Abstract
E/D163 polymorphism of dog prion protein (PrP) has been recently proposed as the variant responsible for canid prion resistance. To further investigate the protective role of this variant against prion replication, the transgenic mouse model OvPrP-Tg532 expressing sheep/goat PrP carrying the substitution D162 (equivalent to D163 position of dog PrP) was generated and intracranially inoculated with a broad collection of small ruminant prion strains. OvPrP-Tg532 mice showed resistance to classical bovine spongiform encephalopathy (BSE) from sheep and some classical scrapie isolates from sheep and goat but were susceptible to ovine atypical L-BSE and numerous classical scrapie isolates. Strikingly, some of these classical scrapie isolates showed a shift in their prion strain properties. These results suggest that other PrP residues apart from E/D163 variant of dog PrP or factors distinct than PrP may participate in prion resistance of canids and that different factors may be required for D162 sheep PrP to provide effective protection to sheep against ruminant prions.
Similar content being viewed by others
Introduction
Prion diseases or transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative diseases. The key molecular event in the pathogenesis of prion diseases is the post-translational modification of the host encoded cellular prion protein PrPC into a disease-associated isoform called PrPSc1. PrPSc self-catalyzes its formation by recruiting and transforming PrPC into PrPSc aggregating and acting as a template to produce newer PrPSc particles2. PrPSc suffers a change in its secondary structure, richer in β-sheet content. Such transformation modifies the protein biochemical features. While PrPC is monomeric, protease-sensitive and soluble in non-ionic detergents3, PrPSc has a high tendency to aggregate, is partially resistant to proteases and insoluble1.
TSEs affect a wide range of mammal species like humans and other species in the human food chain. Human prion diseases include genetic, infectious and sporadic disorders, like Creutzfeldt-Jakob disease (CJD), kuru, Gerstmann- Straussler-Scheinker syndrome (GSS), fatal familial insomnia (FFI) and variably protease-sensitive prionopathy (VPSPr)4. Animal prion diseases encompass scrapie in sheep and goats, chronic wasting disease (CWD) in cervids, bovine spongiform encephalopathy (BSE), transmissible mink encephalopathy (TME)5 and camel prion disease (CPD)6.
Experiments in PrP transgenic mice proved that the efficacy of PrPC conversion into PrPSc mainly rely on the homology grade among the primary sequence of PrPSc and PrPC7, a concept known as “species barrier”. A PrPC-PrPSc interaction sharing the primary sequence is homologous and the efficiency of conversion will be fairly high. By contrast, amino acid differences promote a heterologous interaction which is associated with a decreased efficiency of PrPC transformation8.
PrPC amino acid sequence has been well conserved through evolution9, pointing to an important role of PrPC although its primary function remains unknown. Sequence alignments of mammalian PrPs along with reports of susceptibility/resistance of certain animals to TSEs have resulted in the identification of several amino acids with key roles in susceptibility/resistance to prions. Of special importance is the residue 163 of dog PrP, which can harbor aspartate (D) or glutamate (E), instead of the asparagine (N) encoded in other mammals. This variant has been recently attributed to being the main, if not the only, responsible for the prion resistance of canids10,11. Transgenic mice expressing dog PrP as well as mouse PrP harboring the equivalent D158 variant were resistant to prion infection with three mouse-adapted prion strains10,11. Coexpression of both wild-type and mutated D158 PrP variant in transgenic mice inoculated with mouse-adapted prions increases survival times indicating that this substitution confers a dominant-negative effect on PrP12. Finally, transgenic mice expressing N163-dog PrP were susceptible to sheep classical BSE prions in vivo and to sheep and cattle classical BSE prions in vitro11.
It is well known that prion strains also affect prion conversion. Prion strains are defined as conformational variants which show distinct prion-disease phenotypes when transmitted to hosts with identical PrP sequence13. So, the efficiency of prion replication is not only controlled by the identity between the PrPs involved in the process but also by the prion strain, which renders this replication favorable only when the PrPSc structure is one of the possible that host PrPC can adopt as stated by the conformational selection model14. Thus, resistance/susceptibility to TSEs will always be dependent on the prion strain that is being studied in each case.
