Abstract
Despite substantial efforts to control locusts they remain periodically a major burden in Africa, causing severe yield loss and hence loss of food and income. Distribution maps indicating the value of the basic reproduction number R0 was used to identify areas where an insect pest can be controlled by a natural enemy. A dynamic process-based mathematical model integrating essential features of a natural enemy and its interaction with the pest is used to generate R0 risk maps for insect pest outbreaks, using desert locust and the entomopathogenic fungus Metarhizium acridum (Synn. Metarhizium anisoliae var. acridum) as a case study. This approach provides a tool for evaluating the impact of climatic variables such as temperature and relative humidity and mapping spatial variability on the efficacy of M. acridum as a biocontrol agent against desert locust invasion in Africa. Applications of M. acridum against desert locust in a few selected African countries including Morocco, Kenya, Mali, and Mauritania through monthly spatial projection of R0 maps for the prevailing climatic condition are illustrated. By combining mathematical modeling with a geographic information system in a spatiotemporal projection as we do in this study, the field implementation of microbial control against locust in an integrated pest management system may be improved. Finally, the practical utility of this model provides insights that may improve the timing of pesticide application in a selected area where efficacy is highly expected.
Similar content being viewed by others
Introduction
For centuries, humans have attempted to control pest insect populations that cause agricultural productions losses, and often, chemical insecticides are used to control these insect pests1. Unfortunately, extensive use of chemical pesticides contributes to a plethora of issues such as farmers’ health risks, food safety issues, reduced biodiversity, reduction or loss of natural enemies, pollinators, and other non-target organisms, and emergence of pesticide resistance2. Historically, the use of chemical pesticides to control pests made it possible to increase yields3 and chemical pesticides will probably continue to be a vital tool that can maintain and improve yields in future sustainable plant production systems4 but only in combination with new technologies and non-chemical alternatives2. Driven by the desire to develop alternative methods, a number of studies have explored the use of a natural enemies to control insect pests5,6. Different biological control methods, as defined by7, is considered as an important component of integrated pest management (IPM) in many countries and is mentioned as principle four (non-chemical methods to be preferred) out of the eight principles of IPM in the European Union’s Directive on Sustainable Use of Pesticides (2009/128/EC SUD). Biological control can be defined as the usage of living organisms (predators, parasitoids and entomopathogens) to reduce the density of pest insects7. Insect pathogenic fungi are now widely used as biocontrol agents of pests insect in many countries8,9,10,11,12.
Microbial control can be viewed as applied epizootiology with the goal of inducing an epizootic incidence, i.e. an outbreak of a disease in which there is an unusually large number of cases in the targeted insect population, through manipulation13. Processed-based models development is an ideal tool toward mimicking and predicting the success of a biological process and have been developed to answer biological questions about: (i) predicting the outbreak and success of a specific introduction of a pathogen, (ii) predicting the impact of an introduced pathogen on ecosystems and non-target species, (iii) predicting optimum release and management strategies, (iv) assistance in the selection of the most appropriate agent(s) and, (v) model development that allows a good understanding of the processes involved and the reasons for success or failure14. Biocontrol success or failure can be modeled by a set of differential equations with the aim to understand or predict the underlying mechanisms of interaction14,15. Challenges of modeling the epizootiological development of an entomopathogenic fungus in an insect population resides on all four primary areas that are known to influence epizootiology: (i) the pathogen population, (ii) the host population, (iii) transmission and, (iv) the environment (biotic and abiotic)16,17. Both virulence and pathogenicity are important expressions when modelling and discussing the epizootiological development of an entomopathogenic fungus. We will therfore use the definitions by Lacey18 when using these expressions. They go as follows: “Virulence is the speed by which a microorganism penetrates hosts defenses” and “Pathogenicity is the intrinsic capability of a microorganism to penetrate the host defenses”.
M. anisopliae var. acridum have shown considerable potential when applied as a biocontrol agent of locusts20,21,21 and is also effective against locusts that have developed resistance to chemical insecticides22. M. anisopliae biopesticide is reported to kill 70%–90% of treated locusts within 14–20 days in field condition19. Green Muscle is a biopesticide developed and commercialized since 2001 by LUBILOSA (Lutte Biologique ,contre les Locustes et Sauteriaux) that kill locust and grasshoppers and pose a low risk to the environment23 It was successfully used to contain the locust infestation in the Iku-Katavi National Park in Tanzania in 2009; where around 10 000 hectares were treated to protect and ensure the safety of animals (elephants, giraffes and hippopotamuses) within the area24. The product was also used during the recent locust outbreak in 2019/2020 to treat more than 230 000 thousand of hectares in the horn of Africa, therefore helping about 20 million people in Kenya, Uganda, South Sudan, Somalia, Ethiopia and Tanzania to fight food insecurity25. Although M. anisopliae may infect insects living in a wide array of habitats, its performance as a biocontrol agent is highly variable depending on the environmental conditions to which it is exposed22,26. Temperature and relative humidity is known to affect the performance of M anisopliae significantly28,29,30,30 and can substantially impact the success of biological control31. For instance, it has been demonstrated that temperature significantly affects the fungal germination rate33,34,34, the fungal growth rate within the host32,33,34,34, sporulation and virulence of this entomopathogenic fungus35. The optimal temperature for M. anisopliae is concidered to range from 25 to 35 °C36.
However, the optimal temperature may vary depending on the geographic, and hence, climatic origin of the isolate and to be able to build good descriptive models for a pest natural enemy system, it is imperative to include information about the temperature in the region where the isolate comes from37. Non-optimal temperatures may affect the rate of locust pest mortality by inhibiting spore germination if the temperature is higher than 35 °C or lower than 20 °C, which in turn affects penetration through the insect’s cuticle34. Relative humidity also affects fungal control agents27,38 since it is essential for fungal germination and sporulation on insect cadavers19,39,40. The optimal relative humidity levels are suggested to be more similar for isolates of all geographical origins37, and most entomopathogenic fungi require about 95% relative humidity at the host surface (microclimate) to germinate41. The relative humidity at the host surface where the entomopathogenic fungal conidia germinate may be higher than the ambient relative humidity37. Further, tomato greenhouse studies with fungi in the Hypocreales support that a higher humidity is found in the leaf boundary layer (microclimate) and that this benefit microbial control of small arthropod pests living in that stratum42,43. Therefore, the effect of microclimatic relative humidity on host surface or in leaf boundary layer should be taken into consideration when using ambient relative humidity from weather stations in modelling, forecasting and decision support systems for when to successfully apply a fungal based biocontrol agent44,45. Despite advancing new insights into the importance of climatic variables on the efficiency of entomopathogenic fungi as biocontrol agents44,45, few studies have used both temperature and relative humidity as paramount climatic factors to model and predict the ideal conditions for successful use and application of entomopathogenic fungi in the Hypocreales47,48,48.
