Abstract
Autophagy is a highly conserved process of degradation of cytoplasmic constituents in eukaryotes. It is involved in the growth and development of plants, as well as in biotic and abiotic stress response. Although autophagy-related (ATG) genes have been identified and characterized in many plant species, little is known about this process in Medicago truncatula. In this study, 39 ATGs were identified, and their gene structures and conserved domains were systematically characterized in M. truncatula. Many cis-elements, related to hormone and stress responsiveness, were identified in the promoters of MtATGs. Phylogenetic and interaction network analyses suggested that the function of MtATGs is evolutionarily conserved in Arabidopsis and M. truncatula. The expression of MtATGs, at varied levels, was detected in all examined tissues. In addition, most of the MtATGs were highly induced during seed development and drought stress, which indicates that autophagy plays an important role in seed development and responses to drought stress in M. truncatula. In conclusion, this study gives a comprehensive overview of MtATGs and provides important clues for further functional analysis of autophagy in M. truncatula.
Similar content being viewed by others
Introduction
Autophagy is an evolutionarily conserved degradation process in eukaryotes, which is involved in material and energy homeostasis through recycling of damaged cytoplasmic constituents and unwanted cellular materials1. In Arabidopsis, more than 30 autophagy-related genes (ATGs) have been identified via homology-based cloning using yeast ATGs2,3. They are involved in different stages of autophagosome formation, including phagophore induction, cargo capture, vesicle expansion and closure, and delivery of the vesicles to the vacuole4. ATGs are functionally classified into four core functional groups namely the ATG1 kinase complex, PI3K complex, ATG9 recycling complex, and two ubiquitin-like conjugation systems5. To date, ATGs have been characterized in many plant species including Arabidopsis thaliana, rice (Oryza sativa), maize (Zea mays), tobacco (Nicotiana tabacum), and wheat (Triticum aestivum)6,7,8,9.
Previous studies have indicated that autophagy is broadly involved in the growth and development of plants. It has been reported that autophagy-defective mutants show accelerated leaf senescence in Arabidopsis10. The Osatg7 mutant showed complete sporophytic male sterility and reduced pollen germination activity, which suggests that autophagy plays critical roles in pollen development in rice11. Increasing evidence highlights the crucial role of autophagy in starch and lipid metabolism in plants12,13. Moreover, autophagy, as a quality control mechanism, mediates the degradation of cellular components and contributes to cellular homeostasis, which is necessary for plants to survive various abiotic and biotic stresses, such as nutrient deficiencies and heat, hypoxia, salt, and drought stresses14,15,16,17,18.
Medicago truncatula is a model plant for genetic research on legumes that interact with rhizobia to develop nodules for nitrogen fixation19,20,21. Despite its agronomical importance, the production of M. truncatula is threatened by abiotic stresses including high salt and drought stresses22. To facilitate our understanding of the mechanism and function of autophagy in M. truncatula, it is necessary to first identify all the MtATGs. Based on the complete genome sequence of M. truncatula23,24, herein, we provide a comprehensive description of MtATGs, including their genome-wide identification, characterization, and expression analysis. The results of this study lay the foundation for future research on the molecular mechanism of autophagy in M. truncatula.
Materials and methods
Identification of MtATGs
The identification of putative MtATGs was conducted using a bidirectional BLAST analytical strategy, and was performed using the BLASTP program that is integrated into the BioEdit software. First, the protein sequences of published autophagy-related genes in Arabidopsis were used to search against M. truncatula proteome sequences (MedtrA17_4.0) with the E-value cutoff at 1 × e−5. Then, all output M. truncatula protein sequences were aligned back to Arabidopsis proteome sequences. Only the M. truncatula genes that shared the highest similarities to the AtATGs in the second BLAST analysis were considered putative MtATGs. To further verify that the candidate genes are indeed MtATGs, the protein domain architectures were analyzed in the Pfam database (http://pfam.xfam.org)25. The chemical features of the MtATG proteins, including their molecular weights and theoretical isoelectric points, were obtained using the online tool ExPASy (http://web.expasy.org/compute_pi/). Subcellular localization of MtATGs was predicted using the CELLO system (http://cello.life.nctu.edu.tw). The gene and protein structures of MtATGs were extracted from the annotation file of the M. truncatula genome (MedtrA17_4.0) and visualized with the integrating bioinformatic analysis toolkit Tbtools26.