This study further investigates the protective role exerted by the dog PrP D/E163 polymorphic variant in the sheep/goat PrP context towards prion replication. For that purpose, a novel transgenic mouse model called OvPrP-Tg532 was generated. These transgenic mice expressed small ruminant PrP carrying the substitution D162 (equivalent to D/E163 position of dog PrP) and were intracranially inoculated with sheep/goat prion isolates representing different scrapie and BSE strains. The use of isolates from the same species (sheep/goat) avoided the species barrier facilitating prion transmission to precisely test the effect of the D162 substitution. Animals were only resistant to some isolates, suggesting that although the dog PrP D/E163 variant plays an important role in canid resistance to prion diseases, distinct PrP residues or factors other than PrP must collaborate in this phenomenon.
Results
Generation of OvPrP-Tg532 transgenic mice
The same plasmid vector used to generate OvPrP-Tg501 mouse line15 was used to generate the new transgenic mouse line as described in the Materials and Methods section. Several founder lines hemizygous for the transgene (D162GoPrP+/-) were obtained. Founder animals (muPrP+/- D162GoPrP+/-) were crossed with prnp null mice (muPrP-/-) to obtain transgenic hemizygous lines in a murine prnp null background (muPrP-/- D162GoPrP+/-). The absence of the murine prnp was determined by PCR using specific primers. Then, hemizygous mice were crossed to produce homozygous animals (muPrP-/- D162GoPrP+/+). At this point, PrPC expression level was determined in brain homogenates of mice by serial dilution in western blotting (WB) and compared to OvPrP-Tg501 brain PrPC expression levels. OvPrP-Tg532 mouse line was selected based on the fact that they showed brain PrPC levels and an electrophoretic profile similar to that of OvPrP-Tg501 mice (Fig. 1). OvPrP-Tg532 mice reached the end of their lifespan (> 650 days post-inoculation) with neither evidence of spontaneous prion disease nor behavioral defects.
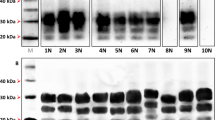
Brain PrPC expression in OvPrP-Tg501 and OvPrP-Tg532 mice. Immunoblotting of PrPC detected using 12B2 mAb. Neat sample (10% brain homogenates) and serial dilutions were loaded on 12% Bis–Tris gels. The figure illustrates a representative set of three independent experiments. Relative molecular mass in kDa at the left side. Full-length blots/gels are presented in Supplementary Figure 1.
Susceptibility of OvPrP-Tg532 transgenic mice to several prion strains
OvPrP-Tg532 mice were intracranially challenged with isolates of classical and atypical BSE adapted to the ovine PrP sequence (Table 1). Classical BSE from experimentally infected goat and sheep, which is well known to successfully propagate into OvPrP-Tg501 mice expressing the wild type small ruminant PrP15, did not infect OvPrP-Tg532 mice expressing the mutated D162-PrP (Table 1). Analysis of brain proteinase K resistant PrP (PrPres) by WB as well as the histological examination of brain tissue were negative (data not shown). By contrast, OvPrP-Tg532 mice got infected with ovine adapted L-BSE isolate (Table 1) showing the same brain PrPres biochemical profile by WB (Fig. 2A) and the same histological features (data not shown) as OvPrP-Tg501 animals challenged with the same inocula. OvPrP-Tg532 mice were also inoculated with several scrapie isolates from sheep and goat. These isolates were collected in different European countries representing the variety of classical scrapie strains circulating in Europe. OvPrP-Tg532 mice proved to be susceptible to all scrapie isolates except from the Italian isolates 198 and I9 from sheep and goat respectively as well as to classical BSE prions as previously mentioned (Table 1). Detection of PrPres by WB and histological analysis of the brains of these latter mice produced negative results (data not shown). In addition, sheep Dawson and goat S2 isolates did not produce 100% attack rates (Table 1).
Comparison between inocula and brain PrPres in OvPrP-Tg501 and OvPrP-Tg532 mice. Immunoblotting of PrPres detected using Sha31 mAb. The figure illustrates a representative set of three independent experiments. Relative molecular mass in kDa at the left side. Full-length blots/gels are presented in Supplementary Figure 1.