A key to use entomopathogenic fungi efficiently as a biocontrol agent is to predict how it will perform across space and time. A model used to study the density dependence and spatial structure in the dynamics of an entomopathogenic fungus is presented in49 and a description of the behavior of infected and non-infected hosts and the prediction of the relevant spatial scale during the spread of entomopathogenic fungus is presented in50. The analysis of the spread of a contagious disease caused by an entomopathogenic fungus in an insect pest population at different host densities is reported in51. The understanding of the effect of conidial dispersal of an entomopathogenic fungus on survival of its host is clarified in52. The outcomes of these models often give potential geographical areas where an entomopathogenic fungus may perform well but without integrating climatic factors such as temperature and relative humidity. In previous studies, meteorological station data in a Geographical Information System (GIS) were used to investigate the most environmentally suitable condition for a good performance of entomopathogenic fungi over a wide spatial and temporal scale31. In53 the authors explored climate-driven (moisture, radiation, precipitation and temperature) geographic distribution of the desert locusts during the locust recession period in Africa.
The basic reproduction number, R0, is a key epidemiological metric for understanding pest risks54 by determining the threshold values at which, the model exhibit changes in its stability. For our entomopathogenic fungus system, R0 is defined as the expected number of new infected individuals (locusts) that a single fungal infected locust may generate in a population of entirely susceptible locusts. Typically, when R0 is greater than one, the introduced fungus can proliferate among the locust pest population and presumably infect and kill more hosts with time, whereas when R0 is smaller than one, the locust outbreak effects cannot be attenuated if the fungus is introduced, and the fungus will certainly die out with time. Therefore, the basic reproduction number also indicates the amount of control needed to eradicate locusts during an outbreak. In previous studies, R0 was used to investigate how the timing and intensity of a virulent entomopathogenic fungus affect insect reproduction and mortality56,57,57. It can provide an approximation of the level of the efficacy of the entomopathogenic fungus towards the pest insect and therefore it offers a good measure for the biocontrol potential of the entomopathogenic fungus towards locusts58.
Our research hypothesizes that combining mechanistic model development and experimental biocontrol results using entomopathogenic fungus may improve the strategy and guidelines for effective insect pest control. As a complement to experimental studies which focused on the growth and development of entomopathogenic fungi20,22,29,31,59,60, the current study proposes a generic modelling approach to map the dynamics of interactions of the entomopathogenic fungus Metarhizium acridium (Synn. Metarhizium ansiopliae var. acridum) (Hypocreales) against the desert locusts Schistocerca gregaria; considering the effects of climatic factors (temperature and relative humidity) and spatial variability (land cover) when estimating R0. The spatial variation of the pathogenicity is herein explored.
Results
Sensitivity analysis
Table 1 displays the Partial Rank Correlation Coefficient (PRCC) for each parameter included in the sensitivity analysis. Four parameters (e, ω, α, γ) will be key contributors to the uncertainty of the efficacy and successful application of the entomopathogenic fungus Metarhizium acridium against the desert locust Schistocerca gregaria. R0 was highly sensitive to the fungal growth rate and to the proportion of resources allocated (host insect body) towards the colony-forming unit (CFU) production (absolute PRCC > 0.7). This means the efficacy of the biocontrol strategy would be highly influenced by the CFU (mycelia or conidia) concentration in the host insect population. Furthermore, R0 was moderately sensitive to the conversion rate of resources into CFU and to the mycelium death rate (absolute PRCC > 0.5). Finally, R0 was less sensitive to the insect carrying capacity (maximum density of pest insect the habitat can support) and the Half-saturation constant (density host insect body at which half of the maximum intake is reached) (absolute PRCC < 0.5).
Behavior of the basic reproduction number (R 0) with changes on climate variables
Figure 1a displays the results of the numerical simulation of the dependence of R0 on temperature with relative humidity fixed at 85, 90 and 95%. The dependence of R0 on both temperature and relative humidity is plotted in Fig. 1b. Figure 1 suggests that relative humidity only affects the magnitude of R0 and has no effect on the optimal temperature for efficacy of the entomopathogenic fungus (M. acridum). Using the parameters estimated for desert locust (Tables 2, 3), the simulations indicate that the maximum value of R0 is 1.2 and it is obtained for temperature within the range 24–33 °C at relative humidity above 95%.
The areas where R0 was close to 1.2 correspond to the area where the M. acridum can provide optimal control. In areas where R0 < 1, desert locust outbreak cannot be attenuated using the entomopathogenic fungus only, whereas the areas were R0 > 1 M. acridum can spread widely and hence be used against an outbreak. Temperatures higher than 35 °C reduce the efficacy of M. acridum, the entomopathogenic fungal species in question. Temperature within the range 15–33 °C, R0 > 1 (Fig. 1a), is suitable for M. acridum but is optimal within the range 24–33. The maximum R0 value of is reached at 28 °C and 95% or higher relative humidity (Fig. 1a,b), which also corresponds to the value in which the fungal development rate and the proportion of resource allocated for spore production are optimal.
Spatiotemporal projection in distribution the basic reproduction number (R 0)
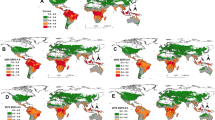
The spatial projection and the mapping of the variation in R0 reflecting the efficiency of the entomopathogenic fungus M. acridum as a biocontrol agent across selected African countries are shown in the maps representing distribution inferred by the degree of magnitude of R0 that indicates higher values in most areas where locusts outbreak can be attenuated after introduction of M. acridum (R0 > 1).
Figures 2, 3, 4 and 5 display the monthly variation of R0 across, Morocco, Kenya, Mali and Mauritania respectively. The locations in red colors (values of R0 > 1) represent the areas that may be suggested for application of M. acridum against desert locust outbreaks that month. Suggested locations vary from one month to another following climate variability within the respective countries and correspond to areas where M. acridum spore production would multiply exponentially and be transmitted for the period the environmental conditions are favorable.