Chromosomal location and gene duplication analysis
MtATGs were mapped to the chromosomes based on their physical positions in the M. truncatula genome (MedtrA17_4.0). To investigate the synteny of related genome regions in M. truncatula, putative orthologous genes were identified using the BLASTP program, and the results were used to generate a synteny map with the MCScanX program27. The genome locations of MtATGs and the duplicated gene pairs were visualized using Tbtools26.
Protein sequence alignment and analysis of the phylogenetic relationship
The phylogenetic analysis of MtATGs was performed using the MEGA7 software28. The amino acid sequences of MtATGs and AtATGs in different gene families were aligned independently using the ClustalW algorithm with the default parameters. An unrooted phylogenetic tree was constructed with the neighbor-joining statistical method, and the following parameters were used: uniform rates are used as rates among sites, gaps/missing data are treated as pairwise deletion, and the bootstrap analysis was performed with 1000 replicates to obtain a support value for each branch.
Identification of cis-elements
The 1.5 kb genomic DNA sequence upstream of the initiation codon of each MtATG was retrieved from the M. truncatula genome (MedtrA17_4.0). The assumed cis-elements of MtATGs were predicted using the PlantCARE web servers (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)29.
Construction of the protein–protein interaction (PPI) network
The PPI networks were constructed using the STRING database (http://www.string-db.org). A total of 39 MtATGs were selected as input, and the PPI network of the MtATGs was constructed with a medium confidence (0.4).
Analysis of the expression profiles using microarray data
The M. truncatula microarray data were downloaded from the MtGEA v3 database (https://mtgea.noble.org/v3/)30. Expression values were normalized using the z-score method, and plotted using GraphPad Prism 8.
Plant materials and growth conditions
Medicago truncatula (cv. Jemalong A17) seeds were scarified with sulfuric acid, and vernalized on wetted filter paper at 4 °C for 7 days. Seedlings were grown in a greenhouse at 24 °C, 16-h light/8-h dark cycle, with humidity ranging from 60 to 80%. Different plant tissues (roots, stems, leaves, petioles, buds, flowers, and pods) were harvested from multiple plants. Material for the seed developmental was collected from pods at 5 different stages. For drought stress, 7-day-old seedlings were treated by withholding watering for 2 days. For mannitol treatment, 2-weeks-old seedlings were transferred to liquid 1/2 MS medium supplemented with 300 mM mannitol for additional 2 days. All plant samples were frozen immediately in liquid nitrogen after harvest and stored at − 80 °C until use. Plant material collections in this study complied with relevant institutional, national, and international guidelines and legislation.
RNA isolation and quantitative PCR (qPCR) analysis
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The isolated RNA was reverse transcribed using ReverTra Ace qPCR RT Master Mix with gDNA Remover Kit (TOYOBO). qPCR was performed using the CFX Connect Real-Time PCR System (Bio-Rad) with the SYBR Premix ExTaq Mix (Takara). MtACTIN (Medtr2g008050) was used as a reference gene. Three technical replicates were used for each reaction. The gene-specific primers for the qPCR analysis are listed in Supplementary Table S4.
Protein blotting analysis
Western blotting analysis of ATG8 lipidation was performed as previously described31. 2-weeks-old seedlings were ground in liquid nitrogen and homogenized in ice-cold RIPA buffer (50 mM Tris–HCl pH8.0, 150 mM NaCl, 1% NP-40, 0.5% Sodium Deoxycholate, 0.5% PvPP, 0.1% SDS). After centrifuged for 15 min at 12,000g, the supernatant fraction was transferred to a new microcentrifuge tube, and electrophoresis with 15% SDS-PAGE supplemented with 6 M Urea. Anti-ATG8a antibodies (ab77003, Abcam) were used in the immunoblotting analysis.