Nearly all successfully transmitted isolates conserved the original PrPres electrophoretic profile in WB (Fig. 2) and the histological characteristics were indistinguishable from the ones displayed in control OvPrP-Tg501 animals (data not shown). However, scrapie isolates PS21 from sheep and F10, F14, F2 and S3 from goat displayed a change in their brain PrPres signature in WB which shifted the 21 kDa of the non-glycosylated band to a 19 kDa molecular weight (Fig. 2). Sheep scrapie PS21 and goat scrapie F2 were selected for further study and second passage were performed in OvPrP-Tg501 (Table 1). Brain PrPres from these animals retained the 19 kDa molecular weight of the non-glycosylated band (Fig. 2).
Histologically, once transmitted into the OvPrP-Tg532 and back into OvPrP-Tg501 mice the lesion profiles for sheep scrapie PS21 and goat scrapie F2 were slightly modified (Fig. 3). The PrPSc deposition profile showed an increase in the intraneural deposition for both OvPrP-Tg532 adapted inocula (sheep scrapie PS21 and goat scrapie F2) transmitted back into OvPrP-Tg501 while the severity of the other PrP deposits decreased (Fig. 3).
Histological analysis of the brains of mice inoculated with Sh-Sc 21 and Go-Sc F2 inocula. Brain lesion profiles were performed using hematoxylin and eosin staining using samples from at least 4 inoculated mice. The brain areas analyzed were cerebral cortex (CCtx); striatum (Stri); hippocampus (Hpp); thalamus (Thal); hypothalamus (Hpth); Midbrain (Midb); Cerebelar cortex (CbCtx) and pons/medulla oblongata (PoMe). The representative pictures showing vacuolization were taken in the midbrain section. PrPSc deposit profile was performed by IHC with 2A11 mAb. The magnitude of PrPSc accumulation was scored using samples from at least 4 inoculated mice in the mentioned above brain areas considering the following PrPSc types: intraneuronal (ITNR), intraglial (ITGL, intra-microglial and intra-astrocytic combined), stellate (STEL), fine particulate (PRTC), coalescing (COAL), linear (LINR) and plaques (PLAQ, vascular and non-vascular combined).
Discussion
The dog-PrP D/E163 polymorphic variant has been recently proposed as responsible for the prion resistance of canids10,11. Such variant was found to protect against mouse-adapted strains RML, 22L and 301C in transgenic mice expressing the mouse D158 PrP variant equivalent to the dog D16310. Coexpression of both the wild-type and mutated PrPs in transgenic mice inoculated with mouse-adapted prion strains increased mice survival time12. Later, transgenic mice expressing dog PrP harboring the E163 polymorphic variant were resistant to classical and atypical L-BSE and classical and atypical scrapie prions11. Moreover, once the N163 mutation was introduced in transgenic mice expressing dog PrP, animals were susceptible to sheep classical BSE in vivo and to cattle and sheep classical BSE prions in vitro11. To further study the role exerted for this polymorphic variant in the susceptibility/resistance of prions, we generated the OvPrP-Tg532 mouse line. This line expresses physiological levels of small ruminant PrP harboring the mutation D162, analogue to the D/E163 polymorphic variant of dog PrP. These mice were intracranially inoculated with a broad panel of prion isolates with different strain features.
OvPrP-Tg532 proved to be resistant to classical BSE from ovine and caprine origin (Table 1) while its control counterpart model (OvPrP-Tg501) was susceptible with 100% attack rates. This result is in line with the previously published mouse model expressing the D158-mouse PrP, in which the BSE mouse-adapted strain 301C was unable to cause disease after intracerebral challenge10. However, dog PrP, which expresses in a natural manner the “resistant” D163 could eventually be misfolded in vitro by BSE and BSE-derived prions that retained their ability to infect bovine PrP transgenic mice16. Altogether, suggests the molecular compatibility between D163 dog PrP variant and classical BSE PrPSc at least in vitro. An in silico analysis of this acidic substitution revealed an electrostatic potential change on the surface of PrP when compared to its counterpart amino acid (asparagine). The acidic properties conferred by D/E residue disturb this region introducing a positively charged residue that might block the PrP conversion10.