Morocco: Monthly spatial projection of the variation in R0 as a function of temperature and relative humidity reflecting the potential efficiency of the entomopathogenic fungus Metarhizium acridum as a biocontrol agent against desert locust. The area in red in each month are areas where R0 > 1 correspond to zones with potential higher efficiency and hence successful fungal biocontrol agent application against locusts while the area in orange (R0 ≈ 1) can also be targeted but with less potential efficacy. Application in areas in yellow and blue (R0 < 1) will probably not be efficient. The dots correspond to the reported point incidence of locusts in Morocco.
Kenya: Monthly spatial projection of the variation in R0 as a function of temperature and relative humidity reflecting the potential efficiency of the entomopathogenic fungus Metarhizium acridum as a biocontrol agent against locusts. The area in red in each month are areas where R0 > 1 correspond to zones with potential higher efficiency and hence fungal biocontrol agent application against desert locusts while area in orange (R0 ≈ 1) can also be targeted but with less potential efficacy. Application in areas in yellow and blue (R0 < 1) will probably not be efficient. The dots correspond to the reported point incidence of locusts in Kenya.
Mali: Monthly spatial projection of the variation in R0 as a function of temperature and relative humidity reflecting the potential efficiency of the entomopathogenic fungus Metarhizium acridum as a biocontrol agent against locusts. The area in red in each month are areas where R0 > 1 correspond to zones with potential higher efficiency and hence fungal biocontrol agent application against locusts while the area in brown (R0 ≈ 1) can also been targeted but with less potential efficacy. Application in areas in yellow and blue (R0 < 1) will probably not be efficient. The dots correspond to the reported point incidence of locusts in Mali.
Mauritania: Monthly spatial projection of the variation in R0 as a function of temperature and relative humidity reflecting the potential efficiency of the entomopathogenic fungus Metarhizium acridum as a biocontrol agent against desert locusts. The area in red in each month are areas where R0 > 1 correspond to zones with potential higher efficiency and hence fungal biocontrol agent application against locusts while area in orange (R0 ≈ 1) can also be targeted but with less potential efficacy. Application in areas in yellow and blue (R0 < 1) will probably not be efficient. The dots correspond to the reported point incidence of locusts in Mauritania.
Discussion
Developing mathematical models for understanding the long term dynamic of climatic variables affecting the efficacy and successful application of an entomopathogenic fungus as a control agent of desert locust is a task that necessitates a robust conceptual framework, capable of exploring population dynamics both temporally and spatially. The present study proposed a modelling framework to help in exploring the spatial variability in performance of M. acridum applied as a biocontrol agent of desert locusts and apply it in selected African countries where locust outbreaks are common. Our model study confirmed that relative humidity and temperature are key factors impacting the biocontrol potential for the entomopathogenic fungus M. acridum to infect and kill the desert locust.
Processed-based modeling has been used to describe the mechanisms responsible for the dynamics of factors affecting the epidemiology of a entomopathogenic fungus14,61. The effect of time on fungal-pest interactions has been modeled by various forms of differential equations considering discrete time maps62, metapopulations63, networks and spatial data14. Developing reliable modeling frameworks which account for the prediction of large-scale climatic variables such as temperature and relative humidity on the performance of entomopathogenic fungi can be useful to schedule guidelines for smallholder farmer in the implementation of suitable pest management strategies.
In previous studies, a simulation model that captures the effects of temperature and host-mediated behavior was presented31, the effect of relative humidity on the performance of fungus for controlling locusts and grasshoppers using linear Eqs. 38 was explored. Through these models, the authors showed that high temperature (above 35 °C) is a limiting factor for the performance of the entomopathogenic fungal isolates they tested; Klass et al.31 on M. anisopliae (Hypocreales) and Hajek et al.38 Entomophaga maimaga (Entomophthoromycota), but that high relative humidity was required for the isolates to perform well. These models are based on the association between environmental factors (temperature) and the spatial arrangement of the fungus, aiming to identify the conditions most suitable for the species (M. anisopliae or? E. maimaga) to control locusts. The favorable conditions identified by these models were projected into geographical space to infer spatial assumptions (based on temperature) about the possible distribution of the entomopathogenic fungi, to generate maps that shows the variation of the lethal time towards the host (locusts) over wider spatial and temporal scales36. The derivation and the mapping of an efficient pathogenicity index from a population model unifying essential features of the locust-natural enemy interactions mechanism and integrating jointly the relative humidity and temperature require more investigation.
Controlling locust invasions associated with climate variability will be critical in alleviating the potential threats to global food security that may lead to long-term nutritional emergencies and food crisis64. Previous studies reported that the optimum temperature for the efficient growth of most entomopathogenic fungi in the Hypocreales was around 30 °C19,22,31,36,65,66, and others found out that most entomopathogenic fungi in the Hypocreales require at least 95% relative humidity at the surface of the insect to germinate41. Our numerical results suggested that R0 is at maximum for temperatures in the range of 24–33 °C at relative humidity above 95%, which agree to a certain level with the experimental results obtained in e.g.19,22,36,41,65,66. The accuracy of the model lies in the ability to reproduce almost all the areas where the entomopathogenic fungus can spread and then successfully control locust pest.
The main goal of locust control is to successfully implement a preventive and proactive strategy that can disrupt their breeding cycle19. Furthermore, according to the locust expert at FAO, Keith Cressman, desert locusts can multiply 20-fold with a new generation every three months. Therefore, it is crucial to develop67 an effective tool that can help farmers and governments in their field monitoring activities, by building models that may contribute to future decision support systems (DSS) for when and where to apply M. acridum to control locusts. Although field validation of the model was not conducted, we aim to achieve this in future collaborations with governments and organizations such as FAO through the team in charge of predicting the migratory pattern of desert locusts across Africa through an early warning system. Once a future locust invasion is forecast in an uninvaded country, an early or preventive application of the biopesticide can be scheduled in a timely manner for areas predicted by this model to be more suitable for surivival of the fungus. To obtain validation data for our model, this should be accompanied with recording field data on locust mortality caused by M. acridum and the actual environmental factors before, during and after M. acridum application.This should be compared with data from areas without application of M. acridum to rule out the effect of naturally occurring M. acridum infections of locusts. A preliminary validation can also be performed before investing a lot of resources into real field trials by using the current model to generate the map of Somalia and compare predicted potential efficiency of the area that fits with the 236 000 hectares treated by FAO during the recent locust outbreak67.
