Abstract
The aim of the current study was to determine the prevalence of anatomic variant in cephalic arch on preoperative mapping venography and evaluate patency rates and predictors of patency in patients with brachiocephalic fistulas. The prevalence of anatomic variant in cephalic arch was retrospectively evaluated in 1004 consecutive patients who underwent bilateral preoperative mapping venography from July 2006 to December 2018 in a single center. The overall prevalence of anatomic variant in cephalic arch was 17.2% (173/1004). For patency analysis, 128 patients with brachiocephalic fistulas were divided into two groups: a standard anatomy (SA) group (n = 97) and a variant anatomy (VA) group (n = 31). There were no significant differences in clinical characteristics between the two groups. The primary patency rate did not differ significantly between the two groups. The secondary patency rate was significantly (p = 0.009) lower in the VA group than in the SA group. Older age (HR 1.03; 95% CI 1.01–1.05; p = 0.007) was a negative predictor of primary patency, and antiplatelet agent (HR 0.53; 95% CI 0.33–0.84; p = 0.007) and large-diameter cephalic vein (HR 0.52; 95% CI 0.31–0.86; p = 0.012) were positive predictors of primary patency. Older age (HR 1.04; 95% CI 1.01–1.07; p = 0.011) and anatomic variant in cephalic arch (HR 2.9; 95% CI 1.19–7.06; p = 0.019) were negative predictors of secondary patency. The current study provides insight into the clinical significance of anatomic variant in cephalic arch. Anatomic variant in cephalic arch should be considered as a potential risk factor for decreased patency of brachiocephalic fistula during preoperative planning.
Similar content being viewed by others
Introduction
Preoperative vascular mapping for hemodialysis access placement has been increasingly adopted to evaluate the precise vascular anatomy and to improve dialysis result by proper selection of target vessel1,2,3. Doppler ultrasound can provide an accurate assessment of luminal diameter with a flow velocity. It is preferred in patients with minimal residual renal function4. Venography is extremely useful for evaluating central vein stenosis and provide a road map of entire venous anatomy5. Brachiocephalic fistula is usually placed in a patient with a forearm fistula that has failed or a patient whose forearm vessels are unsuitable for fistula formation on preoperative vascular mapping6,7.
Cephalic arch is one of the most important anatomic landmarks of brachiocephalic fistula. It is the final bridge of the cephalic vein as it arcs through the deltopectoral groove to join the axillary vein8. Careful preoperative assessment of the cephalic arch is required because it is a unique venous outflow tract and a frequent site of stenosis in brachiocephalic fistula9,10,11. On preoperative mapping venography, anatomic variant in cephalic arch has been reported to be as high as 8.7% in published literature1,12. However, there is paucity of data on the clinical significance of anatomic variant in cephalic arch13. Thus, the aim of the current study was to determine the prevalence of anatomic variant in cephalic arch on preoperative mapping venography and to evaluate patency rates and predictors of patency in patients with brachiocephalic fistulas.
Methods
The Institutional Review Board of Soonchunhyang University Cheonan Hospital (IRB No. 2019-12-005) approved this retrospective single-center study and waived the requirement of written informed consent for use of clinical and imaging data. All methods were carried out in accordance with relevant guidelines and regulations.
Venography protocol
During the study period, mapping venography was performed as a routine imaging procedure before access creation. Intravenous accesses were placed in both hands with 20-guage indwelling needles. Venography was performed using a 50:50 dilution mixture (20–30 ml) of nonionic low-osmolar contrast media (Omnipaque 350; GE Healthcare, Jupiter, Florida, USA) with normal saline. A tourniquet was applied to the level of the upper arm near the axilla14. After veins were filled with contrast media, digital spot images were taken. Evaluations of the cephalic arch and the central vein were achieved with contrast media injection as an untied tourniquet at the same time.
