Abstract
Considering the severe hazards of abnormal concentration level of H2S as an extremely toxic gas to the human body and due to the disability of olfactory system in sensing toxic level of H2S concentration, a reliable, sensitive, selective and rapid method for the detection of H2S is proposed and its efficacy is analyzed through simulation. The proposed system is based on the deflection of a laser beam in response to the temperature variations in its path. In order to provide selectivity and improve sensitivity, gold nanostructures were employed in the system. The selectivity was introduced based on the thiol–gold interactions and the sensitivity of the system was enhanced due to the modification of plasmon resonance behavior of gold nanostructures in response to gas adsorption. Results from our analysis demonstrate that compared with Au and SiO2–Au, the Au nanomatryoshka structures (Au–SiO2–Au) showed the highest sensitivity due to promoting higher deflections of the laser beam.
Similar content being viewed by others
Introduction
Hydrogen sulfide (H2S), is a colorless water-soluble, corrosive, flammable and extremely toxic gas and is identified with a “rotten egg” odor. H2S is widely produced in nature or industry, such as in hot springs, volcanic gases, crude petroleum, petrochemical industry, paper manufacturing, and waste disposal1,2,3,4,5. Many investigations have demonstrated that H2S in abnormal concentration levels, has serious adverse effects on human health. Numerous neural disorders, such as ischemic stroke, Alzheimer’s disease, Parkinson’s disease, Down’s syndrome, could occur because of abnormal levels of H2S2,3,6. Also, H2S could affect the cardiovascular system due to the opening of the ATP-sensitive potassium channel, leading to vascular smooth muscle relaxation and a decrease in blood pressure3. Furthermore, H2S can highly affect the eyes, skin, the respiratory system, and mucous membranes could be destroyed or inflamed7,8. H2S with concentrations higher than 250 ppm could lead to blood poisoning and even death1. In this regard, considering the human and environmental safety, the safe exposure threshold of H2S announced by the American National Institute for Occupational Safety and Health (NIOSH) is 10 ppm for 8 h9.
The olfactory organs of humans can feel H2S in concentration of 130 ppb with a characteristic similar to a rotten egg smell, while at the concentration of 83 ppb it interacts with blood hemoglobin with destructive effects on human health5. In addition, small increase in H2S levels, or prolonged exposure to low concentrations, can cause anosmia3. Therefore, the design and fabrication of a rapid and reliable sensing platform for in-situ real-time detection of H2S in ppm concentration with high selectivity and sensitivity is a major challenge1,3,8.
So far, many strategies have been developed for detection of H2S which could be classified under three main categories; semiconductor metal oxide (SMO) (such as ZnO, SnO2, In2O3)10, electrochemical11 and optical based sensors3,12. Among various types of optical-based sensors, fluorescence-based detection13, colorimetry14, surface enhanced Raman spectroscopy (SRES)15, and UV–visible absorption spectrometry16 are well known. Despite advances in H2S detection in the past few years, these techniques have suffered from certain limitations. For instance, in mobile monitoring of H2S by SMO-based sensors, the main limitation is the power consumption17. In case of the electrochemical sensors, the impact of ambient humidity and temperature is a potential limitation18. Although electrochemical-based sensors are able to overcome the limitation of temperature and humidity dependence to some extent, but high temperatures interfere with the performance of these sensors19. Despite the high sensitivity and selectivity of fluorescence-based sensor, difficulty in the synthesis of tags and their durability, restricts its application6. Moreover, colorimetry-based detection techniques lack sufficient sensitivity for H2S gas2.
Considering these limitations of the current methods, design of novel strategies for detection of H2S with the capability of overcoming the above restrictions seems necessary. Non-contact optical-based sensors are not affected by temperature or humidity and maintain their functionality even in high temperature and ambient humidity1. As one of the most sensitive members of molecular absorption spectroscopy family, beam deflection spectroscopy (BDS) can be utilized for detecting agents as well as the measurement of special sample properties such as porosity and thermal properties20,21,22,23. It is also known as “photothermal deflection spectroscopy (PDS)” or “mirage-effect technique”20,24,25. Briefly, in the BDS, the sample is locally irradiated using a modulated laser beam (pump beam), and the absorbed electromagnetic radiation heats the sample locally through non-radiative processes. The refractive index of medium would change as a result of variations in the density and consequently, the path of another laser beam (probe beam) which passes along the sample surface, would be spatially deflected24,26. By measuring the deflection of the probe beam, using a position-sensitive detector (PSD) or a charge-coupled device (CCD) camera, the PDS signal is acquired which is proportional to the electromagnetic radiation absorption of the sample27,28.