In Kimathi et al.68, the developed ecological niche model spatially projected to Kenya identified five classes (very low, low, moderate, high, very high) of suitability for the locust breeding site classified as [0–0.2] (very low) to [0.8–1.0] (very high). Analyzing the map of efficacy obtained for Kenya in this study and comparing the areas predicted by our model with potential higher efficiency for fungal application against locusts, we realize that there is a match between these areas and the ones with very high suitability for locust breeding68. This includes counties such as Garissa, Wajir, Marsabit and Mandera where locust invasions were recently reported. The present study therefore complements the work of Kimathi et al.68 by using the variability of the R0 value to guide where and when it might be advisable to plan the application of the microbial control agent M. acridum against desert locust at a large spatial scale. The decrease of R0 that spatially characterizes the areas with lower efficacy (R0 < 1) also matches with locations predicted by the ecological niche model as with low or very low suitability for locust breeding and should not be given priority. However, considering the fact that the current spatial projections use climate data collected and interpolated from weather stations, the areas predicted to have moderate efficacy (R0 ≈ 1) by our model might provide microclimatic conditions adequate for the efficacy of the fungal pathogen against desert locust as suggested by previous studies37,42.
In previous studies, several authors have used different approaches to tackle pest insects problems similar to the current study64,70,71,72,73,74,75,76,77,78,78. For instance, Moukam Kakmeni et al.72 used the same method as in this work to derive the spatial distribution maps for malaria transmission under different climatic and intervention scenarios. However, their context required R0 < 1 for efficient control of the vector-borne diseases which is the opposite to this study where the target is R0 > 1. In Klass et al.31, the spatial variation of pathogen pathogenicity and the implications for biocontrol of locusts and grasshoppers was explored. Although the outcome is realistic, their method did not include the dynamics of fungus-pest insect interactions and neither considered the effect of relative humidity nor the efficacy of the entomopathogenic fungus. Our study is innovative by its way of using the basic reproduction number R0 derived from a dynamical mathematical model depending on both temperature and relative humidity to study the efficacy of an entomopathogenic fungus against pest locusts. The study in56,57,57 used a similar approach but the limitation of their studies are that they did not consider the effects of climatic variables, limiting their predictive accuracy.
Our results underline that, realistic models need to include a broad spectrum of spatial and temporal factors to predict the efficacy of a fungal based biocontrol agent with high accuracy. A key challenge is to choose the most appropriate scale for modeling. This is influenced not only by the resolution of available climate data, but also by the biological knowledge that modeling process may translate into something globally observable at varying scale. Note that the greatest effect of climate change on the efficacy of a fungal based biocontrol agent is likely to be observed at the extremes of the optimal range of temperatures and RH at which the maximal fungal growth and germination occurs, which in our system is set at 28 °C and RH above 95%.
By applying the basic reproduction number R0, maps that illustrate the possibility of a successful application of the fungal based biocontrol agent M. acridum for the control of desert locust were obtained. The outcome of this study could constitute a realistic basis for understanding the interactions and complexities between the entomopathogenic fungus and desert locust. By developing models that may increase the efficacy of fungal based biocontrol agents, this may provide farmers with a better IPM tool and hence reduce the use of chemical insecticides as well as concerns connected to their side effects on health and environment.
There are increasing commercialization and farmer adoption of Low Risk Plant Protection Product (Annex II, point 5 of Regulation (EC) 1107/2009) to control locust outbreaks in Africa67. The fungal based product Green Muscle (with the active ingredient (a.i.) M. acridum) is the most widely used79 and reported to be very effective in killing adults within one to two weeks depending on the environmental conditions67. Large-scale applications in targeted countries are usually done with planes using oil-based formulation of the product which offer better chance of contact with the locust and makes it less vulnerable to low relative humidity. Since the timing of the spraying of these products is a key for better efficacy, the maps projecting the potential area of efficacy like those presented as output of this model can be useful resources when planning for such activities. This might also help optimizing the allocated resources and focus on specific areas in a specific month where high efficacy is expected.
Looking forward, an improvement from the current state of this work would consider the effect of another environmental factor such as ultraviolet radiation and, also extend similar model development exercises using other entomopathogenic fungi. Moreover, downscaling the spatial extent of the predictions combined with adopting hourly and daily temporal variation would also help improve the accuracy and help agricultural officers to provide reliable advises to farmers.
Materials and methods
Study area and datasets used
The areas chosen for the study include Morocco, Mauritania, Kenya and Mali where both past and recent desert locusts outbreaks occurred and were documented with high levels of crop damage and represented as reported point coordinates of desert locust incidences53,68,80,81. The datasets used include the average monthly temperatures, the average monthly relative humidity, and the reported point coordinates of desert locust incidence in the respective countries of interest. Temperature data were taken from the Climatologies at High resolution for the Earth Land Surface Areas (CHELSA) climate data (Version 1.2)82; corresponding to the monthly average mean for the time period 1979–2013. Relative humidity data were obtained from the NASA Surface meteorology and Solar Energy (SSE) (http://eosweb.larc.nasa.gov/sse/) and corresponded to 22 years mean average. Temperature and relative humidity data used are in raster format.
Assumptions
-
(i)
Temperature and macroclimatic relative humidity were considered as the key factors affecting the efficacy of the entomopathogenic fungus M. acridium against desert locust.
-
(ii)
Application of the entomopathogenic fungus is successful in the target desert locus infested area and fungal spores are in contact with the desert locust cuticula.
-
(iii)
Application of the entomopathogenic fungus is done at large spatial scale with mixture of different type of land coverage
Mathematical model
Equations for modeling the epizootiological development of an insect pathogen in an insect pest population often adopt deterministic or stochastic transmission formulation embedded within static or fluctuating environments14. The model presented in Gilchrist et al.62 is used as root of our new model formulation. Their model originally assumes that the density of the pest within a patch is fixed and the interaction between fungus and the host insect population (desert locust in this context) varies with the time. They characterized the model by three coupled equations describing the host insect population density within a patch, colony forming unit (CFU) being mycelium or conidial concentration in the host insect population (Table 2). In our proposed model the following assumptions are made: (i) the density of host insect pest within a patch is not limited; (ii) the fungus-insect pest interaction depends on the climate variables relative humidity and temperature which influences the epizootiology development within-patch.
The interaction between pest insect density (r) within a patch and the concentration of CFUs (m) (Fig. 6) is described at a daily time resolution (t) by the following coupled differential equations:
where c0, β, K, α and γ are fixed model parameters (see Table 2) while ω and ε are estimated based on equations presented in Table 3.