Monodansylcadaverine (MDC) staining and microscopy
MDC staining was performed as previously described32. Briefly, lateral roots of M. truncatula were detached and stained with 0.75 mM MDC for 1 h. The root cells were observed using LSM 780 inverted microscope (Carl Zeiss) with a DAPI-specific filter.
Results
Genome-wide identification of ATGs in M. truncatula
To identify MtATGs, the BLASTP algorithm was employed in searches against M. truncatula proteome sequences (MedtrA17_4.0) using the amino acid sequences of A. thaliana ATGs (AtATGs) as queries. A total of 39 MtATGs were identified in M. truncatula (Table 1, Supplementary Tables S1, S2). The lengths of the MtATGs ranged from 62 amino acids to 3768 amino acids. Most of the MtATGs (MtATG2, MtATG3, MtATG4, MtATG5, MtATG6, MtATG7, MtATG10, MtATG11, MtATG12, MtATG101, MtVPS15, and MtVPS34) contained a single member. A few of them (MtATG1, MtATG8, MtATG9, MtATG13, MtATG16, and MtATG18) contained multiple members, ranging from two to eight in different groups (three in the MtATG1 family, eight in the MtATG8 family, two in the MtATG9 family, three in the MtATG13 family, three in the MtATG16 family, and eight in the MtATG18 family) (Table 1).
The chromosomal distribution of MtATGs determined using the TBtools software is shown in Fig. 1. In total, 38 MtATGs were found to be distributed across all eight chromosomes except for MtATG7, which could not be mapped to any chromosome according to data from MedtrA17_4.0 (Fig. 1). The number of MtATGs located on each chromosome varies dramatically. Chromosome 4 (Chr4) contains the maximum number (11) of MtATGs, whereas chromosome 6 has only one MtATG gene. Gene duplication is important for adaptation of plants to adverse and complex environments. In M. truncatula, 7 pairs of MtATGs were predicted to be segmentally duplicated. As shown in Fig. 1, these 7 pairs of duplicated MtATGs (MtATG8c and MtATG8d, MtATG8g and MtATG8f., MtATG9a and MtATG9b, MtATG13b and MtATG13c, MtATG16a and MtATG16b, MtATG18a and MtATG18c, MtATG18d and MtATG18c) are distributed across chromosomes 1, 2, 3, 4, 7, and 8. These duplications may have led to the expansion of MtATG families in M. truncatula.
The subcellular localization of the MtATGs was predicted using the CELLO system (http://cello.life.nctu.edu.tw). Most of the MtATGs were predicted to localize to the nucleus, plasma membrane, and cytoplasm, followed by extracellular space, chloroplast, and mitochondria (Table 1, Supplementary Figure S1). Furthermore, some MtATG families exhibited different subcellular localization. For example, MtATG8 proteins were predicted to be mainly cytoplasmic or nuclear, but were also found to localize to the mitochondria and extracellular space (Table 1). The prediction was the same for MtATG18 family members, which were localized to both the plasma membrane and nucleus (Table 1). Taken together, the diverse subcellular localization of MtATGs implies that they have distinct functions.
Phylogenetic analysis of MtATGs
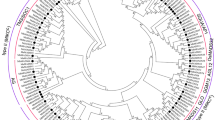
To evaluate the evolutionary relationships of MtATGs, we conducted a phylogenetic analysis using the amino acid sequences of the multi-member subfamilies (MtATG1, MtATG8, MtATG9, MtATG13, MtATG16, and MtATG18) and their orthologs from Arabidopsis. As shown in Fig. 2, members of the MtATG1 and MtATG13 families were clustered in two branches (Fig. 2A,B). There are two ATG9s and three ATG16s in M. truncatula, whereas only one ATG9 and ATG16 in Arabidopsis (Fig. 2C,D). ATG8 plays a central role in autophagy by promoting autophagosome formation and cargo recruitment. As in Arabidopsis, eight MtATG8 members were clustered into two distinct groups in M. truncatula: MtATG8a, MtATG8b, MtATG8c, MtATG8d, and MtATG8e were grouped into clade I, whereas MtATG8f, MtATG8g, and MtATG8h were clustered in clade II (Fig. 2E). MtATG8 proteins showed high identity with ATG8 proteins from Arabidopsis, except for MtATG8h, in which half of the amino acids from the N-terminus were absent (Supplementary Figure S2). The C-terminal glycine residue in ATG8, which is exposed upon protease cleavage by ATG4, is essential for the conjugation of ATG8 to phosphatidylethanolamine33. However, MtATG8b did not contain the C-terminal glycine residue. This result indicates that MtATG8b might function in other biological processes independent of autophagy. In addition, one MtATG8 member of clade II, MtATG8f, had a C-terminal extension after the Gly residue, whereas the AtATG8 members of clade II lack the C-terminal extension (Supplementary Figure S2). Eight MtATG18 members were also clustered in two branches like the MtATG8 family members (Fig. 2F). Clade I of MtATG18 family consisted of MtATG18a, MtATG18b, MtATG18c MtATG18d, and MtATG18e, whereas clade II was made up of MtATG18f, MtATG18g, and MtATG18h (Fig. 2F).