Three configurations for experimental setup of the BDS system have been used: (a) transverse configuration where the probe beam is parallel to the sample surface and is utilized for opaque solid samples, (b) collinear configuration (or transmission configuration) where the probe beam transmits through the sample while the pump and probe beams are parallel and (c) reflection configuration in which the deflection of the reflected probe beam is measured29,30.
The BDS system as a sensor, introduces several advantages. This system does not require any complicated equipment. The low cost diode lasers could be exploited for pump and probe beams. Also simple equipment could be utilized as detector (e.g. quadrant photo detectors (QPD) which are usually operated as position sensitive detectors). Simple sample preparation, low required amount of samples along with high sensitivity as well as comparable spectral, spatial, and temporal resolution are advantages of the BDS as a detecting system20,23,31,32. Moreover, being contactless and non-destructive, make this system into a potential candidate for toxic and critical detections. In addition, the background measurement is zero which results in minimal calibration requirements26. The BDS system is humidity and temperature independent and could operate at high temperatures as well.
Various nanostructures have been extensively used to improve the selectivity and sensitivity of the H2S sensors33,34,35. For instance, detection of low concentrations of H2S has been performed using BaTiO2 nanoparticles36. Electroactive nanoparticles (NPs), such as metal NPs, have been utilized as electrode modifiers in electrochemical sensors in order to improve the generation of stable and strong electrochemical signals2. Among the various types of NPs which can be used in H2S detection, gold NPs have attracted considerable attention due to their favorable properties37,38. Biocompatible Au NPs have excellent conductivity, convenient functionalization properties and large specific surface area39, along with unique surface plasmon resonance (SPR) absorption peak at 524 nm40.
In comparison with other reducing (NH3) and oxidizing gases (Cl2 and NO2), the studies have demonstrated that Au NPs exhibited great selectivity for H2S molecules which could be attributed to the strong gold–thiol interaction3,6,41,42,43,44. Results of previous study showed that gold thin film reveal more selectivity toward H2S than NH3 and demonstrated a stronger response in similar condition. This phenomenon is due to the fact that H2S is more reducing in nature comparing with NH342. On the other hand, due to the application of citrate in the process of synthesizing and stabilizing of gold nanoparticles, sulfide ions have high tendency to bind to gold nanoparticles and replace the carboxyl groups45. In this regard, using gold nanoparticles in the proposed sensing platform provides high selectivity for H2S detection. Furthermore, another study40, confirmed that the adsorption and desorption of H2S molecules led to significant changes in electron hopping of Au NPs which could be used in H2S detection. The morphology of gold nanostructures plays a critical role in their SPR absorption peaks. Spherical Au NPs show a single absorption peak while Au nano rods exhibit two peaks which are related to their longitudinal and transverse modes46,47. In SiO2–Au core–shell and Au–SiO2–Au nanomatryoshka structures, the peaks could be tuned with size of the core and shell structures accordingly48,49.
In this paper, we propose a novel H2S sensor structure based on the beam deflection technique and using Au nanostructures. The absorption spectrum of the Au nanostructures is dependent on the presence of gas in the medium. By irradiating the Au nanostructures-modified substrate, the generated heat would result in modifying the refractive index of the surrounding medium which could be detected by the deflection of the beam. Proposing the beam deflection approach makes the measurements easy (compared with other spectroscopic techniques such as SERS) while exploiting the Au NPs makes the sensing method selective and sensitive. Based on all optical methods exploited for this system, the functionality of sensing platform is independent of the humidity and temperature and unlike the electrochemical sensors, the operation of sensor would be possible in high temperatures as well. The system is easily operated and does not require complicated equipment. The operation of the system is fundamentally based on the variation of electron density and the refractive index of Au nanostructures due to the adsorption of H2S molecules. Furthermore, exploiting the nanostructures provides a stronger response due to their high surface to volume ratio which provides higher surface for adsorption of H2S gas molecules. This improves the sensitivity of the proposed system. While the adsorption of H2S molecules on the surface of Au nanostructures is physical and no chemical reaction takes place, the recovery time of sensor is short. The performance of the proposed technique was computationally evaluated.