Model diagram of the within-patch system describing the interactions between the entomopathogenic fungus and host insect inside a patch model. Yellow color on the diagram denotes the entomopathogenic fungus M. acridium, green color denotes host insect population (desert locust), blue circle denotes interactions between the entomopathogenic fungus and the host insect, red arrow denotes death. See Table 2 for detailed model explanation and parameters definitions (adapted from62).
Moreover, the spore production rate of the fungus s(t), is proportional to the amount of growth media (pest insect body) available for spore production by the fungus (see Fig. 6)62. Thus,
See Table 2 for a list of parameters of this within-patch model.
Equilibrium points and stability analysis
The equilibrium points are found by solving the equations \(dr/dt = dm/dt = 0\) of the within-patch models Eqs. (1) and (2). The equation has three steady state points: (i) the trivial steady state, (ii) the pathogen-free equilibrium point and (iii) the basic reproduction number R0. To support the local stability of the steady states, we examine the linearized form of Eq. (1) at the equilibrium points.
The trivial steady state
The trivial steady state of the within-patch models Eqs. (1) and (2) is given by \(E_{0} = \left( {r,m} \right) = \left( {0,0} \right)\). E0 is a saddle point, and at this point, there is no insect pest or entomopathogenic fungus. The Jacobian matrix of the within-patch model Eqs. (1) and (2) at the point is given by:
with the characteristic equation:
where the eigenvalues (λ) are given by \(\lambda_{1} = - \alpha\), \(\lambda_{2} = c_{0}\).
The pathogen-free equilibrium point
The pathogen-free equilibrium point is given by \(E_{1} = \left( {r,m} \right) = \left( {K,0} \right)\). At this point, there are no fungal infected pest insects. The Jacobian matrix of the within-patch models Eqs. (1) and (2) at this point is given by:
The characteristic equation corresponding to the pathogen-free equilibrium point E1 is given by
The Routh–Hurwitz criterion ensures that all roots of the polynomial given by Eq. (7) have negative real parts83. Using Routh–Hurwitz, the pathogen free-equilibrium point E1 is stable if the following conditions are satisfied:
Figure 7a displays the stability diagram of the pathogen free-equilibrium point E1 given by Eqs. (8) and (9). For the simulations we chose parameters values given in Table 2. The diagram presents a blue region in the parameters space of (ω, ε) for, which all roots of the polynomial given by Eq. (7) have negative real parts and therefore corresponding to the stability zone of the pathogen free-equilibrium points.
Illustration of the stability diagram of \((\omega ,\varepsilon )\) for within patch model using parameters values in Table 2. (a) Stability diagram of the pathogen free equilibrium point and, (b) stability diagram of the endemic equilibrium point.
Basic reproduction number R 0
An important metric of interacting and dynamic epizootiology systems is the basic reproduction number R0. It is applied in this context to quantify how frequently the entomopathogenic fungus transmission occurs and what it will result in (e.g. mortality of insect, reduced reproduction potential).
The basic reproduction number R0 is further assumed to describe the propensity of the entomopathogenic fungus to survive and to be propagated or go extinct. R0 is calculated for the proposed model by examining the stability of the pathogen-free equilibrium point \(\left( {E_{1} = \left( {r,m} \right) = \left( {K,0} \right)} \right)\). The next generation operator approach described in Hartemink et al.58 is employed to evaluate R0. The formulation of the basic reproduction number R0 is given by the following expression:
It is shown in Eqs. (8) and (9) that, if R0 < 1, then the pathogen-free equilibrium is stable and hence the entomopathogenic fungus goes extinct, while if R0 > 1 then the equilibrium is unstable and hence the entomopathogenic fungus continues to transmit the disease. Estimation of R0 helps to determine whether locust outbreak effects can be attenuated if the entomopathogenic fungi is introduced (R0 > 1).
The endemic equilibrium point
At this point, both pest insect and entomopathogenic fungus exist. The endemic equilibrium point (E2) for the within-patch model Eqs. (1) and (2) is given by:
The Jacobian matrix of the within-patch model Eqs. (1) and (2) at the endemic equilibrium point E2 is given by:
where
The characteristic equation corresponding to the endemic equilibrium point E2 is expressed as:
where
Using the Routh–Hurwitz criterion, the endemic equilibrium point E2 is stable if the following conditions are satisfied:
Figure 7b illustrates the stability diagram of the endemic equilibrium point E2 given by Eqs. (19) and (20) through simulations with parameters values given in Table 2. The blue region in parameter space of (ω, ε) corresponds to the stability zone of the endemic equilibrium point.
Expression of R 0 as function of climatic variables
Some parameters in the R0 expression (Eq. 10) are assumed to be constant while other parameters are considered to be temperature and relative humidity sensitive over space and time. The actual expression of R0 (Eq. 10) is obtained with the following assumptions: (i) fungal lethal time is temperature sensitive; (ii) the proportion of fungal growth media (pest insects) allocated towards CFUs (mycelium/ conidia) is temperature and relative humidity sensitive; (iii) the parameters α, β, K and γ are constants. Temperature sensitive relation of fungal growth rate is described in31. Data obtained in60 were employed to estimate the parameters linking temperature, relative humidity and the proportion of resources (insects) available for spore production. Table 3 contains the summary of the parameters ε and ω.
Mapping R 0 under climate (relative humidity and temperature) variability
To map R0, its expression as function of climatic (relative humidity and temperature) variables was used. A matrix of geographical coordinates was built within the extent of the area of interest, afterward for each point coordinate of the matrix; we extracted the monthly average value of the temperatures and relative humidity from the respective raster layer and estimated the value of R0 using its mathematical expression (Eq. 10). The resulting matrix of R0 is converted into American Standard Code for Information Interchange (ASCII) files through spatial interpolation which was uploaded into Quantum-Geographical Information System (Q-GIS version 3.10)84 for visualization and mapping. Subsequently, the potential areas where locusts outbreak effects can be attenuated if the entomopathogenic fungus is introduced as a biocontrol agent are visualized. Overall, the approach exploits the spatial variation of temperature and relative humidity to provide predictions of the entomopathogenic fungus efficiency at large scale. The process was implemented in R (V.3.6.3)85 using several R-packages that include.