Phylogenetic analysis of ATGs from Medicago truncatula and Arabidopsis thaliana. Phylogenetic tree of ATG1 (A), ATG13 (B), ATG9 (C), ATG16 (D), ATG8 (E), and ATG18 (F) families in M. truncatula and Arabidopsis. The unrooted tree was constructed using MEGA7 based on the multiple sequence alignment of the ATG protein sequences by the neighbor-joining (NJ) method. The number at each node represents the bootstrap value from 1000 replicates.
Analyses of gene structures and distribution of conserved domains
Gene structure is closely related to the expression pattern and function divergence of members of multigene families. Gene structure analysis revealed that all the MtATGs contain introns, with the number of exons ranging from 2 to 17 (Fig. 3A). In addition, similar exon–intron patterns and the same number of exons were observed in some ATG subfamilies, such as MtATG1a/b, MtATG8a/c/d/e/f/g, MtATG13a/b/c, MtATG18a/c/d/e, and MtATG18g/h (Fig. 3A). The similar gene structures suggest functional redundancy among these genes. However, differences in exon–intron patterns and exon numbers were also seen within some subfamilies, such as MtATG1t, MtATG8b/h, and MtATG18b/f (Fig. 3A).
Gene structure and conserved domains of MtATGs. (A) Gene structure of MtATGs is illustrated according to M. truncatula genome annotation (MedtrA17_4.0), and the lengths of the exons and introns of each MtATG are exhibited proportionally. MtATGs are grouped based on their biological function in the autophagy pathway. (B) The domain architectures were predicted using the Pfam database, and protein lengths of the MtATGs were acquired from the M. truncatula genome annotation (MedtrA17_4.0). The black box represents the WD40 domain.
The conserved domains of MtATGs were detected using the Pfam database25. In general, the composition of the conserved domains in MtATGs is comparable to that in Arabidopsis. Furthermore, members of the same MtATG families have similar domains. For example, all three MtATG1 proteins contain a protein kinase domain (Pkinase) at their N-terminus (Fig. 3B). In addition, almost all MtATG8 proteins (except MtATG8h) are similar in length and have identical ATG8 domains (Fig. 3). A similar phenomenon was also observed in the MtATG9 and MtATG13 subfamilies. However, exceptions were also found in the MtATG16 and MtATG18 subfamilies. All the MtATG16 family proteins have a C-terminal WD40 domain, but lack an N-terminal ATG16 domain in MtATG16c (Fig. 3B). MtATG18 proteins contain the WD40 domain except for MtATG18b and MtATG18h, but members of clade II (MtATG18f/g/h) have a C-terminal BCAS3 domain that is absent in members of clade I (Fig. 3B). The differences in the gene structure and conserved domains may be related to functional divergence among the different gene products within some MtATG families.
