Abstract
Type 2 C protein phosphatases (PP2Cs) represent the major group of protein phosphatases in plants and play important roles in various plant processes. In this study, 94 MtPP2C genes were identified from Medicago truncatula and further phylogenetically classified into 13 subfamilies, as supported by exon-intron organization and conserved motif composition. Collinearity analysis indicated that segmental duplication events played a crucial role in the expansion of MtPP2C gene families in M. truncatula. Furthermore, the expression profiles of MtPP2Cs under different abiotic treatments were analyzed using qRT-PCR. Results showed that these MtPP2Cs genes displayed different expression patterns in response to drought, cold and ABA stress conditions and some of the key stress responsive MtPP2Cs genes have been identified. Our study presents a comprehensive overview of the PP2C gene family in M. truncatula, which will be useful for further functional characterization of MtPP2Cs in plant drought and cold stress responses.
Similar content being viewed by others
Introduction
Reversible phosphorylation of proteins is an important protein modification process that regulates a large number of physiological and biochemical reactions in plants. Phosphorylation and dephosphorylation are catalyzed by protein kinases (PKs) and protein phosphatases (PPs), respectively. According to the specificity of substrates, PPs are divided into serine/threonine protein phosphatase (PSPs) and tyrosine protein phosphatases (PTPs). PSPs are classified into two categories: Category 1 includes PP1, PP2A, PP2B, PP4, PP5, and PP6; while category 2 is PPM (protein phosphatase M), including PP2C and other Mg2+-dependent phosphatases1,2.
PP2C proteins belong to monomer enzymes and the activity depends on Mg2+ and Mn2+. In eukaryotes, the catalytic domain of PP2C proteins is located at either the N-terminus or the C-terminus3. Further research revealed that the regions of catalytic domain in eukaryotic PP2C proteins are relatively conserved, whereas the regions of non-catalytic domain have diverse amino acid sequences2,3.
PP2Cs are evolutionarily conserved from prokaryotes to higher eukaryotes, having been found in archaea, bacteria, fungi, plants and animals4. In plants, PP2Cs form the largest family of phosphatase genes, accounting for 60–65% of all phosphorylases5,6. The high proportion of PP2C genes is indicative of their evolutionary significance, requirement and involvement in diverse plant cellular functions2. As a major class of protein phosphatases, PP2Cs catalyze dephosphorylation of substrate proteins to regulate signaling pathways and participate in various physiological and biochemical processes in plants. Current studies have shown that PP2Cs play crucial roles in different processes, such as ABA signaling, biotic and abiotic stress responses, plant immunity, K+ nutrient signaling and plant development2,7.
Drought, salt, and temperature stresses are major environmental factors that affect the geographical distribution of plants in nature, limit plant productivity in agriculture, and threaten food security8. Plants evolve a variety of signaling mechanisms to adapt to adverse environments, such as drought, high salt, extreme temperatures and pest attacks. Many studies have shown that some PP2C genes are involved in the regulation of the ABA signaling pathway by modulating the kinase activity of SnRK or MAPK to respond to abiotic stresses9. For example, PpABI1A and PpABI1B, the only two subfamily A PP2Cs in moss, are directly involved in ABA responses, including induced vegetative desiccation tolerance10. In higher plants, the function of PP2C in abiotic stress is more diverse. For instance, in Arabidopsis, ABI1, ABI2 and HAB1 participate in plant abiotic stress/tolerance by negatively regulating ABA signaling11,12,13. Transgenic studies in maize and Arabidopsis confirmed that ZmPP2C-A10 functions as a negative regulator of drought tolerance as well9. Similar results have been obtained from studies in other plants, such as tomato14, poplar15, Artemisia annua L16., Populus euphratica17, and sweet cherry18. These studies show that subfamily A PP2Cs in Arabidopsis and other plants negatively regulate ABA and stress signaling pathways. On the contrary, BdPP2CA6, a subfamily A PP2C from Brachypodium distachyon, was found to be a positive regulator in both ABA and stress signaling pathways19. Another study has identified a potential rice subfamily A PP2C, which regulates ABA signaling negatively and abiotic stress signaling positively20. Most subfamily A PP2C members of Arabidopsis participate in stress tolerance via ABA-dependent signaling pathways, but in other studies, some PP2Cs can also regulate plant stress tolerance by ABA-independent signaling pathways, such as OsPPOs from rice21. These studies indicate that PP2Cs in different plants have diverse functions in stress signal pathways.
As genome sequencing of more species is completed, the PP2C gene family has been isolated, identified, and characterized in a number of plant species including Arabidopsis3,22,23, rice6,22, hot pepper24, wild soybean25, maize26 and Brachypodium distachyon4. However, as a model legume plant, the PP2C gene family in M. truncatula has not been analyzed comprehensively and their functions remain elusive at present.
In this study, we identified 94 MtPP2C genes from M. truncatula genome and grouped them into 13 subfamilies. Comprehensive analyses of gene structures, gene duplications, chromosomal distribution, and phylogeny of these MtPP2Cs were further carried out. At the same time, their expression profiles were also investigated by qRT-PCR under drought and cold treatments. The results presented here provide a solid foundation for further functional characterization of MtPP2C genes in this model species.
Result
Genome-wide Identification of PP2C Family Members in M. truncatula
To identify the PP2C genes, we searched the M. truncatula genome database (Plaza3.0 database) using the InterPro PP2C domain “IPR001932” as the key word and found 95 putative PP2C genes. After confirming the presence of PP2C domains using Pfam and Batch CD-search, we found that one putative PP2C gene lacks the PP2C catalytic domain. Therefore, 94 genes were identified as PP2C members in M. truncatula and were named as MtPP2C1 to MtPP2C94, based on their locus ID.