However, when searching for “universal” PrP sequences that could drive resistance against prions it is important to bear in mind that susceptibility to TSEs depends on the PRNP genotype and the infectious strain17,18. Thus, a wide panel of prion isolates was inoculated in the above mentioned small ruminant transgenic mice to test the role of the singular D162 mutation. The two Italian scrapie isolates included in this study, which showed long incubation times in OvPrP-Tg501 (Table 1)15, were not able to infect D162 OvPrP-Tg532 mice. Two possible explanations could account for this fact. As these strains are classified as slow replicating prions, the PrP containing the D162 mutation may just elongate the survival times making the disease not able to appear within the mouse lifespan. A not fully protective effect but a significant delay in disease manifestation was also found in the highly prion susceptible bank vole PrP transgenic mouse model in which this substitution was introduced12. In this case, the substitution could trigger a protective effect by inducing protein alterations which attenuate the rate of fibril formation and the stability of newly formed fibrils10,19,20. Alternatively, a complete resistance against these Italian scrapie isolates could explain these results. In fact, in the mouse context, the D158 mouse model was also completely resistant to the mouse scrapie adapted strains RML and 22L10. With the aim of reducing incubation times of these slow replicating scrapie strains in the OvPrP-Tg532 model (that could interfere with a polymorphic barrier induced by the substitution D162), second passages will be necessary.
L-BSE and the non-Italian scrapie isolates tested in this panel were able to produce disease in the OvPrP-Tg532 mouse model with attack rates close to 100%. However, slightly longer incubation periods were found in almost all the cases when compared to OvPrP-Tg501 control model (Table 1). These results highlight the importance of using a wide diversity of prion isolates to test the role of a singular PrP mutation in the prion susceptibility/resistance. Therefore, the mutation D162 does not provide total resistance against prion infection in the small ruminant PrP context; it depends on the prion strain tested on each case. Although the successful first passage already shows that the D162 variant in sheep PrP does not provide complete resistance to these prion strains, the second passages would be very informative. Once fully adapted to replication in both D162 OvPrP-Tg532 and N162 OvPrP-Tg501 models, a proper comparison of the transmission properties among the two models could be done.
In addition, for sheep scrapie PS21 and goat scrapies F2, F10, S2 and S3 isolates, the molecular weight of the non-glycosylated PrP band changed from 21 to 19 kDa (Fig. 2). This shift in the strain characteristics was permanent even after transmission back into the OvPrP-Tg501 mice and solely caused by the D162 mutation.
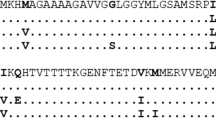
There is no report of TSE infection in canids and the D/E163 polymorphic variant was pointed as responsible for this even in the mouse PrP context10,11. However, we prove that the phenomenon of susceptibility/resistance to prions is strongly strain-dependent and the reports in the mouse PrP context10,11 included other prion strains. In addition, the transmission barrier in the dog brain can be overcome using the PMCA technique for classical BSE prion agent16. These experiments were done using dog brain expressing the D163 PrP polymorphic variant, the same we introduced in the sheep PrP sequence. However, the transgenic mouse line that was found to be resistant to prion infection using a variety of strains, including cattle and sheep classical BSE, expressed the E163 PrP polymorphic variant11. Within the Canidae family, the D163 PrP variant is present in several species while the E163 PrP is restricted to domestic dogs. Thus suggesting that the D163 variant could pose some resistance to prion infection in a strain dependent manner, while the E163 variant may be a more robust protector. Although canids are resistant to TSEs in natural conditions, its PrP can be misfolded in vitro by the classical BSE agent16 whilst the present study points that its resistance cannot be fully attributed to this polymorphic allele since the introduction of the equivalent D162 mutation in sheep PrP did not confer absolute prion resistance. However, it must be taken into account that D162-sheep PrP is not analogue to the dog PrP sequence and therefore other factors may be required for D162-sheep PrP to provide effective protection to sheep against ruminant prions. In addition, other canid PrP residues, or even other genetic factors different than PrP may be involved in fully canid prion resistance. A multiple protein sequence alignment done for mouse, sheep and dog PrP reveals that few amino acid residues apart from D/E163 polymorphism in dog PrP are distinct between these three species (Fig. 4). Since D158 mice were found resistant to the three prion strains tested10,12, amino acid differences between dog and mouse PrP did not account for the prion resistance, at least for the strains tested there. However in the case of our OvPrP-Tg532 mice, 7 amino acid positions differ with the dog PrP sequence and these positions may also be responsible for canid resistance and/or for sheep susceptibility to prion infection (Fig. 4). Little is known from other genetic factors other than PrP affecting prion disease progression. Quantitative trait locus (QTL) mapping, the crossing of heterogeneous stock mice, expression profiling and human genome wide association studies (GWAS) have pointed to the existence of non-PrP genetic modifiers (reviewed in 21). The role that these genes and their coding proteins may pose in prion propagation is not known but they generally belong to neurodegeneration, immune response and protein folding and degradation pathways21. It has also been reported that susceptibility to prion strains is mainly governed by host PrPC while other host factors modulate strain features22.