Operative technique
All surgical procedures were performed by one of the two vascular surgeons (10 and 5 years of experience, respectively). Brachiocephalic fistulas were placed in patients whose preoperative mapping indicated brachial artery and cephalic vein in the antecubital space. The minimum cephalic vein diameter required was set at 2 mm. If possible, the non-dominant side was used for construction of the fistula. Under local anesthesia, a transverse skin incision was made at about 1 cm above the elbow crease. The bicipital fascia was incised, and the brachial artery was exposed. The artery was incised after clamping, an end-to-side vein-to artery anastomosis was performed with a running 7–0 polypropylene suture with a limited arteriotomy. Subsequently, clamps were removed and bleeding was controlled. The fistula could then be easily palpated through the skin.
Study design
The prevalence of anatomic variant in cephalic arch was evaluated in 1004 consecutive patients who underwent bilateral preoperative mapping venography from July 2006 to December 2018. All venograms were reviewed by two interventional radiologists (10 and 5 years of experience, respectively) who were blinded for patient characteristics to minimize potential bias. Of 1004 patients, 131 underwent creation of brachiocephalic fistula. Three patients who failed to have a mature fistula were excluded. A total of 128 patients (age, 60.71 ± 13.3 years; men, 53.1%) were eligible for patency analysis. They were divided into two groups: a standard anatomy (SA) group (n = 97) and a variant anatomy (VA) group (n = 31). The data collection regarding patency and interventions of each patient was based on patient medical records. The study flow diagram is depicted in Fig. 1.
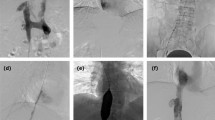
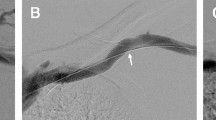
Primary endpoints included primary and secondary patency rates defined based on Reporting Standards of the Society for Vascular Surgery and the American Association for Vascular Surgery15. Primary patency was defined as the interval from the time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis. Secondary patency was defined as the interval from the time of access placement until access abandonment including endovascular interventions. Secondary endpoints included the number of intervention and the location of stenosis requiring intervention. The location of stenosis requiring intervention was categorized anatomically into juxta-anastomosis stenosis (< 3 cm from anastomosis), outflow vein (cephalic arch) stenosis, or central vein stenosis (Fig. 2).
Statistical analysis
For descriptive statistics, continuous variables are presented as mean ± standard deviation and categorical variables are presented as absolute and relative frequencies. For comparative analysis, the Wilcoxon rank-sum test was used for continuous variables and the Chi-squared test or Fisher’s exact test was used for categorical variables. The Kaplan–Meier technique and the log-rank test were used to evaluate and compare access patency rates between the two groups. Censoring of endpoints occurred in the setting of patient death with a functioning access, renal transplantation with a functioning access, and functioning access at the time of the last documented follow-up. Potential predictors of access patency were analyzed with a Cox proportional-hazards model. Variables with a p-value of less than 0.1 on univariable analysis were included in the multivariable analysis. A p-value < 0.05 was considered statistically significant. All statistical analyses were executed using R version 3.6.3 software (Foundation for Statistical Computing, Vienna, Austria).
Results
The overall prevalence of anatomic variant in cephalic arch was 17.2% (173/1004). For patency analysis, 128 patients with brachiocephalic fistulas were divided into two groups: the SA group (n = 97) and the VA group (n = 31). There were no significant differences in clinical characteristics between the two groups (Table 1). The mean follow-up duration was 36.5 months for the SA group and 29.7 months for the VA group, showing no significant difference between the two groups (p = 0.215). The primary patency rate did not differ significantly between the two groups either (p = 0.58) (Fig. 3). However, the secondary patency rate was significantly lower in the VA group than in the SA group (p = 0.009). The mean number of intervention required was 1.07 ± 1.32 times in the SA group and 1.06 ± 1.69 in the VA group, showing no significant difference (p = 0.607). There was no significant difference in the location of stenosis between the two groups either: juxta-anastomosis, outflow vein, or central vein (p > 0.99) (Fig. 2).