All of the basic information on these 94 MtPP2C genes is provided in Table 1. Sequence analysis revealed that the lengths of the deduced MtPP2C proteins vary from 118 amino acids (MtPP2C71) to 1,256 amino acids (MtPP2C23), with an average of 419 amino acids. The predicted molecular weights (MW) and isoelectric points (pI) range from 13.047 kDa (MtPP2C71) to 133.232 kDa (MtPP2C23) and from 3.80 (MtPP2C23) to 9.82 (MtPP2C84), respectively. Subcellular localization prediction showed that most of the MtPP2C proteins might be located in chloroplasts, nuclei or cytoplasm, followed by mitochondria, extracellular compartments and vacuoles (Table 1).
To further understand the relationship between MtPP2C genes and AtPP2C genes, we further annotated the Arabidopsis homologous genes of each MtPP2C by Blast search against TAIR (http://www.arabidopsis.org/index.jsp) (Supplementary Table S1).
Chromosomal location and duplication of MtPP2C genes
Based on physical locations on M. truncatula chromosomes, the 94 MtPP2C genes were displayed using the MapInspect software. Ninety-three MtPP2C genes are distributed across all eight chromosomes (Ch1–Ch8), ranging from two to 22 per chromosome (Fig. 1). The number of MtPP2Cs located on each chromosome varies dramatically; chromosomes 1 contains the largest number of MtPP2C family members with 22 genes, whereas the least number was detected on chromosomes 6, containing only two MtPP2C genes. Furthermore, one MtPP2C (MtPP2C94) is located on an unassembled genomic scaffold, thus cannot be mapped to any particular chromosome according to what we currently know about this genome. These results showed that the MtPP2C genes are unevenly distributed on different chromosomes, and that each subfamily gene is also unevenly distributed.
Previous studies in rice, Arabidopsis and B. distachyon showed that PP2C gene families mainly expanded through whole-genome and chromosomal segment duplications4,22. Closely related genes located within a distance of less than 200 kb on the same chromosome are defined as tandem duplications, otherwise they are segmental duplications27. In M. truncatula, 25 pairs of paralogous MtPP2C genes were found to be involved in segmental duplication events and no tandem duplication gene pairs were found (Fig. 1). As shown in Fig. 1, these 25 pairs of duplicated MtPP2C genes are distributed on chromosome1, 2, 3, 4, 5 and 7, but not on chromosome 6 and 8. The ratio of Ka/Ks showed that 24 pairs of duplicated MtPP2C genes, except for MtPP2C17/26, have evolved mainly from purifying selection (Supplementary Table S2). Amino acid alignment and phylogenetic analysis indicated that two counterparts of each gene pair are from the same subgroup (Fig. 2 and Supplementary Table S2).
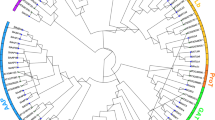
Phylogenetic relationships among 94 MtPP2C genes in M. truncatula. The unrooted phylogenetic tree was generated with MEGA 6.06 program using the full-length amino acid sequences of the 94 MtPP2C proteins by the neighbor-joining (NJ) method with 1,000 bootstrap replicates. Thirteen different subfamilies (A–L) are highlighted with different colored backgrounds.
Phylogenetic analysis
To evaluate the evolutionary relationships of 94 PP2C proteins in M. truncatula, we conducted a phylogenetic analysis using MEGA6.06 based on full-length protein sequences (Fig. 2). At the same time, we constructed another phylogenetic tree to compare the phylogenetic relationships of PP2Cs among Arabidopsis, rice and M. truncatula (Supplementary Fig. S1). Consistent with the previous studies in Arabidopsis and rice22, all MtPP2C genes are grouped into 13 subfamilies and several independent single branches. As expected, most MtPP2Cs cluster together with those from Arabidopsis because both M. truncatula and Arabidopsis are dicotyledonous plants, while those PP2Cs from rice tend to form independent branches. As shown in Fig. 2 and Supplementary Fig. S1, there is only a little difference between the two phylogenetic trees and most of the MtPP2C proteins fall into the same subfamily. In Fig. 2, MtPP2C66 can be grouped into subfamily H, while MtPP2C71 and MtPP2C10 can be grouped into subfamily I because of relatively high bootstrap support (66% and 50%, respectively), but in Supplementary Figure S1 they cannot be grouped.
As shown in Fig. 2, 87 out of 94 MtPP2C genes are distributed in 13 subfamilies (A-L), and the remaining seven MtPP2C genes, MtPP2C9, MtPP2C10, MtPP2C13, MtPP2C48, MtPP2C66, MtPP2C71 and MtPP2C74, cannot be grouped into any subfamilies. The subfamilies D, E and A are the largest three subfamilies, containing 19, 12 and 9 members, respectively. Subfamily J is the smallest one, including only one gene, MtPP2C12. Moreover, subfamilies C and D as well as subfamilies L and H constitute sister clades in a monophyletic cluster with high bootstrap support (96% and 86%, respectively), suggesting close evolutionary relationships between the respective subfamilies.
As shown in Supplementary Fig. 1, the number of MtPP2C genes in each subfamily is similar among M. truncatula, Arabidopsis and rice except for subfamily D. We found that the number of subfamily D genes in M. truncatula (19) is significantly higher than that of other plants, such as Arabidopsis (9), rice (11), maize (13) and B. distachyon (9)4,6,22,26. MtPP2C57, MtPP2C58, MtPP2C59 and MtPP2C86 are grouped into an independent branch, of which no PP2Cs from Arabidopsis and rice exist (bootstrap, 88%). Similarly, the other six genes, MtPP2C34, MtPP2C35, MtPP2C36, MtPP2C83, MtPP2C84 and MtPP2C85 also form an independent branch (bootstrap, 89%). These MtPP2C genes belonging to independent branches may have specific functions in M. truncatula. The remaining six MtPP2C genes from M. truncatula are clustered together with the PP2C genes from Arabidopsis and rice.