Dog, mouse and sheep PrP sequence alignment. PrP amino acid alignment from residue 91 to 230 of dog PrP from dog, sheep and mouse species. The dog amino acids that are different in the other two species are indicated in bold. Accession numbers: dog PrP (FJ870767.1); ovine PrP (NP001009481.1) mouse PrP (NP035300).
With the reports existing up to now, the D162 mutation could have been considered interesting for the sheep and goat breeding selection programs conducted to reduce classical scrapie incidence within Europe. Although this polymorphism does not naturally exist in sheep and goats, genetically modified animals could have been produced. Unfortunately, our study demonstrates that D162 substitution does not provide total resistance against prion infection in the small ruminant PrP context, discarding this mutation as suitable for sheep and goat breeding selection programs.
Methods
Ethics statement
Animal experiments were carried out in accordance with the recommendations of the Code for Methods and Welfare Considerations in Behavioral Research with Animals (Directive 86/609EC and 2010/63/EU) and all efforts were made to minimize suffering. Experiments were approved by the Committee on the Ethics of Animal Experiments (CEEA) of the Spanish Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA); Permit Number: CEEA2009/007. The reporting of the animal experiments in this manuscript follows the recommendations in the ARRIVE guidelines.
OvPrP-Tg532 transgenic mice generation
OvPrP-Tg532 mouse line expressing D162 small ruminant PrP about onefold the PrP expression level in goat brain on a mouse PrP null background has been generated as previously described15.
The pMo-GoPrP.Xho plasmid used for the generation of OvPrP-Tg501 mice expressing the wild type small ruminant PrP15 was used as the template for directed mutagenesis reaction. pMo-GoPrP.Xho plasmid contains the goat PrP inserted in the MoPrP.Xho expression vector23. Goat and sheep PrP share the same primary sequence just differing in the nucleotide sequence for certain amino acids. The MoPrP.Xho expression vector contains the murine PrP promoter, exon 1, intron 1, exon 2, and 3’-untranslated sequences. The plasmid was mutated to generate a D162-PrP plasmid by using a QuikChange II XL kit (Stratagene, CA) with specific oligonucleotides (5 ‘-GTACCGTTACCCCGACCAAGTGTACTACAGACCAG-3 ‘ and 5’- CTGGTCTGTAGTACACTTGGTCGGGGTAACGGTAC-3 ‘), following the manufacturer’s instructions.
The transgene was excised from the expression vector by use of NotI. The corresponding gel slice was excised and digested using β-agarase I (New England Biolabs) as described by the manufacturer. Purified DNA was resuspended in TE (10 mM Tris, pH 7.4, 0.1 mM EDTA) at a final concentration of 2 to 6 ng/ml and microinjected into pronuclear-stage embryos collected from superovulated B6CBAF1 females mated with PrP null 129/Ola males24. DNA was extracted from founders’ tail biopsy by use of an Extract-N-Amp tissue PCR kit (Sigma-Aldrich) following the manufacturer’s instructions. The transgene presence in founders was identified by PCR amplification using specific primers 5’-CATTCTGCCTTCCTAGTGGTACC-3’ and 5’-GCTTGTTCCACTGACTGTGGC-3’. MoPrP+/- D162GoPrP+/- founders were backcrossed with homozygous PrP null animals (MoPrP-/-) to obtain mice hemizygous for the mutation which lack murine PrP expression (MoPrP-/- D162GoPrP+/-). The absence of the murine PrP ORF in the transgenic mice was confirmed by PCR amplification using the primers 5’- TAGATGTCAAGGACCTTCAGCC-3’ and 5’-GTTCCACTGATTATGGGTACC-3’. Later, MoPrP-/- D162GoPrP+/- were crossed to produce homozygous MoPrP-/- D162GoPrP+/+ mice for the transmission experiments.