Analysis of cis-elements in the promoter regions of MtATGs
Cis-elements regulate genes through interactions with their corresponding transcription factors. To further understand the gene regulation network of MtATGs, cis-elements were identified using the online tool PlantCARE29. Ninety-two putative cis-elements were found among MtATG promoters (Supplementary Table S3). Among these, the TATA-box and CAAT-box are the most common cis-elements. Many of the identified cis-elements, such as ABRE (abscisic acid-related), TCA-element (salicylic acid-related), TCCACCT-motif and TGACG-motif (MeJA-related), TGA-element (auxin-related), TATC-box, and P-box and GARE-motif (gibberellin-related), are involved in hormone responsiveness (Fig. 4). Among these, cis-elements that respond to MeJA and ABA were found to be the most abundant. In addition, some stress-related elements are mainly related to anaerobic (ARE), defense (STRE and TC-rich repeats), drought (MBS), low temperature (LTR), and wound (WUN-motif) stresses (Fig. 4). The diversity of cis-elements in the promoter regions of MtATGs provided evidence for their potential biological functions in response to phytohormone, abiotic and biotic stresses.
Analysis of the protein–protein interaction network of MtATGs
To investigate the protein–protein interaction (PPI) between MtATGs, all the 39 MtATGs were submitted to the STRING (Search Tool for the Retrieval of Interacting Genes database) website. Twenty-two MtATGs were found to form a complex interaction network that can be divided into four major modules according to the functional classification in Arabidopsis (Fig. 5). In the first module, MtATG1a, MtATG11, MtATG101, and three MtATG13 members (MtATG13a, MtATG13b, MtATG13c) interact with each other and function as the ATG1 kinase complex. The second module consists of two members of the PI3K complex, MtATG6 and MtVPS34. MtATG2 and six MtATG18 family members (MtATG18a, MtATG18b, MtATG18c, MtATG18f, MtATG18g, and MtATG18h), making up the third module, play a role in autophagic membrane recruitment. The last module, composed of MtATG4, MtATG5, MtATG12, and four MtATG8 members (MtATG8a, MtATG8d, MtATG8f, and MtATG8g), serves as the ubiquitin-like conjugation system. This interaction pattern of MtATGs is similar to that of Arabidopsis, suggesting that ATGs are possibly evolutionarily conserved in Arabidopsis and M. truncatula.
Expression patterns of MtATGs in different tissues and during seed development
To investigate the possible roles of MtATGs in the growth and development of plants, the expression patterns of their genes in different tissues and during different stages of seed development were determined34. All the MtATGs were expressed in the tested tissues, indicating that autophagy is critical for growth and development of plants (Fig. 6A). However, MtATGs showed significantly distinct tissue-specific expression patterns in different tissues. Specifically, the expression levels of many MtATGs, such as MtATG4, MtATG8b, MtATG8g, MtATG9a, MtATG13a, MtATG13c, MtATG18b, MtATG18c, MtATG18e, MtATG18h, MtATG101, VPS15, and VPS34, were significantly higher in roots than in other tissues (Fig. 6A). In addition, some MtATGs (MtATG1a, MtATG1t, MtATG2, MtATG7, MtATG9b, MtATG10, and MtATG18f) were highly expressed in leaves, whereas others (MtATG3, MtATG8a, MtATG8e, MtATG8f, and MtATG11) were highly expressed in flowers (Fig. 6A). The results revealed that different MtATGs might function in different tissues. To validate the results of the microarray data, the expressional profiles of several MtATGs (MtATG1a, MtATG2, MtATG4, MtATG5, MtATG8a, and MtATG18b) were inspected by qPCR. Most of the selected genes were highly expressed in roots, which was very similar to those of microarray analysis (Fig. 6B).
Expression patterns of MtATGs in different tissues. (A) Expression patterns of MtATGs in different tissues. Roots, stems, leaves, petioles, and shoot buds were harvested from multiple M. truncatula plants at 28 days after planting, and fully opened flowers and pods (2.5–9.0 mm in length) were collected. (B) qRT-PCR validation of MtATGs expression in different tissues. MtACTIN was used as a reference gene. Error bars represent SD of three independent experiments. Significant differences were indicated with an asterisks (*), P < 0.05.