Gene structure and conserved motifs distribution analysis
In order to better understand the conservation and diversity of motif compositions and gene structures of MtPP2Cs, the conserved motifs and exon-intron organization of MtPP2Cs were analyzed. By comparing the CDS and the genomic DNA, the MtPP2C gene structures were obtained (Fig. 3). The number of introns is highly divergent, from zero to 19, which is consistent with PP2C genes in Arabidopsis and rice. Of the 94 MtPP2C genes, only four genes (MtPP2C6, MtPP2C31, MtPP2C43 and MtPP2C78) have no introns, whereas MtPP2C12 contains 19 introns. In the same subfamily, most members share similar exon/intron structures, such as intron phase, intron number and exon length (Fig. 3). For example, in the largest subfamily D, 16 MtPP2C genes harbor three introns, with the exception of MtPP2C57 and MtPP2C84, which have two introns, and MtPP2C59, which has five introns. In subfamily F2, all five members have seven introns. A great degree of variation in the number of introns exists in subfamilies I, H, E and K.
The exon-intron structure of MtPP2C genes. Exon-intron analyses of MtPP2C genes were carried out with GSDS. Lengths of exons and introns of each MtPP2C gene are exhibited proportionally. Gene families are grouped and color-coded based on the phylogenetic tree. For all genes, black lines represent introns, yellow boxes represent exons and purple boxes represent UTRs.
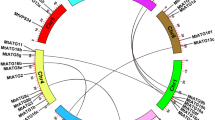
The conserved motifs of MtPP2C proteins were analyzed using the software MEME, and 15 distinct conserved motifs were identified (Supplementary Fig. S2). The composition patterns of motifs tend to be consistent with the results from our phylogenetic tree, that is to say, the MtPP2Cs within each subfamily share similar motif compositions, but among different subfamilies, the motif compositions vary (Fig. 4). Motif 1, 2, 3, 4, 6, 7, 8 and 13 are present in most subfamilies, among them, motif 2 is present in 91 MtPP2C proteins except for MtPP2C66, MtPP2C86 and MtPP2C84. In contrast, some other motifs exist only in specific subfamilies. For instance, motif 12 and motif 14 is present only in subfamilies E and D, respectively, while motif 9 is present in both subfamilies F1 and D. These results suggest that the specific functions of different subfamily genes may be due to specific motifs. This indicates that patterns of introns and motifs, which correlate well with the phylogenetic clades, strongly support their close evolutionary relationships among the MtPP2C genes within the same subfamilies.
Conserved motifs of MtPP2C proteins. The conserved motifs in MtPP2C proteins were identified by MEME software. Grey lines represent the non-conserved sequences, and each motif is indicated by a colored box numbered on the right side of the figure. The length of motifs in each protein is presented proportionally.
Cis-element analysis in the promoter regions of MtPP2Cs
Cis-elements in combination with transcription factors regulate the transcription level of a gene. To investigate the possible roles of MtPP2Cs in abiotic stresses, corresponding promoter regions (1.5 kb upstream ATG) of 94 MtPP2C genes was subjected to cis-element analysis by PlantCARE online.
Fourteen putative cis-acting elements were investigated in this study (Supplementary Table S3), including six abiotic stress-responsive (ARE, C-repeat/DRE, HSE, LTR, MBS and TC-rich repeats) and nine hormone-responsive (ABRE, CGTCA-motif, ERE, GARE-motif, P-box, TATC-motif, TCA element and TGA-element) cis-acting elements. Overall, cis-elements responsive to abiotic stresses and hormones are widely present in the promoters of the MtPP2C genes and the number of cis-elements ranges from 3 to 18 (Supplementary Table S4), suggesting that these MtPP2Cs are involved in responses to different stresses in M. truncatula.
Expression Profiles of the MtPP2C Genes in Different Tissues
Sixteen MtPP2C genes (MtPP2C9, 13, 16, 25, 31, 45, 46, 50, 55, 56, 57, 67, 77, 78, 79 and 86) do not have their corresponding probe sets in the dataset, but the expression profiles of the rest 78 MtPP2C genes were analyzed (Supplementary Fig. S3). Different MtPP2C genes show different expression patterns in each tissue. Some genes are highly expressed in all eight tissues, such as MtPP2C20, MtPP2C29, MtPP2C39, MtPP2C73, MtPP2C91 and MtPP2C93. In contrast, the expression of some genes is low in all eight tissues, such as MtPP2C34, MtPP2C35 and MtPP2C36. Some MtPP2C genes show significantly distinct tissue-specific expression patterns across the eight tissues examined. For instance, MtPP2C32 is preferentially expressed in roots but lowly expressed in other seven tissues. In another example, the expression of MtPP2C11 in roots and nodules is much lower than that in the other six tissues, but the expression of MtPP2C5 is exactly the opposite of MtPP2C11. The results revealed that different PP2C genes from M. truncatula might function in different tissues.
Expression Profiles of MtPP2C Genes Under Cold, Drought and ABA Stress
In plants, many PP2Cs play important roles in response to drought and cold stresses. To investigate the expression profiles of MtPP2C genes under different abiotic stress, quantitative real time-PCR (qRT-PCR) analysis was used to examine their transcription levels.