Analysis of PrPC expression in OvPrP-Tg532 transgenic mice
Whole mice brains were homogenized in extraction buffer (0.5% NP-40, 1% sodium deoxycholate, 10 mM EDTA in phosphate-buffered saline [PBS], pH 7.4, with Complete protease inhibitor cocktail (Roche)). Samples were precleared by centrifugation at 2,000 X g for 5 min, added an equal volume of SDS reducing sample loading buffer and boiled for 5 min before loading onto an SDS-12% polyacrylamide gel. For immunoblotting experiments, the monoclonal antibody 12B225 was used at a concentration of 1 µg/ml. This antibody recognizes the 93-WGQGG-97 epitope of the goat PrP sequence. Immunocomplexes were detected using horseradish peroxidase-conjugated anti-mouse IgG. Immunoblots were developed with enhanced chemiluminescence.
Transmission studies
OvPrP-Tg532 and OvPrP-Tg501 (expressing wild type goat PrP15) transgenic mice were challenged with a collection of prion agents (see Table 1 for details). Inocula were prepared from infected brain tissues as 10% (wt/vol) homogenates in 5% glucose.
Groups of 6 to 9 individual identified animals (6 to 7 weeks old) were anesthetized and intracerebrally inoculated with 20 µl of 10% brain homogenate in the right parietal lobe, using a 25-gauge disposable hypodermic needle. As a control, 6 or 7 animals of each line were inoculated with healthy goat brain to discard the appearance of spontaneous prion disease. Mice were observed daily and their neurological status assessed. When the progression of prion disease was evident, or at the end of their life span (beyond 650 days of age), mice were euthanized for ethical reasons. During necropsy, part of the brain was harvested at 20 °C for determination of the presence of PrPres by WB. Survival time was expressed as the mean number of survival days post-inoculation (dpi) for all the PrPres-positive mice. Attack rate was determined as the proportion of PrPres-positive mice among all the mice inoculated. The rest of the brain was fixed by immersion in 10% formalin to quantify spongiform degeneration by histopathology and PrPres accumulation by immunohistochemistry (IHC).
WB analysis
Brain tissue was homogenized in 5% glucose in distilled water in grinding tubes (Bio-Rad) and adjusted to 10% (w/v) by using a TeSeE™ Precess 48TM homogenizer (Bio-Rad) following the manufacturer’s instructions. To determine the presence of PrPres in transgenic mouse brains, 100 μL of 10% brain homogenate were analyzed by WB as previously described14. For immunoblotting, membranes were incubated with Sha31 PrP monoclonal antibody (epitope 148-YEDRYYRE-155 of the goat PrP sequence)26 at a final concentration of 1 μg/mL. Immunocomplexes were detected with horseradish peroxidase- conjugated anti-mouse IgG (Amersham Pharmacia Biotech) and blots were developed with chemiluminescent substrate ECL Select (GE Healthcare Amersham Biosciences). Images were captured using ChemiDoc XRS + System and then processed using Image Lab 5.2.1 Software.
