GZ9399, EHR1 and A22, and Giza178 is recommended to lead cultivation under aerobic conditions.
Data availability
Data used in the current research presented in the manuscript. The source of seeds is Rice Research and Training Center, Giza, Egypt.
References
Kahani, F. & Hittalmani, S. Genetic analysis and traits association in F2 inter varietal populations in rice under aerobic condition. J. Rice Res. 3, 152 (2015).
Dey, S., Ram, K., Chhabra, A. K., Lokeshwar, R. A. & Janghel, D. K. Aerobic rice: Smart technology of rice cultivation. Int. J. Curr. Microbiol. Appl. Sci. 7, 1799–1804 (2018).
Hermans, T. Modelling Grain Surplus/Deficit in Cameroon for 2030 (2016).
De Borja Reis, A. F. et al. Relationship of nitrogen and crop performance in aerobic rice and continuous flooding irrigation in weathered tropical lowland. Eur. J. Agron. 95, 14–23 (2018).
Verulkar, S. B., Mandal, N. P., Dwivedi, J. L., Singh, B. N. & Sinha, P. K. Breeding resilient and productive genotypes adapted to drought prone rainfed ecosystems of India. Field Crop Res. 117, 197–208 (2010).
Joshi, R., Singh, B. & Shukla, A. Evaluation of elite rice genotypes for physiological and yield attributes under aerobic and irrigated conditions in tarai areas of western Himalayan region. Curr. Plant Biol. 13, 45 (2018).
Mitchell, J., Proud, C., Fukai, S. Traits of importance for aerobic rice. Paper presented at the Proceedings of the 19th Australian Society of Agronomy Conference, Wagga, NSW, Australia (2020).
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D. & Basra, S. M. A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212 (2009).
Rashwan, E., Alsohim, A. S., El-Gammaal, A., Hafez, Y. & Abdelaal, K. A. A. Foliar application of nanozink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ. Bull. 29, 8889–8904 (2021).
Abdelaal, K. A. A. et al. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 12, 1736 (2020).
Abdelaal, K. A. et al. Pivotal role of yeast and ascorbic acid in improvement the morpho-physiological characters of two wheat cultivars under water deficit stress in calcareous soil. Fresenius Environ. Bull. 30, 2554–2565 (2021).
Abdelaal, K. et al. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 10, 520 (2021).
AlKahtani, M. D. F. et al. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 10, 398 (2021).
Hasan, M. K. et al. Comparative adaptable agronomic traits of Blackgram and mungbean for saline lands. Plant Arch. 17, 589–593 (2017).
Abdelaal, K. A. A., Mazrou, Y. S. A. & Hafez, Y. M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 9, 733 (2021).
Abdelaal, K. A. A., El-Afry, M., Metwaly, M., Zidan, M. & Rashwan, E. Salt tolerance activation in faba bean plants using proline and salicylic acid associated with physio-biochemical and yield characters improvement. Fresenius Environ. Bull. 30, 3175–3186 (2021).
El-Shawa, G. M. R., Rashwan, E. M. & Abdelaal, K. A. A. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical characters of calendula plants. Sci. J. Flowers Ornam. Plants 7, 461–482 (2021).
Al Kahtani, M. D. F. et al. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 10, 1180 (2021).
Pongprayoon, W., Roytrakul, S., Pichayagkura, R., Chadchawan, S. & Alejo, L. A. Assessing the impacts of climate change on aerobic rice production using the DSSAT-CERES-Rice model. J. Water Clim. Chang. 12, 696–708 (2021).
Sulmon, C. et al. Abiotic stressors and stress responses: What commonalities appear between species across biological organization levels?. Environ. Pollut. 202, 66–77 (2015).
Sabouri, A. et al. Superior adaptation of aerobic rice under drought stress in Iran and validation test of linked SSR markers to major QTLs by MLM analysis across two years. Mol. Biol. Rep. 45, 1037–1053 (2018).
El-Banna, M. F. & Abdelaal, K. A. A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod. Mansoura Univ. 9, 989–1001 (2018).
Abdelaal, K. A. A. et al. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10, 26 (2021).
El-Flaah, R. F., El-Said, R. A. R., Nassar, M. A., Hassan, M. & Abdelaal, K. A. A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POD and PPO in faba bean (Vicia faba L.) under salinity stress conditions. Fresenius Environ. Bull. 30, 5751–5764 (2021).
Hafez, Y. et al. Alleviating the detrimental impacts of salt stress on morpho-hpysiological and yield characters of rice plants (Oryza sativa L.) using actosol. Nano-Zn and Nano-Si. Fresenius Environ. Bull. 29, 6882–6897 (2021).
Ramulu, V., Reddy, M. D., Umadevi, M. & Sudharani, Y. Response of rice cultivars to nitrogen levels under aerobic and transplanted conditions. Indian J. Agric. Res. 54, 521–525 (2015).
Bradford, M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Bergmeyer, H. U. Standardization of methods for estimation of enzyme activity in biological fluids. Z. Klin. Chem. Biochem. 1970(8), 658–660 (1970).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Kar, M. & Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57, 315–319 (1976).
González, L., González-Vilar, M. Determination of relative water content. in Handbook of Plant Ecophysiology Techniques, 207–212. (Springer, 2001).
Premachandra, G. S., Saneoka, H. & Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J. Agric. Sci. 115, 63–66 (1990).
Hubbard, R. M., Ryan, M. G., Stiller, V. & Sperry, J. S. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 24, 113–121 (2001).
Gomez, K.A., Gomez, A.A. (1984). Statistical procedures for agricultural research.