Results
Absorption coefficient of gold nanostructures
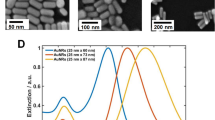
The absorption cross section of three Au nanostructures calculated by solving Maxwell’s equations using the Mie method are illustrated in Fig. 1.
Optimization of substrate
In order to find the appropriate substrate for the system, we compared the temperature variation and deflection angle for the modified-glass and modified-Au substrates in the vicinity of H2S. In this regard, Fig. 2 depicts the results of this comparison.
Temperature variation profile of geometry
Figure 3 demonstrates the temperature variation profile of geometry in the time domain at time intervals of 0.6 and 4.99 s, respectively.
Temperature profile and deflection angle for Au nanostructures
The BDS system with a modified substrate was used to detect air and H2S and the results were compared for three different Au nanostructures. Figure 4 shows the temperature variation and the deflection angle for these different nanostructures in the vicinity of air and H2S on the modified substrate.
Discussion
We have proposed a BDS-based system for detection of H2S and its operational performance is simulated. Considering the application of Au nanostructures for improving the selectivity and sensitivity of detection, the absorption coefficient of three types of Au nanostructure (including Au nanosphere, SiO2–Au core–shell structure and Au–SiO2–Au nanomatryoshka structures) have been calculated. As shown in Fig. 1, the Au–SiO2–Au nanomatryoshka displays two absorption peaks compared with the two other types of Au nanostructures having one absorption peak which is based on the presence of Au in two separate regions of the nanostructure50. Moreover, a blue shift of the absorption peak for all three nanostructures in the vicinity of H2S is associated with an increase of electron density of Au nanostructure due to the adsorption of H2S molecules on the nanostructures51. As can be seen in Fig. 1, the blue shift for the Au–SiO2–Au nanomatryoshka is more distinctive than in the other two nanostructures.
Based on the important role of the substrate in transferring heat, we have compared the efficiency of the BDS for two different substrates. Figure 2 demonstrates the comparison of temperature variation and deflection angle for glass and Au substrates in the presence of H2S. As can be seen in Fig. 2, temperature variations and angle deflections for the glass substrate are more than those for the Au substrate. This difference is due to the fact that Au exhibits a higher heat conductance compared with the glass52. The smaller conductivity of the glass substrate would result in confining the heat near the surface of the substrate, while the Au substrate conducts the heat more easily, and the region between the Au and the gas achieves a lower heat. Considering the above reasons, the glass is a more appropriate substrate for the proposed BDS system.
Figure 3 demonstrates the temperature profile of the modelled geometry, showing the temperature raise in the structure in response to the absorbed heat from the pump-laser beam over time.
Finally, the efficiency of three different types of Au nanostructures on H2S detection by the BDS system was evaluated. Figure 4 shows the temperature variation and deflection angle of the laser beam in the vicinity of air and H2S for three different Au nanostructures. The results demonstrate that there are obvious differences in both the temperature variation and deflection angle between air and H2S for three types of Au nanostructures indicating the sensitivity of the proposed BDS system for detection of H2S. In addition, the difference is more pronounced for the Au–SiO2–Au nanomatryoshka structure, compared with the other two nanostructures. Thus, the maximum sensitivity of the BDS system for detection of H2S is through exploiting the Au–SiO2–Au nanomatryoshka. Using an Au nanostructure not only improves the selectivity of the system, but also introduces sensitivity through variation of electron density upon adsorption of the gas on its surface, which modifies its plasmon resonance behavior.
In our proposed modelling approach, the temperature dependency of thermal properties is negligible. For the high temperatures, this dependency should be considered and introduced in the heat transfer equation. In order to implement the system, for measurement and detection of the small variations of deflected beam, based on the periodical excitation of the sensitive layer by probe beam, the output signal could be detected with a lock-in approach. The lock-in detection could be performed either with an analog lock-in amplifier or digitally inside the software accordingly.
Materials and methods
As illustrated in Fig. 5, the sensing element of the proposed system is a substrate covered with Au nanostructures. The substrate is periodically heated with a laser beam (pump) passing through a chopper. The absorbed energy from the pump laser changes the refractive index of the adjacent medium. The modulation of the refractive index of the medium is detected through the deflection of a second laser beam (probe) which passes through the medium. The deflection could be detected by either the PSD or the CCD arrays. Based on the modulation of heat with the chopper, the deflection is modulated as well, which makes the detection easier using a lock-in amplifier.