In our study, transcripts of 80 MtPP2C genes could be detected by qRT-PCR (CT vaule ≤ 35), but transcripts of 14 MtPP2C genes was barely detectable (MtPP2C45, MtPP2C50, MtPP2C53, MtPP2C55, MtPP2C57, MtPP2C59, MtPP2C77, MtPP2C78, MtPP2C79, MtPP2C83, MtPP2C84, MtPP2C85, MtPP2C90 and MtPP2C94). As shown in Fig. 5, we found that many MtPP2C genes tested in this study show similar trends under three different treatments, especially under drought and ABA treatments. On the contrary, some genes have different expression patterns under different treatments. Furthermore, the MtPP2C genes with significantly altered expression after treatments (fold change ≥ 2 than controls in all three independent treatments) were selected and listed in Supplementary Table 5.
Relative transcriptional expression levels of MtPP2Cs under different abiotic treatments. Each column indicates a sampling time point, and each row indicates an MtPP2C member. The expression level of the control (at 0 h; marked in gray) in every treatment for each gene is used as the rescaled value when calculating the relative expression levels. The relative expressions are log2 transformed and visualized for heatmap using Graphpad prism 7. The colors vary from blue to red representing the scale of the relative expression levels.
All together, we obtained 24 MtPP2C genes showing significant differences in expression levels under cold stress, including 14 up-regulated and 10 down-regulated genes. Three genes belonging to subfamily B, MtPP2C72, MtPP2C46 and MtPP2C47, were most significantly up-regulated under cold treatment, implying their important roles in the response to cold stress. The expression levels of five genes belonging to subfamily D changed significantly under cold treatment, four (MtPP2C34, MtPP2C35, MtPP2C36 and MtPP2C87) of which were down-regulated and one (MtPP2C18) was up-regulated. Similarly, the expression levels of the four genes belonging to subfamily A changed remarkably, three (MtPP2C4, MtPP2C41 and MtPP2C92) of which were down-regulated and one (MtPP2C8) was up-regulated. In addition, some MtPP2C genes from other subfamilies were also induced or inhibited by cold treatment.
Under drought treatment, 11 MtPP2C genes showed obviously different expression levels, including six up-regulated and five down-regulated genes. The expression levels of five genes belonging to subfamily A, MtPP2C8, MtPP2C37, MtPP2C65, MtPP2C67 and MtPP2C92, were all up-regulated obviously, and another subfamily A MtPP2C genes, MtPP2C73, was also up-regulated but at a lower degree (fold change ≥ 1.5). The expression level of MtPP2C69, which belongs to subfamily G, was the most obviously down-regulated under drought treatment.
Under ABA treatment, 14 MtPP2C genes exhibited different expression levels, including nine up-regulated and five down-regulated genes. The MtPP2C genes with increased expression levels after ABA treatment are highly correlated with those responsive to drought treatment, such as MtPP2C8, MtPP2C37, MtPP2C65, MtPP2C67, MtPP2C67, MtPP2C92 and MtPP2C30.
Among the MtPP2C genes with significantly altered expression levels after different treatments, MtPP2C8 is the only gene that was up-regulated by all three treatments. Unlike MtPP2C8, the expression level of MtPPC92 was increased significantly by drought and ABA treatments, while decreased significantly by cold treatment. The expression levels of some MtPP2C genes changed significantly by two treatments, such as MtPP2C67, MtPP2C73, MtPP2C 37, MtPP2C23, MtPP2C69 and MtPP2C80 under drought and ABA treatment, and MtPP2C34, MtPP2C35 and MtPP2C36 under drought and cold treatment. In addition, the expression level of some genes changed only by one treatment, such as MtPP2C40 by cold treatment. Different expression patterns of MtPP2C genes may indicate different roles in response to different treatments.
Discussion
Based on the completion of M. truncatula genome sequencing28, many gene families were identified and characterized at the whole-genome level, including CCCH29, LBD30, WRKY31, AP2/ERF32, Dof 33, GH334, CAMTA35, LEA36, MAPKKK37, U-box38, MYB39,40 and GRAS41,42. In this study, PP2C genes in M. truncatula were comprehensively studied, from genome-wide identification, chromosomal locations, evolutionary relationships, gene structure and conserved motifs analysis to expression patterns under cold and drought stresses.
Compared to other gene families, the PP2C gene family is one of the largest families in the plant kingdom. Genome-wide analyses have identified 80, 90, 91, 88, 104 and 86 PP2C gene family members in Arabidopsis22, rice6, tomato, hot pepper24, maize26, and B. distachyon4 genomes, respectively. Evolutionary analysis showed that PP2C genes are divided into 11,12 or 13 groups in different plants. In lower plants, such as Chlamydomonas reinhardtii, Physcomitrella patens and Selaginella tamariscina, the PP2C gene family members are much less than those in higher plants. The increase and expansion of PP2C genes from lower plants to higher plants may correlate with adaptations to complex environmental conditions43. Here, we identified 94 MtPP2C genes from M. truncatula and divided them into 13 subfamilies (Table 1 and Fig. 1), consistent with other higher plants, such as tomato and hot pepper24.
Most proteins in the same MtPP2C subfamily share similar parameters and the number of introns except for subfamily K. Different subfamilies of MtPP2Cs are distinguished from each other in the values of MW and pI (Table 1 and Supplementary Fig. S4). Overall, members of most subfamilies have a more concentrated MW distribution (30–60) and a wider pI distribution (4.5–10). In contrast, members in subfamilies C and L have a wider MW distribution (22.6–119.58) and a concentrated pI distribution (4.94–6.64). Neither MW nor PI distribution is concentrated in the members of subfamilies K and J (Table 1 and Supplementary Fig. S4). Similar to MW, pI, and the number of introns, MtPP2C proteins grouped into the same subfamily exhibit similar motif distributions, suggesting functional similarities for members in the same subfamily.