Sensitivity analysis (SA), model evaluation and validation
A sensitivity analysis was performed to determine how sensitive the model output R0 (Eq. 10) is to changes in the model parameters, which further allows to determine which climate variable (relative humidity, temperature) that have most influence on the stability/instability of the equilibrium points and on R0. Sensitivity analysis provides a way to measure how changes in the parameters translate into variations in R0. It also assesses the relative importance of different factors responsible for the efficacy of the entomopathogenic fungus as a biocontrol agent against locusts and to better determine how to improve it. Sensitivity analysis was performed by the Partial Rank Correlation Coefficient (PRCC) algorithm86, where large PRCC values (> 0.5 or < − 0.5) indicate that the parameter has a high influence (positive/negative) on the model outcome. A positive PRCC value indicates the increase of the efficacy with the increase of the parameter and a negative value indicates the decrease of the efficacy with an increase of the parameter.
A recent study in some East African countries predicted the breeding area for desert locust under climate change scenario using temperature, rainfall and, sand and soil moisture content as key bioclimatic factors68. The maps obtained in68, that predicted the current and future area of suitability in Kenya was used to compare if, the locations predicted to be highly suitable for the long-term establishment of desert locust, fits with the areas predicted by the current model to be deemed adequate for applying the entomopathogenic fungus as a biocontrol agent, against locust at a specific month. Furthermore, to evaluate the ability of the spatial projection of R0 as a tool to predict the suitable month for applying the entomopathogenic fungus as a biocontrol agent at a given location, the point coordinates of the locust incidences in the respective countries were uploaded to the maps of R0 to compare and check how these points fit with the areas having R0 greater than one (R0 > 1).
Data availability
All data generated or analysed during this study are included in this published article.
References
Biological control in IPM systems in Africa. (CABI, 2002). https://doi.org/10.1079/9780851996394.0000
Kvakkestad, V., Sundbye, A., Gwynn, R. & Klingen, I. Authorization of microbial plant protection products in the Scandinavian countries: A comparative analysis. Environ. Sci. Policy 106, 115–124 (2020).
Barzman, M. et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 35, 1199–1215 (2015).
Popp, J., Pető, K. & Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 33, 243–255 (2013).
Bale, J., van Lenteren, J. & Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 363, 761–776 (2008).
Vacante, V. & Bonsignore, C. P. Natural enemies and pest control. In Handbook of Pest Management in Organic Farming 60–77 (CABI, 2018). https://doi.org/10.1079/9781780644998.0060
Eilenberg, J., Hajek, A. & Lomer, C. Suggestions for unifying the terminology in biological control. Biocontrol 46, 387–400 (2001).
Lacey, L. A. et al. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 132, 1–41 (2015).
Hatting, J. L., Moore, S. D. & Malan, A. P. Microbial control of phytophagous invertebrate pests in South Africa: Current status and future prospects. J. Invertebr. Pathol. 165, 54–66 (2019).
Karimi, S., Askari Seyahooei, M., Izadi, H., Bagheri, A. & Khodaygan, P. Effect of arsenophonus endosymbiont elimination on fitness of the date palm hopper, ommatissus lybicus (Hemiptera: Tropiduchidae). Environ. Entomol. 48, 614–622 (2019).
Kumar, K. K. et al. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 165, 74–81 (2019).
Mascarin, G. M. et al. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 165, 46–53 (2019).
Shapiro-Ilan, D. I., Bruck, D. J. & Lacey, L. A. Principles of epizootiology and microbial control. Insect Pathol. https://doi.org/10.1016/B978-0-12-384984-7.00003-8 (2012).
Hawkins, B. A. & Cornell, H. V. Theoretical Approaches to Biological Control. https://doi.org/10.1017/CBO9780511542077 (Cambridge University Press, 2009).
Tonnang, H. E. Z., Nedorezov, L. V., Ochanda, H., Owino, J. & Löhr, B. Assessing the impact of biological control of Plutella xylostella through the application of Lotka—Volterra model. Ecol. Model. 220, 60–70 (2009).
Hesketh, H., Roy, H. E., Eilenberg, J., Pell, J. K. & Hails, R. S. Challenges in modelling complexity of fungal entomopathogens in semi-natural populations of insects. Biocontrol 55, 55–73 (2010).
Fuxa, J. R. & Tanada, Y. Epizootiology of Insect Diseases (Wiley, 1987).
Lacey, L. A. Manual of Techniques in Insect Pathology. Manual of Techniques in Insect Pathology (Academic, 1997). https://doi.org/10.1016/b978-0-12-432555-5.x5000-3.
Lomer, C. J., Bateman, R. P., Johnson, D. L., Langewald, J. & Thomas, M. Biological control of locusts and grasshoppers. Annu. Rev. Entomol. 46, 667–702 (2001).
Arthurs, S. & Thomas, M. B. Effects of a mycoinsecticide on feeding and fecundity of the brown locust Locustana pardalina. Biocontrol Sci. Technol. 10, 321–329 (2000).
Jiang, W. et al. Effects of the entomopathogenic fungus Metarhizium anisopliae on the mortality and immune response of Locusta migratoria. Insects 11, 36 (2020).
Thomas, M. B. & Blanford, S. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 18, 344–350 (2003).
Douthwaite, M. B. Development and Commercialization of the Green Muscle Biopesticide 21 (2001).
Douthwaite, B., Langewald, J., & Harris, J. Development and commercialization of the Green Muscle biopesticide. (International Institute of Tropical Agriculture, 2002).
CABI. Green Muscle providing strength against devastating locusts in the horn of Africa—CABI.org. CABI.org https://www.cabi.org/news-article/green-muscle-providing-strength-against-devastating-locusts-in-the-horn-of-africa/ (2020).
Geoff, G. & Steve, W. Biological Control (Springer, 1996). https://doi.org/10.1007/978-1-4613-1157-7.
Fargues, J., Ouedraogo, A., Goettel, M. S. & Lomer, C. J. Effects of temperature, humidity and inoculation method on susceptibility of Schistocerca gregaria to Metarhizium flavoviride. Biocontrol Sci. Technol. 7, 345–356 (1997).
Aragón, P., Coca-Abia, M. M., Llorente, V. & Lobo, J. M. Estimation of climatic favourable areas for locust outbreaks in Spain: Integrating species’ presence records and spatial information on outbreaks. J. Appl. Entomol. 137, 610–623 (2013).
Arthurs, S. & Thomas, M. B. Effect of dose, pre-mortem host incubation temperature and thermal behaviour on host mortality, mycosis and sporulation of Metarhizium anisopliae var. acridum in Schistocerca gregaria. Biocontrol Sci. Technol. 11, 411–420 (2001).
van der Valk, H. Review of the efficacy of Metarhizium anisopliae var. acridum. FAO—U.N. Publ. (2007).