Histological analysis
All procedures concerning the histopathological analysis of mouse brains were performed as previously described27. Mouse brain samples were fixed in neutral-buffered 10% formalin (4% 2-formaldehyde) during necropsy and paraffin embedded. 4 µm-thick tissue slices were stained with hematoxylin and eosin. Lesion profiles of the brains were established following published standard methods28. Semi-quantitative scoring of vacuolation from 0 to 3 in different brain areas was done to construct the vacuolation profile. The brain areas analyzed were the cerebral cortex (CCtx); corpus striatum (Stri); hippocampus (Hpp); thalamus (Thal); hypothalamus (Hpth); midbrain (Midb); cerebellar cortex (CbCtx); and pons/medulla oblongata (PoMe). For each brain area, a single value was calculated as the average vacuolation score. In addition, the total vacuolation score was determined by adding the average scores for the different brain areas. The ratios of the average score for each area to the total vacuolation score were expressed as percentages and plotted against the brain areas to produce lesion profiles. For IHC demonstration of PrPSc accumulation, tissue sections were subjected to antigen retrieval and quenching of hydrogen peroxide, as described previously29 and incubated with monoclonal PrP antibody 2A11 (epitope 163-QVYYRPVDQ-171 of the goat PrP sequence)30. The magnitude of PrPSc accumulation was scored from 0 (absent) to 3 (severe) in the abovementioned brain areas considering the following PrPSc types: intraneuronal (ITNR), intraglial (ITGL, combined intra-microglial and intraastrocytic), extracellular glia-associated (GLAS), fine particulate (PRTC), coalescing (COAL), linear (LINR) and plaque (PLAQ, vascular and non-vascular combined). For each PrPSc type, a single value was calculated as the average of the scores in the different brain areas, and the total brain PrPSc value was calculated as the sum of the different PrPSc averages, with a maximum potential score of 21. The average values for each PrPSc type were converted to percentages with respect to the total PrPSc content in each mouse, and these percentage values were used for graphical representation of PrPSc.
Data availability
No datasets were generated or analyzed during the current study.
References
Colby, D. W. & Prusiner, S. B. Prions. Cold Spring Harb. Perspect. Biol. 3(1), a006833 (2011).
Prusiner, S. B. Molecular biology of prion diseases. Science 252, 1515–1522 (1991).
Prusiner, S. B. Prions. Proc Natl Acad Sci USA 95, 13363–13383 (1998).
Sikorska, B. & Liberski, P. P. Human prion diseases: from Kuru to variant Creutzfeldt-Jakob disease. Subcell Biochem. 65, 457–496 (2012).
Aguilar-Calvo, P., García, C., Espinosa, J. C., Andreoletti, O. & Torres, J. M. Prion and prion-like diseases in animals. Virus Res. 207, 82–93 (2015).
Babelhadj, B. et al. Prion disease in dromedary camels, Algeria. Emerg Infect Dis. 24, 1029–1036 (2018).
Prusiner, S. B. et al. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63, 673–686 (1990).
Marín-Moreno, A., Fernández-Borges, N., Espinosa, J. C., Andréoletti, O. & Torres, J. M. Transmission and replication of prions. Prog. Mol. Biol. Transl. Sci. 150, 181–201 (2017).
Rongyan, Z., Xianglong, L., Lanhui, L., Xiangyun, L. & Fujun, F. Evolution and differentiation of the prion protein gene (PRNP) among species. J. Hered. 99, 647–652 (2008).
Fernández-Borges, N. et al. Unraveling the key to the resistance of canids to prion diseases. PLoS Pathog. 13(11), e1006716 (2017).
Vidal, E. et al. Dogs are resistant to prion infection, due to the presence of aspartic or glutamic acid at position 163 of their prion protein. FASEB J. 34, 3969–3982 (2020).
Otero, A. et al. An amino acid substitution found in animals with low susceptibility to prion diseases confers a protective dominant-negative effect in prion-infected transgenic mice. Mol Neurobiol. 55, 6182–6192 (2018).
Scott, M. et al. Transgenetic investigations of the species barrier and prion strains. In Prion Biology and Diseases (ed. Prusiner, S. B.) 435–482 (Cold Spring Harbor Laboratory Press, 2004).
Collinge, J. & Clarke, A. A general model of prion strains and their pathogenicity. Science 318, 930–936 (2007).
Aguilar-Calvo, P. et al. Role of the goat K222-PrP(C) polymorphic variant in prion infection resistance. J. Virol. 88, 2670–2676 (2014).
Vidal, E. et al. Bovine spongiform encephalopathy induces misfolding of alleged prion-resistant species cellular prion protein without altering its pathobiological features. J. Neurosci. 33, 7778–7786 (2013).