Consistent with previous studies, most of the MtATGs were upregulated during seed development (Fig. 7A). In particular, MtATG2, MtATG3, MtATG4, MtATG5, MtATG6, MtATG13a, and MtATG18b, were highly expressed in the late stage of seed development (Fig. 7A). In contrast, a few MtATGs, including MtATG7 and MtATG8b, were downregulated after pollination (Fig. 7A). To validate the results of the microarray data, seeds were collected from pods at 5 different stages of seed developmental (Fig. 7B). As shown in Fig. 7C, the expression levels of five selected genes (MtATG2, MtATG4, MtATG5, MtATG8a, and MtATG18b) were considerably increased, only MtATG4 showed no gene expression change during seed development. These results were very similar to those of microarray analysis, and indicate that autophagy is essential for seed development in M. truncatula.
Expression patterns of MtATGs during seed development. (A) Gene expression of MtATGs from microarray data during seed development. Seeds were excised form pods at 10, 12, 16, 20, 24, and 36 days after pollination (DAP). Scale bar represents the relative expression value after z-score normalization. (B) Different stages of seed development stages in M. truncatula. (C) qRT-PCR validation of MtATGs expression during seed development. MtACTIN was used as a reference gene. Error bars represent SD of three independent experiments. Significant differences were indicated with an asterisks (*), P < 0.05.
Expression of MtATGs in response to drought stress
To investigate the putative roles of autophagy in the response of M. truncatula to drought stress, the expression profiles of MtATGs were analyzed using microarray data from the MtGEA database35,36. Generally, most MtATGs were upregulated after drought treatment (Fig. 8A). Specifically, 26 of 34 MtATGs (e.g., MtATG1t, MtATG8d, MtATG9a, and MtATG18b) were continuously upregulated when plants were subjected to drought stress by withholding watering, and the transcripts of MtATGs rapidly dropped to their basal levels after resuming the watering (Fig. 8A). Interestingly, MtATG8g showed an opposite trend: the expression level of MtATG8g dramatically decreased under drought stress compared with other MtATGs (Fig. 8A). To validate the results of the microarray data, six genes (MtATG1a, MtATG2, MtATG4, MtATG5, MtATG8a, and MtATG18b) were selected for independent validation by qPCR. The expression levels of most of the selected genes were significantly higher after 2 days of drought treatment (Fig. 8B). To examine autophagy activity under drought stress, antibodies against ATG8a were used to detect ATG8 protein by western blotting. ATG8 proteins are lipidated with phosphatidylethanolamine (PE) to promote autophagosome formation in response to drought treatment, whereas no changes in the level of ATG8-PE were detected under control condition (Fig. 8C). Furthermore, MDC staining showed that the number of autophagosomes was significantly increased after drought treatment (Fig. 8D). These results suggested that autophagy might play a crucial role in M. truncatula response to drought stress.
The expression levels of MtATGs under drought stress. (A) Gene expression of MtATGs from microarray data under drought stress. For drought stress treatment, soil-grown plants were subjected to drought stress by withholding watering (Drought) for 14 days, followed by rewatering. Scale bar represents the fold change (log2 value) relative to the corresponding control. (B) qRT-PCR validation of MtATGs expression under drought stress. For drought stress treatment, 7-day-old seedlings were subjected to drought stress by withholding watering for 2 days. MtACTIN was used as a reference gene. Error bars represent SD of three independent experiments. Significant differences were indicated with an asterisks (*), P < 0.05. (C) Analysis of ATG8 lipidation by western blot. Two week seedlings were transferred to liquid 1/2 MS medium with or without 300 mM mannitol, and whole seedlings were collected at 0, 1, and 2 day after treatment. The anti-ATG8a antibodies were used for immunoblotting. (D) MDC staining of root cells with or without drought treatment. Two-wk-old seedlings were transferred to liquid 1/2 MS medium with or without 300 mM mannitol for 2 days followed by staining with MDC. The labeled autophagosomes (arrows) were visualized by epifluorescence microscopy. Scale bar: 50 μm.