Klass, J. I., Blanford, S. & Thomas, M. B. Development of a model for evaluating the effects of environmental temperature and thermal behaviour on biological control of locusts and grasshoppers using pathogens. Agric. For. Entomol. 9, 189–199 (2007).
Devi, K. U., Sridevi, V., Mohan, C. M. & Padmavathi, J. Effect of high temperature and water stress on in vitro germination and growth in isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin. J. Invertebr. Pathol. 88, 181–189 (2005).
Dimbi, S., Maniania, N. K., Lux, S. A. & Mueke, J. M. Effect of constant temperatures on germination, radial growth and virulence of Metarhizium anisopliae to three species of African tephritid fruit flies. Biocontrol 49, 83–94 (2004).
Ekesi, S., Maniania, N. K. & Ampong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 9, 177–185 (1999).
Thomas, M. B. & Jenkins, N. E. Effects of temperature on growth of Metarhizium flavoviride and virulence to the variegated grasshopper Zonocerus variegatus. Mycol. Res. 101, 1469–1474 (1997).
Klass, J. I., Blanford, S. & Thomas, M. B. Use of a geographic information system to explore spatial variation in pathogen virulence and the implications for biological control of locusts and grasshoppers. Agric. For. Entomol. 9, 201–208 (2007).
Castro, T., Moral, R., Demétrio, C., Delalibera, I. & Klingen, I. Prediction of sporulation and germination by the spider mite pathogenic fungus Neozygites floridana (Neozygitomycetes: Neozygitales: Neozygitaceae) based on temperature, humidity and time. Insects 9, 69 (2018).
Hajek, A. E., Larkin, T. S., Carruthers, R. I. & Soper, R. S. Modelling the dynamics of Entomophaga maimaga (Zygomycetes: Entomophtorales) epizootics in gypsy moth (Lepidoptera: Lymantridae) populations. Environ. Entomol. 22, 1172–1187 (1993).
Gul, H. T., Saeed, S. & Khan, F. A. Z. Entomopathogenic fungi as effective insect pest management tactic: A review. Appl. Sci. Bus. Econ. 1, 10–18 (2014).
Davidson, G. et al. Study of temperature—Growth interactions of entomopathogenic fungi with potential for control of Varroa destructor (Acari: Mesostigmata) using a nonlinear model of poikilotherm development. J. Appl. Microbiol. 94, 816–825 (2003).
Hallsworth, J. E. & Magan, N. Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J. Invertebr. Pathol. 74, 261–266 (1999).
Fargues, J. et al. Climatic factors on entomopathogenic hyphomycetes infection of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) in Mediterranean glasshouse tomato. Biol. Control 28, 320–331 (2003).
Boulard, T. et al. Effect of greenhouse ventilation on humidity of inside air and in leaf boundary-layer. Agric. For. Meteorol. 125, 225–239 (2004).
Mishra, S., Kumar, P. & Malik, A. Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica L. J. Parasit. Dis. 39, 697–704 (2015).
Klingen, I., Westrum, K. & Meyling, N. V. Effect of Norwegian entomopathogenic fungal isolates against Otiorhynchus sulcatus larvae at low temperatures and persistence in strawberry rhizospheres. Biol. Control 81, 1–7 (2015).
Thaochan, N., Benarlee, R., Shekhar Prabhakar, C. & Hu, Q. Impact of temperature and relative humidity on effectiveness of Metarhizium guizhouense PSUM02 against longkong bark eating caterpillar Cossus chloratus Swinhoe under laboratory and field conditions. J. Asia. Pac. Entomol. 23, 285–290 (2020).
Kryukov, V. et al. Ecological preferences of Metarhizium spp. from Russia and neighboring territories and their activity against Colorado potato beetle larvae. J. Invertebr. Pathol. 149, 1–7 (2017).
Saldarriaga Ausique, J. J., D’Alessandro, C. P., Conceschi, M. R., Mascarin, G. M. & Delalibera Júnior, I. Efficacy of entomopathogenic fungi against adult Diaphorina citri from laboratory to field applications. J. Pest Sci. 2017 903 90, 947–960 (2017).
Dwyer, G. Density dependence and spatial structure in the dynamics of insect pathogens. Am. Nat. 143, 533–562 (1994).
Dwyer, G., Elkinton, J. & Hajek, A. Spatial scale and the spread of a fungal pathogen of gypsy moth. Am. Nat. 152, 485–494 (1998).
Knudsen, G. R. & Schotzko, D. J. Spatial simulation of epizootics caused by Beauveria bassiana in Russian wheat aphid populations. Biol. Control 16, 318–326 (1999).
Weseloh, R. M. Effect of conidial dispersal of the fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) on survival of its gypsy moth (Lepidoptera: Lymantriidae) host. Biol. Control 29, 138–144 (2004).
Meynard, C. N. et al. Climate-driven geographic distribution of the desert locust during recession periods: Subspecies’ niche differentiation and relative risks under scenarios of climate change. Glob. Chang. Biol. 23, 4739–4749 (2017).
Anderson, R. M. & May, R. M. Infectious diseases of humans: Dynamics and control. Aust. J. Public Health 16, 208–212 (1991).
Cáceres, C. E. et al. Complex Daphnia interactions with parasites and competitors. Math. Biosci. 258, 148–161 (2014).
Briggs, C. J. & Godfray, H. C. J. The dynamics of insect-pathogen interactions stage-structured populations c. J. Am. Nat. 145, 855–887 (1995).
Rapti, Z. & Cáceres, C. E. Effects of intrinsic and extrinsic host mortality on disease spread. Bull. Math. Biol. 78, 235–253 (2016).
Hartemink, N. A., Randolph, S. E., Davis, S. A. & Heesterbeek, J. A. P. The basic reproduction number for complex disease systems: Defining R0 for tick-borne infections. Am. Nat. 171, 743–754 (2014).
Arthur, F. H. Toxicity of diatomaceous earth to red flour beetles and confused flour beetles (Coleoptera: Tenebrionidae): Effects of temperature and relative humidity. J. Econ. Entomol. 93, 526–532 (2000).
Arthurs, S. & Thomas, M. B. Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria. J. Invertebr. Pathol. 78, 59–65 (2001).
Whipps, J. M. & Davies, K. G. Success in Biological Control of Plant Pathogens and Nematodes by Microorganisms. In Biological Control: Measures of Success 1st edn, (eds Gurr, G. & Wratten, S.) 429. https://doi.org/10.1007/978-94-011-4014-0_8 (Springer, Dordrecht, 2000).