Subfamilies A and B PP2C genes only exist in plants. Members of subfamily A play a role in ABA-dependent stress responses, while members of subfamily B have been characterized as regulators of MAPK activities43. In this study, expression pattern analysis showed that subfamilies A and B exhibit the most prominent responses to abiotic stresses among all 13 MtPP2C subfamilies (Fig. 5 and Supplementary Table S5).
Studies on model organisms Arabidopsis and rice demonstrated that family A PP2C plays an important role in plant response to abiotic stress, especially in the ABA signaling pathway2,44. After evolutionary analysis and sequence alignment, nine PP2C genes belonging to family A in M. truncatula were identified. Consistent with reports in other plants, most members in subfamily A in M. truncatula are significantly up- or down-regulated under cold and drought stress. Furthermore, those subfamily A genes significantly up-regulated by drought are induced by ABA as well, indicating that they are regulated by ABA-dependent pathways.
MtPP2C8, MtPP2C37, MtPP2C67 and MtPP2C73, which are homologs of HAI PP2Cs (Highly ABA-Induced1,2,3) in Arabidopsis, are significantly induced by drought and ABA treatment, while MtPP2C8 is also significantly induced by cold treatment (Fig. 5 and Supplementary Table 5). Studies in Arabidopsis have shown that HAI PP2Cs have unique drought resistance functions. HAI PP2Cs have the greatest effect on ABA-independent low water potential phenotypes but have lesser effect on classical ABA sensitivity phenotypes44.
The expression of MtPP2C92 and MtPP2C65 is increased significantly under drought and ABA treatment, but the expression of MtPP2C92 is decreased under cold treatment (Fig. 5 and Supplementary Table S5). In Arabidopsis, ABI1 (homolog of MtPP2C92) and ABI2 (homolog of MtPP2C65) are two most extensively studied PP2Cs and have been characterized as the main components of the ABA signaling pathway under abiotic stresses and during development2,43,45. The function of MtPP2C92 and MtPP2C65 in M. truncatula may be similar to that of ABIs in Arabidopsis, but the different expression patterns after cold treatment may indicate their differences in cold responses.
There are six members of subfamily B PP2Cs in Arabidopsis22, four of them (AP2C1–4) maintain a kinase interaction motif at the N-terminal region of the proteins and are characterized as MAPK phosphatases3. Only three members of subfamily B PP2C (PP2C46, PP2C47 and PP2C72) in M. truncatula were identified. Phylogenetic analysis indicates that they are closely related to AP2C1-4 (Supplementary Fig. S1 and Supplementary Table S1). AP2C1, a homolog in Arabidopsis with MtPP2C46 and MtPP2C47, was reported as a negative regulator of stress-induced MAP kinase cascade by interacting with and inactivating Arabidopsis MPK4 and MPK6. AP2C1 modulates innate immunity and stress hormones such as jasmonic acid and ethylene in Arabidopsis46. In alfalfa, MP2C (homolog with AP2C1) functions as a negative regulator of the stress-activated MAPK pathway that is activated by cold, drought, touch, and wounding47. AP2C2, a homolog in Arabidopsis with MtPP2C72, is a regulator of stress response signaling, in particular ROS signaling activated by both biotic and abiotic stresses48. Expression analysis showed that the expression of MtPP2C46, MtPP2C47 and MtPP2C72 is induced by cold, drought and ABA, especially by cold treatment (Fig. 5 and Supplementary Table S5). In Arabidopsis, AP2C1 expression is strongly induced by cold, drought and wounding, but AP2C2 is slightly induced by these treatments48. The above studies indicate that subfamily B PP2C genes in M. truncatula may be regulators of the stress-induced MAP kinase cascade, similar to those in Arabidopsis, but the specific function may be different. In M. truncatula, MtPP2C46, MtPP2C47 and MtPP2C72 may play a vital role in cold responses.
In addition to the PP2C genes from subfamilies A and B, many PP2C genes from other subfamilies have also been reported to respond to abiotic stress in plants. Similar to reports in other plants, our study in M. truncatula also revealed that some MtPP2Cs from other subfamilies are induced by cold and drought. The expression of several genes in subfamily E is significantly altered after treatments, such as MtPP2C89 under cold and ABA treatments (Fig. 5 and Supplementary Table S5). A recent study showed three EGRs (Clade E Growth-Regulating) (homolog of MtPP2C89), which belong to subfamily E PP2C in Arabidopsis, act as negative growth regulators to restrain growth during drought49. However, the function of other subfamily PP2C in plant resistance to abiotic stress is poorly understood and needs to be further investigated.
The results of our study establish a foundation for future studies on the functions of MtPP2C genes in plant abiotic response, and provide a basic understanding that may allow us to elucidate the potential functions of MtPP2C genes under drought and cold stresses in M. truncatula.
Methods
Database Searches and Identification of PP2C Genes in M. truncatula
The InterPro PP2C domain “IPR001932” was used to search the Plaza3.0 database (http://bioinformatics.psb.ugent.be/plaza/) in order to identify PP2C candidate genes in M. truncatula50. Amino acid sequences (Supplementary Data 1), CDS sequences (Supplementary Data 2) and Genomic sequences (Supplementary Data 3) of PP2C genes in M. truncatula were downloaded from the Phytozome12.1 database (https://phytozome.jgi.doe.gov/pz/portal.html)51. All protein sequences were manually checked individually using Pfam (http://pfam.xfam.org/) and the online Batch CD-search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) to confirm the presence of the PP2C domains52,53. All candidate PP2C genes with no PP2C domains were removed.
Proteins of PP2Cs in Arabidopsis and rice were downloaded from the TAIR database (https://www.arabidopsis.org) and the Rice Genome Annotation Project Database (https://rice.plantbiology.msu.edu/), which was described in previous reports4,22.