Adsorption of H2S on the surface of Au nanostructures would result in the change in the electron concentration of the nanoparticles, which in turn leads to a change in the location of SPR peak and culminates in different values of the absorbed heat and deflection angle in the same manner. In order to model the functionality of the system, we have used computational modelling approach. First, the effect of gas adsorption on the absorption spectrum of three different Au nanostructures is modeled through solving Maxwell’s equations. The variation of the temperature in the surrounding medium of the sensing element (substrate + Au nanostructures) is calculated by solving the heat transfer equation via the finite element method (FEM) in the COMSOL Multiphysics version 5.3 environment53. Deflection of the laser beam resulting from the temperature gradient was calculated according to the governing equation for the propagation of laser beam.
Optical properties of Au nanostructures in response to adsorbed gas
Among various types of Au nanostructures with different geometries, as depicted in Fig. 6, we have selected three distinct structures including Au nanosphere, SiO2–Au core–shell and Au–SiO2–Au nanomatryoshka structures as the essential component of the sensing element. In order to find the optical properties of the above-mentioned nanostructures, Maxwell’s equations should be solved. Considering the spherical symmetry of all the three mentioned structures, Mie’s theory has been selected for solving Maxwell’s equations54,55. In this regard, the illuminated, scattered and absorbed waves are all expanded using spherical Bessel’s functions and imposing the boundary conditions for electric and magnetic fields on each boundary. The system of equations are solved to find the coefficients for each wave. Each structure and material is introduced in the equations by its permittivity and permeability. In order to introduce the dispersion behavior of the Au permittivity, various models have been introduced, among which we have used the Drude–Lorentz model
to consider all the interband and intraband transitions56, where ɛ∞ is the dielectric constant far above the plasma frequency, λp denotes the plasma wavelength, ɣp is the damping factor expressed in wavelength. λi denotes the interband transition wavelength, ɣi is the transition broadenings (expressed as wavelength), Ai is the dimensionless critical point amplitude, and ɸi represents the phase.
Effect of gas adsorption
The effect of gas adsorption on the permittivity and the variation of optical properties of Au nanostructures, in response to H2S adsorption, was taken into account to find the sensitivity of the proposed method. The plasma wavelength in Eq. (1) was calculated via
where c is the velocity of light in vacuum, m represents the effective mass of the conduction electrons, ɛ0 is the vacuum permittivity, e is the electron charge, and N is the electron concentration. Adsorption of H2S on the surface of the Au nanoparticles, would locally increase the electron density which in turn reduces the plasma wavelength51.
Temperature variation profile
The main approach to calculate the beam deflection is to obtain the temperature variation profile during the heating of the sample. The temperature variations depend on the thermo-optical and structural features of the sample. In order to find the temperature variation profile, the heat transfer equation
should be solved, where ρ is the density, Cp is the heat capacity, k is the thermal conductivity, u is the flow velocity, and Q represents the heat source57,58. In our model, the source of heat is the laser energy absorbed by the Au NPs. The wavelength-dependence absorption of various types of nanoparticles results in different values for the Q accordingly. The structure is heated with five consecutive Gaussian pulses with 1 second width. The geometrical structure of the model is depicted in Fig. 7. In order to solve Eq. (3) for the geometrical structure of Fig. 7, we used the FEM and the COMSOL Multiphysics environment. Equation (3) is solved in time domain and Table 1 lists the material parameters used in the model based on Eq. (3).
Beam deflection calculation
After obtaining the temperature variation profile from the heat equation, the deflection of the probe-laser beam could be calculated. Generally, the time-dependent angle of deflection is calculated via
where s denotes the path along which the probe-beam propagates in the x direction above the sample. Considering the deflection of the beam in the z direction, the angle of deflection can be calculated via
The value of dn/dT provides the variation of the refractive index with the temperature, and in this study, we have considered a fixed value of − 0.88 × 10–6 for this parameter59.