Duncan, D. B. Multiple range tests for correlated and heteroscedastic means. Biometrics 13, 164–176 (1955).
Software, C. Microcomputer Program Analysis (CoHort Software, 1988).
Maria, H. C. Drought stress and reactive oxygen species. Plant Signal. Behav. 3(3), 156–165 (2008).
Preethi, V., Sankarapillai V. L., Paul, C. S., Udayakumar, M., Xinyou, Y., Sheshshayee, S. Production and scavenging of reactive oxygen species confer to differential sensitivity of rice and wheat to drought stress crop and environment. 1, 15–23 (2022).
Foyer, C. H. & Fletcher, J. M. Plant antioxidants: Colour me healthy. Biologist 48, 115–120 (2004).
Farooq, M., Aziz, T., Basra, S. M. A., Cheema, M. A. & Rehamn, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 194, 161–168 (2008).
Hafez, Y. M. et al. Bacillus subtilisasa bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 111, 101489 (2021).
Hafez, Y. et al. Alternative treatments improve physiological characters, yield and tolerance of wheat plants under leaf rust disease stress. Fresenius Environ. Bull. 29, 4738–4748 (2021).
Hafez, Y. M., Abdelaal, K. A. A., Badr, M. M. & Esmaeil, R. Control of puccinia triticina the causal agent of wheat leaf rust disease using safety resistance inducers correlated with endogenously antioxidant enzymes up-regulation. Egypt. J. Biol. Pest Control 2017(27), 1–10 (2017).
Omara, R. I. & Abdelaal, K. A. A. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 104, 48–57 (2018).
El-Nashaar, F. et al. Assessment of host reaction and yield losses of commercial barley cultivars to Drechslera teres the causal agent of net blotch disease in Egypt. Fresenius Environ. Bull. 29, 2371–2377 (2021).
Abdelaal, K. A. A., Hafez, Y. M., El Sabagh, A. & Saneok, H. Ameliorative effects of Abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under water deficit conditions. Fresenius Environ. Bull. 26, 7372–7383 (2017).
Abdelaal, K. A. A., Hafez, Y. M., El-Afry, M. M., Tantawy, D. S. & Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 25, 30199–30211 (2018).
Abdelaal, K. A. A., Rashed, S. H., Ragab, A., Hossian, A. & El Sabagh, A. Yield and quality of two sugar beet (Beta vulgaris L. ssp. vulgaris var. altissima Doll) cultivars are influenced by foliar application of salicylic Acid, irrigation timing, and planting density. Acta Agric. Slov. 115, 239–248 (2021).
Hafez, Y.M., Attia, K.A., Alamery, S., Ghazy, A., Al-Dosse, A., Ibrahim, E., Rashwan, E., El-Maghraby, L., Awad, A., Abdelaal, K.A.A. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy. 10 (2021).
Zayed, B. A., Khedr, R., Hadifa, A. A. & Okasha, A. M. Some antioxidants, physio-morphsological and yield of varying rice varieties affected by salinity levels. Mansoura J. Plant Prod. 8, 747–754 (2017).
Hossain, M. Z. et al. Influence of water stress on morphology, physiology and yield contributing characteristics of rice. SAARC J. Agric. 18, 61–71 (2021).
Bajji, M., Kinet, J. & Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 36, 61–70 (2022).
Wang, Z. & Huang, B. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Sci. 44, 1729–1736 (2004).
Abdelaal, K. A., El-Okkiah, S., Metwaly, M. & El-Afry, L. Impact of Ascorbic acid and proline application on the physiological machinery in soybean plants under salinity stress. Fresenius Environ. Bull. 30, 12486–12497 (2021).
Arafa, S. A. et al. Seed priming boost adaptation in pea plants under drought stress. Plants 2021(10), 2201 (2021).
Yokota, A. et al. Citrulline and DRIP-1 protein (ArgE Homologue) in drought tolerance of wild watermelon. Ann. Bot. 89, 825–832 (2002).
Abd Allah, A. A., Badawy, S. A., Zayed, B. A. & El-Gohary, A. A. The role of root system traits in the drought tolerance of rice (Oryza sativa L.). J. Plant Prod. 1, 621–631 (2010).
Purwanto, O. D., Palobo, F. & Tirajoh, S. Growth and yield of superior rice (Oryza sativa L.) varieties on different planting systems in Papua, Indonesia. SVU-Int. J. Agri. Sci. 2, 242–255 (2021).
Kamarudin, Z. S., Yusop, M. R., TengkuMuda, M., Ismail, M. R. & Harun, A. R. Growth performance and antioxidant enzyme activities of advanced mutant rice genotypes under drought stress condition. Agronomy 8, 279 (2018).
Author information
Authors and Affiliations
Contributions
B.A.Z., H.A.G., M.E.N., S.M.B., A.A.H., D.E.E.-S., M.M.A., E.A.A.M., A.M.O., S.E.: conceptualization, investigation, methodology, analysis, writing up, review and edit. A.A.F.: conceptualization, supervision, investigation, methodology, analysis, writing up, review and edit. Z.M.Y.: conceptualization, supervision, project leader, investigation, methodology, analysis, writing up, review and edit.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zayed, B.A., Ghazy, H.A., Negm, M.E. et al. Response of varied rice genotypes on cell membrane stability, defense system, physio-morphological traits and yield under transplanting and aerobic cultivation. Sci Rep 13, 5765 (2023). https://doi.org/10.1038/s41598-023-32191-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32191-6
This article is cited by
-
Wheat Drought Tolerance: Morpho-Physiological Criteria, Stress Indexes, and Yield Responses in Newly Sand Soils
Journal of Plant Growth Regulation (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.