Discussion
In this study, 39 ATGs were identified in M. truncatula. These ATGs are similar to orthologous genes in Arabidopsis. For example, phylogenetic analysis revealed that ATG families in M. truncatula are very similar to those in Arabidopsis. In addition, the PPI network analysis shows that the interaction pattern of MtATGs is also similar to that of ATGs in Arabidopsis. These results indicate that the autophagy pathway is highly conserved across different plant species. However, the number of members in some ATG families differs among plant species. For example, the ATG8 family contains eight genes in M. truncatula, but nine in Arabidopsis, seven in rice, and thirteen in wheat6,9,37. In addition, the gene structure and conserved domains of some MtATG families, such as MtATG16 and MtATG18 subfamilies, also differ from those of other plants. Furthermore, different types of cis-elements were identified in the promoters of MtATGs in the same gene family. These results suggest that M. truncatula may have species-specific autophagy mechanism. Hence, it is necessary to illustrate the conserved and specific functions of MtATGs in future studies.
Autophagy has been shown to play crucial roles in the growth and development of plants4. In this study, we found that all ATGs were expressed in the tested tissues of M. truncatula, but their expression levels varied among different tissues. The tissue-specific expression of MtATGs suggests that different functions are required in different tissues. Seed development consists of embryo morphogenesis and seed maturation38. In rice, autophagy has been shown to be involved in the regulation of starch and sugar metabolism during seed maturation39. In Norway spruce (Picea abies), autophagy is also involved in embryogenesis in which it regulates vacuolar cell death of the embryo suspensor40. Furthermore, autophagy plays an important role in microspore embryogenesis in Brassica napus41. The seed weight in autophagy-defective mutants of Arabidopsis and maize was reported to be lower than in the wild-type plants7,42. In the present study, we found that most of the MtATGs were induced during seed development and were highly expressed at the late stage of seed development, which indicates that autophagy is necessary for seed development in M. truncatula. Overall, autophagy plays crucial roles in the growth and development of plants through a pathway that is conserved across different species.
Autophagy has been demonstrated to promote plant survival by maintaining cellular homeostasis under drought stress43,44. In A. thaliana, the transcriptional level of ATG18a was rapidly upregulated by mannitol treatment45. In O. sativa, the expression levels of OsATG6 genes were also induced by drought stress46. Moreover, ATG genes were upregulated by drought stress in many other plant species, such as barley47, pepper48, apple49, and banana50. Besides changes in gene expression, the Arabidopsis autophagy-defective mutants (atg5, atg7, and RNAi-ATG18a) showed more sensitivity to drought treatment than the wild type45,51. Inhibition of autophagy by 3-MA or knockdown of ATG6 sensitized wheat seedlings to drought stress52. Furthermore, virus-induced gene silencing of ATG8d or ATG18h significantly reduced drought tolerance in tomato53. However, overexpression of MdATG5 or MdATG18a enhanced tolerance to drought stress in apple trees54,55. In addition, overexpression of SiATG8a from foxtail millet improved drought tolerance in Arabidopsis56. Recently, it was reported that autophagy improves drought tolerance in M. truncatula through degradation of the aquaporin MtPIP2;7, which interacts with the cargo receptor MtCAS3156. Consistent with previous studies, our results reveal that the promoter of many MtATGs contain the drought-related MBS cis-element. Furthermore, the transcriptional levels of most of the MtATG genes, especially those of the MtATG8 family, significantly increased after drought treatment. The lipidation of ATG8 protein and accumulation of autophagosome are enhanced in M. truncatula during drought stress. Our findings indicate that autophagy is is largely induced by drought stress in M. truncatula, and can be considered an adaptive response under drought stress.
Conclusion
This study provided comprehensive analysis of ATGs in M. truncatula. In total, 39 ATGs were identified in M. truncatula. Members of the same ATG family showed similar gene structure and conserved domains. Analysis of cis-elements implied that MtATGs have potential biological functions in response to phytohormone, abiotic and biotic stresses. Phylogenetic and interaction network analyses suggested that the function of MtATGs is evolutionarily conserved in Arabidopsis and M. truncatula. The expression pattern of MtATGs indicates that autophagy possibly participates in seed development and plays an important role in plant responses to drought stress. In conclusion, this study gives a detailed overview of MtATGs and their expression patterns. The results obtained in this study provide useful information for further functional characterization of autophagy in M. truncatula.