Conclusion
A BDS-based system has been proposed for detection of H2S and its performance has been analyzed using computational modelling. The simulation results indicate that comparing Au and glass as a substrate, the glass is more appropriate due to its lower heat conductivity. Using Au NPs for modifying the substrate results in the selectivity of the system for H2S molecules due to the strong gold–thiol interaction. In addition, the plasmon resonance behavior of Au nanostructures would change due to the adsorption of gas on the surface of nanoparticles that alters the electron concentration locally. Among three proposed Au nanostructures, the Au–SiO2–Au nanomatryoshka exhibits a higher sensitivity for the detection of H2S. The proposed system has various advantages of rapid, reliable, sensitive and selective detection of the gas samples and could be employed in real-time applications.
Data availability
Derived data supporting the findings of this study are available from the corresponding author on request.
References
Zhang, Z., Chen, Z., Wang, S., Qu, C. & Chen, L. On-site visual detection of hydrogen sulfide in air based on enhancing the stability of gold nanoparticles. ACS Appl. Mater. Interfaces 6(9), 6300–6307 (2014).
Zhao, Y., Yang, Y., Cui, L., Zheng, F. & Song, Q. Electroactive Au@ Ag nanoparticles driven electrochemical sensor for endogenous H2S detection. Biosens. Bioelectron. 117, 53–59 (2018).
Yuan, Z. et al. Selective colorimetric detection of hydrogen sulfide based on primary amine-active ester cross-linking of gold nanoparticles. Anal. Chem. 87(14), 7267–7273 (2015).
Han, C. et al. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B Chem. 285, 495–503 (2019).
Zhang, Y. et al. Novel two-dimensional WO3/Bi2W2O9 nanocomposites for rapid H2S detection at low temperatures. ACS Appl. Mater. Interfaces 12(49), 54946–54954 (2020).
Zhang, X., Zhou, W., Yuan, Z. & Lu, C. Colorimetric detection of biological hydrogen sulfide using fluorosurfactant functionalized gold nanorods. Analyst 140(21), 7443–7450 (2015).
Li, D. et al. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrogen Energy 44(7), 3985–3992 (2019).
Phuoc, P. H. et al. One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sens. Actuators A 303, 111722 (2020).
Li, Z. et al. Significantly enhanced temperature-dependent selectivity for NO2 and H2S detection based on In2O3 nano-cubes prepared by CTAB assisted solvothermal process. J. Alloy. Compd. 816, 152518 (2020).
Park, K.-R. et al. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B Chem. 302, 127179 (2020).
Gu, W., Zheng, W., Liu, H. & Zhao, Y. Electroactive Cu2O nanocubes engineered electrochemical sensor for H2S detection. Anal. Chim. Acta 1150, 338216 (2021).
Maimaiti, A. et al. Highly sensitive optical waveguide sensor for SO2 and H2S detection in the parts-per-trillion regime using tetraaminophenyl porphyrin. J. Mod. Opt. 67(6), 507–414 (2020).
Zhao, X.-J. et al. A novel “turn-on” mitochondria-targeting near-infrared fluorescent probe for H2S detection and in living cells imaging. Talanta 197, 326–333 (2019).
Zhang, Y. et al. A new strategy for the fluorescence discrimination of Cys/Hcy and GSH/H2S simultaneously colorimetric detection for H2S. Spectrochim. Acta A Mol. Biomol. Spectrosc. 227, 117537 (2020).
Fu, H. et al. Ultrathin hexagonal PbO nanosheets induced by laser ablation in water for chemically trapping surface-enhanced Raman spectroscopy chips and detection of trace gaseous H2S. ACS Appl. Mater. Interfaces 12(20), 23330–23339 (2020).
Zhang, X., Cui, Z., Cheng, Z., Li, Y. & Xiao, H. Quantitative detection of H2S and CS2 mixed gases based on UV absorption spectrometry. RSC Adv. 7(80), 50889–50898 (2017).
Mickelson, W., Sussman, A. & Zettl, A. Low-power, fast, selective nanoparticle-based hydrogen sulfide gas sensor. Appl. Phys. Lett. 100(17), 173110 (2012).
Bakker, E. & Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 74(12), 2781–2800 (2002).
Ali, F. I., Awwad, F., Greish, Y. E. & Mahmoud, S. T. Hydrogen sulfide (H2S) gas sensor: A review. IEEE Sens. J. 19(7), 2394–2407 (2018).
Proskurnin, M., Korte, D., Rogova, O., Volkov, D. & Franko, M. Photothermal beam deflection spectroscopy for the determination of thermal diffusivity of soils and soil aggregates. Int. J. Thermophys. 39(7), 1–13 (2018).