Results of univariable and multivariable Cox proportional hazards regression analysis to identify predictors associated with access patency are summarized in Tables 2 and 3. Multivariable analysis indicated that older age (HR 1.03; 95% CI 1.01–1.05; p = 0.007) was a negative predictor of primary patency, and antiplatelet agent (HR 0.53; 95% CI 0.33–0.84; p = 0.007) and large-diameter cephalic vein (HR 0.52; 95% CI 0.31–0.86; p = 0.012) were positive predictors of primary patency. Older age (HR 1.04; 95% CI 1.01–1.07; p = 0.011) and anatomic variant in cephalic arch (HR 2.9; 95% CI 1.19–7.06; p = 0.019) were negative predictors of secondary patency. There was no multicollinearity because variance inflation factors were less than the threshold value of 2.
Discussion
Anatomic variants of upper arm veins are frequently encountered on preoperative mapping venography. Their common locations include brachial-basilic confluence and cephalic arch1,12. Early confluence of basilic and unpaired brachial veins has been described as potential prohibition of basilic vein transposition that can lead to failure of subsequent graft placement16,17. On the other hand, the clinical significance of anatomic variant in cephalic arch has not been established yet13. In the current study, the association between patency of brachiocephalic fistula and anatomic variant in cephalic arch was investigated. The secondary patency of the VA group was significantly lower than that of the SA group despite the two groups had comparable preoperative clinical characteristics. Anatomic variant in cephalic arch was a significant negative predictor of secondary patency on multivariable analysis.
Anatomic variant in cephalic arch was found in 17.2% of the study population, which was higher than that in previous reports (4–8.7%)1,12. These differences may be explained by different indications of mapping venography. The prevalences of the anatomic variant in previous studies were from a select group of patients which at high risk of central venous stenosis, which is not a true prevalence in all patients considered for fistula formation.
In the forearm, radiocephalic fistula drains via basilic, brachial, and cephalic veins. On the other hand, brachiocephalic fistula has a higher flow rate and, in general, drain exclusively via the cephalic vein and its arch18,19. Therefore, patency analysis of the current study included only patients with brachiocephalic fistula to assess the clinical significance of anatomic variant in cephalic arch. The cephalic arch is one of the most susceptible site for stenosis in brachiocephalic fistula and the incidence has been reported between 17 and 77%20,21,22. The current study using a cohort of dialysis patients demonstrated that the incidence of cephalic arch stenosis and the primary patency rate was comparable between the two groups, but the secondary patency was not. Older age (HR 1.04; 95% CI 1.01–1.07; p = 0.011) and anatomic variant in cephalic arch (HR 2.9; 95% CI 1.19–7.06; p = 0.019) were negative predictors of secondary patency. Multivariable analysis showed that anatomic variant in cephalic arch was a negative predictor of secondary patency.
One potential explanation for lower secondary patency in VA group is altered hemodynamics within cephalic arch. A few previous studies focused on underlying hemodynamics in the cephalic arch have been proposed23,24. Boghosian et al.24 investigated the hemodynamics of anatomic variant in cephalic arch using computational fluid dynamics modeling. They hypothesized that additional channels of cephalic arch could have a protective effect on the development of neointimal hyperplasia by reducing inlet flow rate and wall shear stresses. On the other hand, persistent reduced flow rate within cephalic arch may eventually lead to poor response to interventional treatment and decreased long-term secondary patency.
The current study indicated that age, vein diameter and adjuvant medication were significantly associated with access patency, thus supporting the evidence from several prospective studies and systematic reviews25,26,27,28. However, controversy remains regarding the benefit of arteriovenous fistula placement in elderly patients and the use of adjuvant medication for prolonging access patency29,30,31. It should also be noted that the current study only targeted patients with brachiocephalic fistula, and caution is required when extrapolating this result to other types of dialysis access.
The current study has certain limitations. The retrospective nature of analysis limited a detailed evaluation of records such as serial follow-up of flow velocity of the fistula. Given the low prevalence of anatomic variant, the small sample size in this single-center cohort might have limited the overall generalizability of study results and subgroup analysis. A larger sample size and a prospectively designed study may be required to validate our results. In addition, there might be missing prognostic factors that affected access patency.