Analysis of protein features and chromosomal locations
The Compute pI/MW tool of the ExPASy server (http://web.expasy.org/compute) was used to calculate the molecular weight (MW) and the theoretical isoelectric point (pI) of MtPP2C proteins. The WoLF PSORT program (https://wolfpsort.hgc.jp/) was used to predict protein subcellular localization54.
According to the starting positions on chromosomes, the MapInspect software was used to draw the chromosomal distribution images of of MtPP2C genes.
Duplications between the PP2C genes were identified and complemented using the PGDD database (http://chibba.agtec.uga.edu/duplication/)55,56. The number of nonsynonymous substitutions per nonsynonymous site (Ka) and the number of synonymous substitution per synonymous site (Ks) of duplicated genes were obtained from PGDD database. Ka/Ks < 1 means purifying selection; Ka/Ks = 1 means neutral selection; while Ka/Ks > 1 means positive selection57.
Phylogenetic tree, gene structure and conserved motifs
The protein sequences of MtPP2C genes were aligned by ClustalW58 and used for phylogenetic analysis using MEGA6.0659, and an unrooted phylogenetic tree was constructed using the neighbor-joining (NJ) method with the following parameters: Poisson correction, pair-wise deletion, and 1,000 bootstrap replicates.
The exon-intron structures of MtPP2C genes were determined by comparing the coding sequences and the corresponding genomic sequences on the GSDS website (http://gsds2.cbi.pku.edu.cn)60.
The MEME software (Version 4.11.4) was used to identify conserved motifs in MtPP2C protein sequences according to the following parameters: -protein, -oc, -nostatus, -mod zoops, -nmotifs 15, -minw 6, -maxw 5061.
Cis-elements analysis
The 1,500 bp sequences upstream from the initiation codon (ATG) of all MtPP2C genes (Supplementary Data 4) were obtained from Phytozome v12.151. The putative stress and hormone responsive cis-elements in the promoter regions were identified using the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) program. The details of six abiotic stress-responsive and nine hormone-responsive cis-elements investigated in this study were list in Supplementary Table 6.
Expression profiling of the MtPP2C genes in different tissues
The expression profile of MtPP2C genes in eight tissues (root, stem, leaf, vegetative bud, petiole, flower, pod and nodule) were analyzed using M. truncatula microarray data62.
The genome-wide microarray data were obtained from the M. truncatula Gene Expression Atlas (MtGEA) Project website (http://mtgea.noble.org/v2/). The relative expressions were log2 transformed and visualized for heat map using Graphpad prism 7.
Plant materials, growth conditions and abiotic stress treatments
M. truncatula ecotype Jemalong A17 was used in this study. The seeds were first treated with sulfuric acid and washed with sterilized water, then sown in a mixture of peat soil and vermiculite (1:1, V/V). Seedlings were grown at 22–24 °C in a growth chamber with a 16/8 h (day/night) photoperiod until they were used for treatment at eight weeks old. The method of stress treatment is in accordance with Shu’s report29. For cold stress treatment, the seedlings were transferred to the 4 °C incubator. For drought stress treatment, the seedlings growing under normal conditions were watered with 300 mM mannitol solution. For ABA treatment, the seedling leaves were sprayed with 100 μM ABA solution. The seedlings were harvested at 0, 1, 3 and 12 hours after treatment. For each treatment, five randomly chosen whole seedlings were pooled to form a biological replicate. All samples were frozen immediately in liquid nitrogen after harvest and stored at −80 °C until used for RNA extraction.
Expression analysis of MtPP2C genes response to abiotic stress
Total RNA was isolated from all of the samples using the total RNA extraction kit (Tiangen, China). The quality and quantity of RNA was evaluated by agarose gel electrophoresis and Quawell micro volume spectrophotometer (Q5000, USA), respectively. Then, 1 µg of total RNA after DNase I digestion was reverse transcribed into cDNA using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa, Japan).
The cDNA was amplified using LightCycler 480 SYBR Green Master, with a Roche LightCycler 480 Real Time PCR system (Roche, Switzerland). The thermal cycling program was 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 15 s. The melting curves were analyzed at 60–95 °C after 40 cycles. All qRT-PCRs were carried out for three technical replicates. The relative expression levels of MtPP2C genes were calculated according to the method of Livak and Schmittgen63. MtActin (Medtr2g008050) and MtGapdh (Medtr3g085850) were used as reference genes. The primers used in this study were listed in Supplementary Table S6. The relative expressions were log2 transformed and visualized for heat map using Graphpad prism 7.
References
Chae, L., Pandey, G. K., Sheng, L., Yong, H. C. & Kim, K. N. Protein kinases and phosphatases for stress signal transduction in plants. Abiotic Stress Adaptation in Plants, 123–163 (2009).
Singh, A., Pandey, A., Srivastava Ashish, K., Lam-Son Phan, T. & Pandey Girdhar, K. Plant protein phosphatases 2C: from genomic diversity to functional multiplicity and importance in stress management. Critical Reviews in Biotechnology 36, 1023–1035, https://doi.org/10.3109/07388551.2015.1083941 (2016).
Schweighofer, A., Hirt, H. & Meskiene, I. Plant PP2C phosphatases: emerging functions in stress signaling. Trends in Plant Science 9, 236–243 (2004).
Cao, J., Jiang, M., Li, P. & Chu, Z. Genome-wide identification and evolutionary analyses of the PP2C gene family with their expression profiling in response to multiple stresses in Brachypodium distachyon. BMC genomics 17, 175, https://doi.org/10.1186/s12864-016-2526-4 (2016).