Hill, A. F. et al. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA 97, 10248–10253 (2000).
Collinge, J. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24, 519–550 (2001).
Asante, E. A. et al. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 522, 478–481 (2015).
Lee, C. I., Yang, Q., Perrier, V. & Baskakov, I. V. The dominant-negative effect of the Q218K variant of the prion protein does not require protein X. Protein Sci. 16, 2166–2173 (2007).
Mead, S., Lloyd, S. & Collinge, J. Genetic factors in mammalian prion diseases. Annu. Rev. Genet. 53, 117–147 (2019).
Espinosa, J. C. et al. PrPC governs susceptibility to prion strains in Bank Vole, while other host factors modulate strain features. J. Virol. 90, 10660–10669 (2016).
Borchelt, D. R. et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 13, 159–163 (1996).
Manson, J. C. et al. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8, 121–127 (1994).
Yull, H. M. et al. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am. J. Pathol. 168, 151–157 (2006).
Féraudet, C. et al. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280, 11247–11258 (2005).
Andreoletti, O. et al. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double labeling immunohistochemistry. J. Histochem. Cytochem. 50, 1357–1370 (2002).
Fraser, H. & Dickinson, A. G. The sequential development of the brain lesion of scrapie in three strains of mice. J. Comp. Pathol. 78, 301–311 (1968).
González, L. et al. Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. J. Gen. Virol. 86, 827–838 (2005).
Brun, A. et al. Proteinase K enhanced immunoreactivity of the prion protein-specific monoclonal antibody 2A11. Neurosci. Res. 48, 75–83 (2004).
Espinosa, J. C. et al. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J. Virol. 81, 835–843 (2007).
Marín-Moreno, A. et al. Radical change in zoonotic abilities of atypical BSE prion strains as evidenced by crossing of sheep species barrier in transgenic mice. Emerg Infect Dis. 26, 1130–1139 (2020).
Cassard, H. et al. Evidence for zoonotic potential of ovine scrapie prions. Nat. Commun. 16(5), 5821 (2014).
Stack, M. J., Scott, A. C., Done, S. H. & Dawson, M. Natural scrapie: detection of fibrils in extracts from the central nervous system of sheep. Vet. Rec. 128, 539–540 (1991).
Vanni, I. et al. Isolation of a defective prion mutant from natural scrapie. PLoS Pathog. 12, e1006016 (2016).
Langeveld, J. P. M. et al. Four types of scrapie in goats differentiated from each other and bovine spongiform encephalopathy by biochemical methods. Vet. Res. 50(1), 97 (2019).
Nonno, R. et al. Characterization of goat prions demonstrates geographical variation of scrapie strains in Europe and reveals the composite nature of prion strains. Sci. Rep. 10(1), 19 (2020).
Acknowledgements
This work was supported by grants from the Spanish Ministerio de Ciencia PDI2019-105837RB-I00, Spanish Ministerio de Economía y Competitividad [AGL2016-78054-R (AEI/FEDER, UE) and AGL2009-11553-C02-02 and fellowship BES-2010-040922 to P.A.C.] and the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (fellowship FPI-SGIT-2015-02 to A.M.M.). We thank Sara Canoyra Sánchez for her help and assistance in the editing of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design was done by J.M.T and J.C.E. All authors (A.M.M., J.C.E, P.A.C, N.F.B, J.L.P, L.G and J.M.T) contributed to data acquisition, analysis and interpretation. Original draft preparation was performed by A.M.M, J.M.T and J.C.E. All authors (A.M.M., J.C.E, P.A.C, N.F.B, J.L.P, L.G and J.M.T) revised the draft and have approved the submitted version. All authors (A.M.M., J.C.E, P.A.C, N.F.B, J.L.P, L.G and J.M.T) have agreed to be personally accountable for the author’s own contributions and ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marín-Moreno, A., Espinosa, J.C., Aguilar-Calvo, P. et al. Canine D163-PrP polymorphic variant does not provide complete protection against prion infection in small ruminant PrP context. Sci Rep 11, 14309 (2021). https://doi.org/10.1038/s41598-021-93594-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93594-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.