In conclusion, the current study provides insight into the clinical significance of anatomic variant in cephalic arch. Anatomic variant in cephalic arch should be considered as a potential risk factor for decreased patency of brachiocephalic fistula during preoperative planning.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Hyland, K. et al. Preoperative mapping venography in patients who require hemodialysis access: Imaging findings and contribution to management. J. Vasc. Interv. Radiol. 19, 1027–1033. https://doi.org/10.1016/j.jvir.2008.03.015 (2008).
Brown, P. W. Preoperative radiological assessment for vascular access. Eur. J. Vasc. Endovasc. Surg. 31, 64–69. https://doi.org/10.1016/j.ejvs.2005.10.002 (2006).
Lima, A., Carrilho, P. & Germano, A. Clinical and ultrasound evaluation for hemodialysis access creation. Nefrologia (Engl Ed) https://doi.org/10.1016/j.nefro.2020.10.013 (2021).
Shenoy, S. & Darcy, M. Ultrasound as a tool for preoperative planning, monitoring, and interventions in dialysis arteriovenous access. AJR Am. J. Roentgenol. 201, W539-543. https://doi.org/10.2214/AJR.13.11277 (2013).
Surlan, M. & Popovic, P. The role of interventional radiology in management of patients with end-stage renal disease. Eur. J. Radiol. 46, 96–114. https://doi.org/10.1016/s0720-048x(03)00074-3 (2003).
Quencer, K. B. & Arici, M. Arteriovenous fistulas and their characteristic sites of stenosis. AJR Am. J. Roentgenol. 205, 726–734. https://doi.org/10.2214/AJR.15.14650 (2015).
Maya, I. D., O’Neal, J. C., Young, C. J., Barker-Finkel, J. & Allon, M. Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin. J. Am. Soc. Nephrol. 4, 86–92. https://doi.org/10.2215/CJN.02910608 (2009).
Loukas, M. et al. The clinical anatomy of the cephalic vein in the deltopectoral triangle. Folia Morphol. (Warsz) 67, 72–77 (2008).
Vasanthamohan, L., Gopee-Ramanan, P. & Athreya, S. The Management of cephalic arch stenosis in arteriovenous fistulas for hemodialysis: A systematic review. Cardiovasc. Intervent. Radiol. 38, 1179–1185. https://doi.org/10.1007/s00270-015-1190-4 (2015).
D’Cruz, R. T. et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: A systematic review and meta-analysis. J. Vasc. Access 20, 345–355. https://doi.org/10.1177/1129729818814466 (2019).
Kanterman, R. Y. et al. Dialysis access grafts: Anatomic location of venous stenosis and results of angioplasty. Radiology 195, 135–139. https://doi.org/10.1148/radiology.195.1.7892454 (1995).
Lee, H. S. et al. Anatomical variants of upper arm veins on preoperative mapping venography for hemodialysis access in Korean adults. J. Vasc. Access 20, 270–275. https://doi.org/10.1177/1129729818803870 (2019).
Shalhub, S., Dua, A., Shin, S. & Aarabi, S. Hemodialysis access: Fundamentals and advanced management (Springer, 2017).
Lee, S. A. et al. Venogram of the upper extremity using the tourniquet technique for the evaluation of central vein patency: A comparison to conventional and CO2 venogram. J. Korean Soc. Radiol. 65, 61–68. https://doi.org/10.3348/jksr.2011.65.1.61 (2011).
Sidawy, A. N. et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J. Vasc. Surg. 35, 603–610. https://doi.org/10.1067/mva.2002.122025 (2002).
Anaya-Ayala, J. E. et al. Prevalence of variant brachial-basilic vein anatomy and implications for vascular access planning. J. Vasc. Surg. 53, 720–724. https://doi.org/10.1016/j.jvs.2010.09.072 (2011).
Kaiser, C. L., Anaya-Ayala, J. E., Ismail, N., Davies, M. G. & Peden, E. K. Unrecognized basilic vein variation leading to complication during basilic vein transposition arteriovenous fistula creation: Case report and implications for access planning. Eur. J. Vasc. Endovasc. Surg. 39, 627–629. https://doi.org/10.1016/j.ejvs.2010.01.011 (2010).