Kerk, D., Templeton, G. & Moorhead, G. B. G. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein sata from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiology 146, 351 (2008).
Singh, A., Giri, J., Kapoor, S., Tyagi, A. K. & Pandey, G. K. Protein phosphatase complement in rice: genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC genomics 11, 435, https://doi.org/10.1186/1471-2164-11-435 (2010).
Sugimoto, H. et al. Overexpression of a novel Arabidopsis PP2C isoform, AtPP2CF1, enhances plant biomass production by increasing inflorescence stem growth. Journal of Experimental Botany 65, 5385–5400, https://doi.org/10.1093/jxb/eru297 (2014).
Zhu, J. K. Abiotic stress signaling and responses in plants. Cell 167, 313–324, https://doi.org/10.1016/j.cell.2016.08.029 (2016).
Xiang, Y., Sun, X., Gao, S., Qin, F. & Dai, M. Deletion of an endoplasmic reticulum stress response element in a ZmPP2C-A gene facilitates drought tolerance of maize seedlings. Molecular Plant 10, 456–469 (2017).
Komatsu, K. & Group, A. PP2Cs evolved in land plants as key regulators of intrinsic desiccation tolerance. Nature Communications 4, 375–381 (2011).
Rodriguez, P. L., Leube, M. P. & Grill, E. Molecular cloning in Arabidopsis thaliana of a new protein phosphatase 2C (PP2C) with homology to ABI1 and ABI2. Plant Molecular Biology 38, 879–883 (1998).
Gosti, F. et al. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. The Plant Cell 11, 1897–1909 (1999).
Merlot, S., Gosti, F., Guerrier, D., Vavasseur, A. & Giraudat, J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. The Plant Journal 25, 295–303 (2001).
Sun, L. et al. Transcriptional regulation of SlPYL, SlPP2C, and SlSnRK2 gene families encoding ABA signal core components during tomato fruit development and drought stress. Journal of Experimental Botany 62, 5659–5669, https://doi.org/10.1093/jxb/err252 (2011).
Arshad, M. & Mattsson, J. A putative poplar PP2C-encoding gene negatively regulates drought and abscisic acid responses in transgenic Arabidopsis thaliana. Trees-Structure and Function 28, 531–543, https://doi.org/10.1007/s00468-013-0969-7 (2014).
Zhang, F. et al. Type 2C phosphatase 1 of Artemisia annua L. is a negative regulator of ABA signaling. Biomed Research International, https://doi.org/10.1155/2014/521794 (2014).
Chen, J. et al. A putative PP2C-encoding gene negatively regulates ABA signaling in Populus euphratica. PLOS one 10, e0139466 (2015).
Wang, Y. et al. Transcriptional regulation of PaPYLs, PaPP2Cs and PaSnRK2s during sweet cherry fruit development and in response to abscisic acid and auxin at onset of fruit ripening. Plant Growth Regulation 75, 455–464, https://doi.org/10.1007/s10725-014-0006-x (2015).
Zhang, F. et al. Brachypodium distachyon BdPP2CA6 interacts with BdPYLs and BdSnRK2 and positively regulates salt tolerance in transgenic Arabidopsis. Front Plant Sci 8, 264 (2017).
Singh, A., Jha, S. K., Bagri, J. & Pandey, G. K. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis. Plos One 10, e0125168 (2015).
You, J. et al. A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant physiology 166, 2100–2114 (2014).
Xue, T. et al. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC genomics 9, 550, https://doi.org/10.1186/1471-2164-9-550 (2008).
Kerk, D. et al. The complement of protein phosphatase catalytic subunits encoded in the genome of Arabidopsis. Plant physiology 129, 908–925 (2002).
Kim, S. et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature Genetics 46, 270–278 (2014).
Chen, C. et al. Genome-wide analysis and expression profiling of PP2C clade D under saline and alkali stresses in wild soybean and Arabidopsis. Protoplasma, 1–12 (2017).
Wei, K. & Pan, S. Maize protein phosphatase gene family: identification and molecular characterization. BMC genomics 15, 773 (2014).
Cheung, J. et al. Genome-wide detection of segmental duplications and potential assembly errors in the human genome sequence. Genome Biology 4, R25 (2003).
Young, N. D. et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480, 520–524 (2011).
Zhang, C. et al. Genome-wide analysis of the CCCH zinc finger gene family in Medicago truncatula. Plant Cell Reports 32, 1543–1555 (2013).
Yang, T., Fang, G. Y., He, H. & Chen, J. Genome-wide identification, evolutionary analysis and expression profiles of lateral organ boundaries domain gene family in Lotus japonicus and Medicago truncatula. Plos One 11 (2016).
Song, H. & Nan, Z. Genome-wide identification and analysis of WRKY transcription factors in Medicago truncatula. Hereditas 36, 152–168 (2014).
Shu, Y., Liu, Y., Zhang, J., Song, L. & Guo, C. Genome-wide analysis of the AP2/ERF superfamily genes and their responses to abiotic stress in Medicago truncatula. Frontiers in Plant Science 6, 1247 (2016).
Shu, Y. J., Song, L. L., Zhang, J., Liu, Y. & Guo, C. H. Genome-wide identification and characterization of the Dof gene family in Medicago truncatula. Genetics and Molecular Research 14, 10645–10657, https://doi.org/10.4238/2015.September.9.5 (2015).
Yang, Y. et al. Genome-wide identification, expression analysis of GH3 family genes in Medicago truncatula under stress-related hormones and Sinorhizobium meliloti infection. Applied Microbiology and Biotechnology 99, 841–854 (2015).
Yang, Y. et al. Genome-wide identification of CAMTA gene family members in Medicago truncatula and their expression during root nodule symbiosis and hormone treatments. Frontiers in Plant Science 6, 459 (2015).