Fournier, D., Boccara, A., Amer, N. M. & Gerlach, R. Sensitive insitu trace-gas detection by photothermal deflection spectroscopy. Appl. Phys. Lett. 37(6), 519–521 (1980).
Gutierrez-Arroyo, A., Pérez, C. S., Alemán-García, N. & Piña-Barba, C. (eds) Optical Characterization of Thermal Properties of Biological Tissue. 8th Iberoamerican Optics Meeting and 11th Latin American Meeting on Optics, Lasers, and Applications (International Society for Optics and Photonics, 2013).
Fontenot, R. S., Mathur, V. K. & Barkyoumb, J. H. New photothermal deflection technique to discriminate between heating and cooling. J. Quant. Spectrosc. Radiat. Transfer 204, 1–6 (2018).
Jackson, W. B., Amer, N. M., Boccara, A. & Fournier, D. Photothermal deflection spectroscopy and detection. Appl. Opt. 20(8), 1333–1344 (1981).
Salazar, A., Sánchez-Lavega, A. & Fernandez, J. Theory of thermal diffusivity determination by the ‘“mirage”’technique in solids. J. Appl. Phys. 65(11), 4150–4156 (1989).
Krzempek, K. A review of photothermal detection techniques for gas sensing applications. Appl. Sci. 9(14), 2826 (2019).
Hanh, B., Faubel, W., Heissler, S., Wartewig, S. & Neubert, R. Pharmaceutical applications of photothermal beam deflection. Laser Phys. 16(5), 794–798 (2006).
Kienle, A. et al. Spatially resolved absolute diffuse reflectance measurements for noninvasive determination of the optical scattering and absorption coefficients of biological tissue. Appl. Opt. 35(13), 2304–2314 (1996).
Commandre, M. & Roche, P. Characterization of optical coatings by photothermal deflection. Appl. Opt. 35(25), 5021–5034 (1996).
Spear, J. D., Russo, R. E. & Silva, R. J. Collinear photothermal deflection spectroscopy with light-scattering samples. Appl. Opt. 29(28), 4225–4234 (1990).
Korte, D., Carraro, G., Fresno, F. & Franko, M. Thermal properties of surface-modified-and-fe photocatalysts determined by beam deflection spectroscopy. Int. J. Thermophys. 35(11), 2107–2114 (2014).
Korte, D. & Franko, M. Photothermal deflection experiments: Comparison of existing theoretical models and their applications to characterization of TiO2-based thin films. Int. J. Thermophys. 35(12), 2352–2362 (2014).
Zhou, Y., Wang, Y. & Guo, Y. Cuprous oxide nanowires/nanoparticles decorated on reduced graphene oxide nanosheets: Sensitive and selective H2S detection at low temperature. Mater. Lett. 254, 336–339 (2019).
Eom, N. S. A., Cho, H.-B., Lim, H.-R., Kim, B. S. & Choa, Y.-H. Facile tilted sputtering process (TSP) for enhanced H2S gas response over selectively loading Pt nanoparticles on SnO2 thin films. Sens. Actuators B Chem. 300, 127009 (2019).
Li, X. et al. Highly selective and sensitive detection of hydrogen sulfide by the diffraction peak of periodic Au nanoparticle array with silver coating. ACS Appl. Mater. Interfaces 12(36), 40702–40710 (2020).
Huang, H.-M., Li, H.-Y., Wang, X.-X. & Guo, X. Detecting low concentration of H2S gas by BaTiO3 nanoparticle-based sensors. Sens. Actuators B Chem. 238, 16–23 (2017).
Della Gaspera, E. et al. Au nanoparticles in nanocrystalline TiO2−NiO films for SPR-based, selective H2S gas sensing. Chem. Mater. 22(11), 3407–3417 (2010).
Mubeen, S. et al. Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal. Chem. 82(1), 250–257 (2010).
Zhao, Y. et al. Dual amplified electrochemical immunosensor for highly sensitive detection of Pantoea stewartii sbusp. stewartii. ACS Appl. Mater. Interfaces 6(23), 21178–83 (2014).
Zhao, Y. et al. Double detection of mycotoxins based on SERS labels embedded Ag@ Au core–shell nanoparticles. ACS Appl. Mater. Interfaces 7(39), 21780–21786 (2015).