Sarala, S. et al. Cephalic arch stenosis: Location of stenosis in indian hemodialysis patients. Indian J. Nephrol. 28, 273–277. https://doi.org/10.4103/ijn.IJN_321_16 (2018).
Miller, G. A., Friedman, A., Khariton, A., Preddie, D. C. & Savransky, Y. Access flow reduction and recurrent symptomatic cephalic arch stenosis in brachiocephalic hemodialysis arteriovenous fistulas. J. Vasc. Access 11, 281–287. https://doi.org/10.5301/jva.2010.592 (2010).
Neves, M. et al. Predictive factors of recurrent endovascular intervention for cephalic arch stenosis after percutaneous transluminal angioplasty. J. Vasc. Surg. 68, 836–842. https://doi.org/10.1016/j.jvs.2017.12.055 (2018).
Wang, S., Almehmi, A. & Asif, A. Surgical management of cephalic arch occlusive lesions: Are there predictors for outcomes?. Semin Dial 26, E33-41. https://doi.org/10.1111/sdi.12085 (2013).
Shawyer, A. et al. Cephalic arch stenosis in autogenous haemodialysis fistulas: Treatment with the viabahn stent-graft. Cardiovasc. Intervent. Radiol. 36, 133–139. https://doi.org/10.1007/s00270-012-0433-x (2013).
Hammes, M. et al. Increased inlet blood flow velocity predicts low wall shear stress in the cephalic arch of patients with brachiocephalic fistula access. PLoS ONE 11, e0152873. https://doi.org/10.1371/journal.pone.0152873 (2016).
Boghosian, M. et al. Hemodynamics in the cephalic arch of a brachiocephalic fistula. Med. Eng. Phys. 36, 822–830. https://doi.org/10.1016/j.medengphy.2014.03.001 (2014).
Lazarides, M. K., Georgiadis, G. S., Antoniou, G. A. & Staramos, D. N. A meta-analysis of dialysis access outcome in elderly patients. J. Vasc. Surg. 45, 420–426. https://doi.org/10.1016/j.jvs.2006.10.035 (2007).
Mohamed, I., Kamarizan, M. F. A. & Da Silva, A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst. Rev. 7, CD002786. https://doi.org/10.1002/14651858.CD002786.pub4 (2021).
Palmer, S. C. et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: Systematic review and meta-analysis. Am. J. Kidney Dis. 61, 112–122. https://doi.org/10.1053/j.ajkd.2012.08.031 (2013).
Abreu, R. et al. Predictors of early failure and secondary patency in native arteriovenous fistulas for hemodialysis. Int. Angiol. 37, 310–314. https://doi.org/10.23736/S0392-9590.18.03927-5 (2018).
Moist, L. M. et al. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Semin Dial. 25, 640–648. https://doi.org/10.1111/sdi.12037 (2012).
Trimarchi, H. et al. Clopidogrel diminishes hemodialysis access graft thrombosis. Nephron. Clin. Pract. 102, c128-132. https://doi.org/10.1159/000089671 (2006).
Kaufman, J. S. et al. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J. Am. Soc. Nephrol. 14, 2313–2321. https://doi.org/10.1097/01.asn.0000081661.10246.33 (2003).
Acknowledgements
‘Clinical Trial Center at Soonchunhyang University College of Medicine, Cheonan Hospital’ kindly provided statistical advice for this manuscript.
Funding
This work was supported by the Soonchunhyang University Research Fund. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.L.: data analysis and interpretation, manuscript writing; S.Y.: study design, data analysis and interpretation; W.L.: data analysis and interpretation; Y.C.: study design, data interpretation, proof reading; S.P.: study design, data interpretation, proof reading; S.L.: study design, data interpretation, proof reading.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H.N., Yang, S.B., Lee, W.H. et al. Prevalence and clinical significance of anatomic variant in cephalic arch on preoperative mapping venography. Sci Rep 12, 17398 (2022). https://doi.org/10.1038/s41598-022-22372-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22372-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.