Liu, Z. M., Liu, W. X., Jia, X. T., Zhang, Z. S. & Wang, Y. R. Genome-wide analysis of LEA gene family in Medicago truncatula. Pratacultural. Science 32, 382–391 (2015).
Li, W. et al. Bioinformatics analysis of MAPKKK family genes in Medicago truncatula. Genes 7, 13, https://doi.org/10.3390/genes7040013 (2016).
Song, J. et al. The U-box family genes in Medicago truncatula: Key elements in response to salt, cold, and drought stresses. Plos One 12, e0182402 (2017).
Wang, F. & Li, X. Genome-wide characterization and expression analysis of MYB transcription factors in Lotus japonicas and Medicago truncatula. Genes & Genomics 39, 831–842 (2017).
Zheng, X. W., Deng-Xia, Y. I., Shao, L. H. & Cong, L. I. In silico genome-wide identification, phylogeny and expression analysis of the R2R3-MYB gene family in Medicago truncatula. Journal of Integrative Agriculture 16, 1576–1591 (2017).
Song, L., Tao, L., Cui, H., Ling, L. & Guo, C. Genome-wide identification and expression analysis of the GRAS family proteins in Medicago truncatula. Acta Physiologiae Plantarum 39, 93 (2017).
Zhang, H. et al. Genome-wide characterization of GRAS family genes in Medicago truncatula reveals their evolutionary dynamics and functional diversification. Plos One 12, e0185439 (2017).
Fuchs, S., Grill, E., Meskiene, I. & Schweighofer, A. Type 2C protein phosphatases in plants. Febs Journal 280, 681–693, https://doi.org/10.1111/j.1742-4658.2012.08670.x (2013).
Bhaskara, G. B., Nguyen, T. T. & Verslues, P. E. Unique drought resistance functions of the highly ABA-induced Clade A protein phosphatase 2Cs. Plant Physiology 160, 379–395 (2012).
Li, Y. L., Li, Y., Liu, Y., Wu, Y. & Xie, Q. The sHSP22 heat-shock protein requires the ABI1 protein phosphatase to modulate polar auxin transport and downstream responses. Plant Physiology 666, 01206.02017 (2018).
Schweighofer, A. et al. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. The Plant Cell 19, 2213–2224 (2007).
Meskiene, I. et al. MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proceedings of the National Academy of Sciences 95, 1938–1943 (1998).
Choopayak & Chonnanit. Characterization and functional analysis of a novel PP2C phosphatase AP2C2 from Arabidopsis (2008).
Bhaskara, G. B., Wen, T. N., Nguyen, T. T. & Verslues, P. E. Protein phosphatase 2Cs and microtubule-associated stress protein 1 control microtubule stability, plant growth, and drought response. The Plant Cell 29, 169–191 (2017).
Van de Velde, J., Van Bel, M., Vaneechoutte, D. & Vandepoele, K. A collection of conserved noncoding sequences to study gene regulation in flowering plants. Plant Physiology 171, 2586–2598 (2016).
Goodstein, D. M. et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Research 40, 1178–1186 (2011).
Finn, R. D. et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research 44, 279–285 (2016).
Marchlerbauer, A. et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Research 45, 200 (2017).
Paul, H. et al. WoLF PSORT: protein localization predictor. Nucleic Acids Research 35, 585–587 (2007).
Lee, T. H., Tang, H., Wang, X. & Paterson, A. H. PGDD: a database of gene and genome duplication in plants. Nucleic Acids Research 41, D1152 (2012).
Ghosh, A. & Islam, T. Genome-wide analysis and expression profiling of glyoxalase gene families in soybean (Glycine max) indicate their development and abiotic stress specific response. BMC Plant Biology 16, https://doi.org/10.1186/s12870-016-0773-9 (2016).
Lynch, M. & Conery, J. S. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155 (2000).
Tang, Z., Blacquiere, G. & Leus, G. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30, 2725–2729 (2013).
Hu, B. et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297 (2014).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research 37, 202–208 (2009).
Benedito, V. A. et al. A gene expression atlas of the model legume Medicago truncatula. Plant Journal 55, 504–513 (2008).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by grant from National Natural Science Foundation of China (31760696).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: R.W., Y.W. and Q.Y. Collected public datasets: Q.Y. and K.L. Performed experiments: Q.Y., K.L., Q.W. and X.N. Analyzed the data: Q.Y., K.L., Y.W., F. Y. and G.L. Wrote the manuscript: Q.Y. Revised the manuscript: G.L., R.W. and Y.W.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Q., Liu, K., Niu, X. et al. Genome-wide Identification of PP2C Genes and Their Expression Profiling in Response to Drought and Cold Stresses in Medicago truncatula. Sci Rep 8, 12841 (2018). https://doi.org/10.1038/s41598-018-29627-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29627-9
This article is cited by
-
Genome-wide analysis and expression pattern of the ZoPP2C gene family in Zingiber officinale Roscoe
BMC Genomics (2024)
-
Identification of PP2C Genes in Tibetan Hulless Barley (Hordeum vulgare var. nudum) Under Dehydration Stress and Initiatory Expression and Functional Analysis of HvPP2C59
Plant Molecular Biology Reporter (2022)
-
A type 2C protein phosphatase activates high-affinity nitrate uptake by dephosphorylating NRT2.1
Nature Plants (2021)
-
Pervasive duplication, biased molecular evolution and comprehensive functional analysis of the PP2C family in Glycine max
BMC Genomics (2020)
-
Comprehensive genomic analysis of the DUF4228 gene family in land plants and expression profiling of ATDUF4228 under abiotic stresses
BMC Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.