Kumar, A. et al. Room temperature detection of H2S by flexible gold–cobalt phthalocyanine heterojunction thin films. Sens. Actuators B Chem. 206, 653–662 (2015).
Joshi, N. et al. Flexible H2S sensor based on gold modified polycarbazole films. Sens. Actuators B Chem. 200, 227–234 (2014).
Geng, J., Thomas, M. D., Shephard, D. S. & Johnson, B. F. Suppressed electron hopping in a Au nanoparticle/H2S system: Development towards a H2S nanosensor. Chem. Commun. 14, 1895–1897 (2005).
Zhang, Y. et al. Gold nanoclusters as fluorescent sensors for selective and sensitive hydrogen sulfide detection. Talanta 171, 143–151 (2017).
Prado, A. R. et al. Surface plasmon resonance-based optical fiber sensors for H2S in situ detection. Plasmonics 16(3), 787–797 (2021).
Wiley, B. et al. Shape-controlled synthesis of silver and gold nanostructures. MRS Bull. 30(5), 356–361 (2005).
Liu, A., Wang, G., Wang, F. & Zhang, Y. Gold nanostructures with near-infrared plasmonic resonance: Synthesis and surface functionalization. Coord. Chem. Rev. 336, 28–42 (2017).
Mohapatra, S. & Moirangthem, R. S. Theoretical study of modulated multi-layer SPR device for improved refractive index sensing. IOP Conf. Ser. Mater. Sci. Eng. 310, 012017 (2018).
Ismail, R. K. et al. (eds) Time Effect on the Red Shift of Surface Plasmonic Resonance Core-Shell SiO2: Gold Nanoparticles (AuNPs) (AIP Publishing LLC, 2019).
Bardhan, R. et al. Nanosphere-in-a-nanoshell: A simple nanomatryushka. J. Phys. Chem. C 114(16), 7378–7383 (2010).
Buso, D., Post, M., Cantalini, C., Mulvaney, P. & Martucci, A. Gold nanoparticle-doped TiO2 semiconductor thin films: Gas sensing properties. Adv. Funct. Mater. 18(23), 3843–3849 (2008).
Siegel, J., Lyutakov, O., Rybka, V., Kolská, Z. & Švorčík, V. Properties of gold nanostructures sputtered on glass. Nanoscale Res. Lett. 6(1), 1–9 (2011).
Manuchehrabadi, N. & Zhu, L. Development of a computational simulation tool to design a protocol for treating prostate tumours using transurethral laser photothermal therapy. Int. J. Hyperth. 30(6), 349–361 (2014).
Raad, S. H., Atlasbaf, Z., Rashed-Mohassel, J. & Shahabadi, M. Scattering from graphene-based multilayered spherical structures. IEEE Trans. Nanotechnol. 18, 1129–1136 (2019).
Kahnert, M. Numerical solutions of the macroscopic Maxwell equations for scattering by non-spherical particles: A tutorial review. J. Quant. Spectrosc. Radiat. Transfer 178, 22–37 (2016).
Etchegoin, P. G., Le Ru, E. & Meyer, M. An analytic model for the optical properties of gold. J. Chem. Phys. 125(16), 164705 (2006).
Dada, O. O. & Bialkowski, S. E. Finite element analysis modeling of pulse-laser excited photothermal deflection (mirage effect) from aerosols. Appl. Spectrosc. 62(12), 1326–1335 (2008).
Pryor, R. W. Multiphysics Modeling Using COMSOL®: A First Principles Approach (Jones & Bartlett Publishers, 2009).
Bialkowski, S. E. Photothermal Spectroscopy Methods for Chemical Analysis (Wiley, 1996).
Acknowledgements
This article has been extracted from thesis written by Mrs. Elham Afjeh-Dana in School of Medicine, Shahid Beheshti University of Medical Sciences (Registration No. M471).
Author information
Authors and Affiliations
Contributions
P.S. proposed the original idea and with the contribution of E.A.D. and H.R.T. the computational model was developed. E.A.D. performed the simulations. E.A., H.R.T. and M.R.R. provided advice in analyzing the results and discussions. All authors contributed to writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afjeh-Dana, E., Asadian, E., Razzaghi, M.R. et al. Deflection-based laser sensing platform for selective and sensitive detection of H2S using plasmonic nanostructures. Sci Rep 12, 15789 (2022). https://doi.org/10.1038/s41598-022-19739-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19739-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.