Gilchrist, M. A., Sulsky, D. L. & Pringle, A. Identifying fitness and optimal life-history strategies for an asexual filamentous fungus. Evolution 60, 970–979 (2006).
Frank, S. A. Spatial processes in host-parasite genetics. In Metapopulation Biology, 1st edn, (eds Hanski, I. A. & Gilpin, M. E.) 325–352. https://doi.org/10.1016/B978-012323445-2/50018-3 (Elsevier, 1997).
Yan, Y., Wang, Y.-C., Feng, C.-C., Wan, P.-H.M. & Chang, K.T.-T. Potential distributional changes of invasive crop pest species associated with global climate change. Appl. Geogr. 82, 83–92 (2017).
Inglis, G. D., Johnson, D. L. & Goettel, M. S. Effects of temperature and thermoregulation on mycosis by Beauveria bassianain grasshoppers. Biol. Control 7, 131–139 (1996).
Lactin, D. J. & Johnson, D. L. Temperature-dependent feeding rates of Melanoplus sanguinipes nymphs (Orthoptera: Acrididae) laboratory trials. Environ. Entomol. 24, 1291–1296 (1995).
FAO. Biopesticides for locust control | FAO Stories | Food and Agriculture Organization of the United Nations. Food and Agriculture Organisation of the UN http://www.fao.org/fao-stories/article/en/c/1267098/ (2021).
Kimathi, E. et al. Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 10, 11937 (2020).
Cordovez, J. M., Rendon, L. M., Gonzalez, C. & Guhl, F. Using the basic reproduction number to assess the effects of climate change in the risk of Chagas disease transmission in Colombia. Acta Trop. 129, 74–82 (2014).
Hartemink, N. A. et al. Mapping the basic reproduction number ( R 0) for vector-borne diseases: A case study on bluetongue virus. EPIDEM 1, 153–161 (2009).
Jamison, A., Tuttle, E., Jensen, R., Bierly, G. & Gonser, R. Spatial ecology, landscapes, and the geography of vector-borne disease: A multi-disciplinary review. Appl. Geogr. 63, 418–426 (2015).
Moukam Kakmeni, F. M. et al. Spatial panorama of malaria prevalence in Africa under climate change and interventions scenarios. Int. J. Health Geogr. 17, 2 (2018).
Ngarakana-Gwasira, E. T., Bhunu, C. P., Masocha, M. & Mashonjowa, E. Transmission dynamics of schistosomiasis in Zimbabwe: A mathematical and GIS approach. Commun. Nonlinear Sci. Numer. Simul. 35, 137–147 (2016).
Ogden, N. H. & Radojevic, M. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector ixodes scapularis. Environ. Health Perspect. 122, 631–639 (2014).
Parham, P. E. & Michael, E. Modeling the effects of weather and climate change on malaria transmission. Environ. Health Perspect. 118, 620–626 (2010).
Phillips, J. Climate change and surface mining: A review of environment-human interactions & their spatial dynamics. Appl. Geogr. 74, 95–108 (2016).
Rogers, D. J. & Randolphz, S. E. The global spread of malaria in a future. Warmer World Sci. 2, 1763–1766 (2000).
Wu, X. et al. Developing a temperature-driven map of the basic reproductive number of the emerging tick vector of Lyme disease Ixodes scapularis in Canada. J. Theor. Biol. 319, 50–61 (2013).
CABI. Green Muscle providing strength against devastating locusts in the horn of Africa. https://www.cabi.org/news-article/green-muscle-providing-strength-against-devastating-locusts-in-the-horn-of-africa/ (2020).
Piou, C. et al. Mapping the spatiotemporal distributions of the Desert Locust in Mauritania and Morocco to improve preventive management. Basic Appl. Ecol. 25, 37–47 (2017).
FAO. FAO Locust Hub. https://locust-hub-hqfao.hub.arcgis.com/ (2021).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017).
DeJesus, E. X. & Kaufman, C. Routh-Hurwitz criterion in the examination of eigenvalues of a system of nonlinear ordinary differential equations. Phys. Rev. A 35, 5288–5290 (1987).
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. Qgisorg (2014).
RCoreTeam. R: A language and environment for statistical computing. The R Foundation for Statistical Computing. (2020).
Marino, S., Hogue, I. B., Ray, C. J. & Kirschner, D. E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254, 178–196 (2008).
Acknowledgements
The first author of this study is a PhD student working in the fellowship Project (VW-89362) funded by the Volkswagen Foundation under the funding initiative Knowledge for Tomorrow—Cooperative Research Projects in Sub-Saharan on Resources, their Dynamics, and Sustainability—Capacity Development in Comparative and Integrated Approaches. The authors also thank the support from the “Climate Smart Agricultural Technologies for improved Rural Livelihoods and Food Security in Mali and Niger”, funded by the Royal Norwegian Embassy in Bamako, Mali (Projects MLI-17-008 and NER-17-005).
Author information
Authors and Affiliations
Contributions
Conceptualization: F.T.N., C.T., R.A.G.; F.M.K. Data curation: S.F.K., F.T.N., R.A.G., Formal analysis: S.F.K., F.T.N., R.A.G., Funding acquisition : C.T., F.M.K., K.H.T. Methodology: S.F.K., F.T.N., R.A.G., A.R.H., Resources: F.T.N., I.K., A.R.H., R.A.G K.H.T. Supervision: F.T.N., C.T., F.M.K. Writing—original draft: S.F.K., F.T.N., R.A.G., F.M.K. Writing—review and editing: F.T.N., C.T., R.A.G., I.K., A.R.H., K.H.T., F.M.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamga, S.F., Ndjomatchoua, F.T., Guimapi, R.A. et al. The effect of climate variability in the efficacy of the entomopathogenic fungus Metarhizium acridum against the desert locust Schistocerca gregaria. Sci Rep 12, 7535 (2022). https://doi.org/10.1038/s41598-022-11424-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11424-0
This article is cited by
-
Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control
Journal of Pest Science (2024)
-
Identification and biocontrol potential evaluation of a naturally occurring Metarhizium pingshaense isolate infecting tea weevil Myllocerinus aurolineatus Voss (Coleoptera: Curculionidae)
Egyptian Journal of Biological Pest Control (2023)
-
Insights into sublethal effects of Metarhizium anisopliae on the biotic potentials of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) on maize
Egyptian Journal of Biological Pest Control (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.