References
Xia, T. et al. Heterologous expression of ATG8c from soybean confers tolerance to nitrogen deficiency and increases yield in Arabidopsis. PLoS ONE 7, e37217. https://doi.org/10.1371/journal.pone.0037217 (2012).
Thompson, A. R. & Vierstra, R. D. Autophagic recycling: Lessons from yeast help define the process in plants. Curr. Opin. Plant Biol. 8, 165–173. https://doi.org/10.1016/j.pbi.2005.01.013 (2005).
Yoshimoto, K., Takano, Y. & Sakai, Y. Autophagy in plants and phytopathogens. FEBS Lett. 584, 1350–1358. https://doi.org/10.1016/j.febslet.2010.01.007 (2010).
Liu, Y. & Bassham, D. C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 63, 215–237. https://doi.org/10.1146/annurev-arplant-042811-105441 (2012).
Li, F. & Vierstra, R. D. Autophagy: A multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 17, 526–537. https://doi.org/10.1016/j.tplants.2012.05.006 (2012).
Xia, K. et al. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L). DNA Res. 18, 363–377. https://doi.org/10.1093/dnares/dsr024 (2011).
Li, F. et al. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell 27, 1389–1408. https://doi.org/10.1105/tpc.15.00158 (2015).
Zhou, X. M. et al. A comprehensive, genome-wide analysis of autophagy-related genes identified in tobacco suggests a central role of autophagy in plant response to various environmental cues. DNA Res. 22, 245–257. https://doi.org/10.1093/dnares/dsv012 (2015).
Yue, W. et al. Genome-wide sequence and expressional analysis of autophagy gene family in bread wheat (Triticum aestivum L.). J. Plant Physiol. 229, 7–21. https://doi.org/10.1016/j.jplph.2018.06.012 (2018).
Hanaoka, H. et al. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 129, 1181–1193. https://doi.org/10.1104/pp.011024 (2002).
Kurusu, T. et al. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 10, 878–888. https://doi.org/10.4161/auto.28279 (2014).
Wang, Y. et al. Autophagy contributes to leaf starch degradation. Plant Cell 25, 1383–1399. https://doi.org/10.1105/tpc.112.108993 (2013).
Farquharson, K. L. Autophagy contributes to plant lipid homeostasis. Plant Cell 31, 1427–1428. https://doi.org/10.1105/tpc.19.00306 (2019).
Huang, X. et al. Genetic analyses of the Arabidopsis ATG1 kinase complex reveal both kinase-dependent and independent autophagic routes during fixed-carbon starvation. Plant Cell 31, 2973–2995. https://doi.org/10.1105/tpc.19.00066 (2019).
Suttangkakul, A., Li, F., Chung, T. & Vierstra, R. D. The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 23, 3761–3779. https://doi.org/10.1105/tpc.111.090993 (2011).
Chung, T., Phillips, A. R. & Vierstra, R. D. ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci. Plant J. 62, 483–493. https://doi.org/10.1111/j.1365-313X.2010.04166.x (2010).
Thompson, A. R., Doelling, J. H., Suttangkakul, A. & Vierstra, R. D. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138, 2097–2110. https://doi.org/10.1104/pp.105.060673 (2005).
Chen, L. et al. Autophagy contributes to regulation of the hypoxia response during submergence in Arabidopsis thaliana. Autophagy 11, 2233–2246. https://doi.org/10.1080/15548627.2015.1112483 (2015).
van Noorden, G. E. et al. Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol. 144, 1115–1131. https://doi.org/10.1104/pp.107.099978 (2007).
Nallu, S. et al. Regulatory patterns of a large family of defensin-like genes expressed in nodules of Medicago truncatula. PLoS ONE 8, e60355. https://doi.org/10.1371/journal.pone.0060355 (2013).
Young, N. D. & Udvardi, M. Translating Medicago truncatula genomics to crop legumes. Curr. Opin. Plant Biol. 12, 193–201. https://doi.org/10.1016/j.pbi.2008.11.005 (2009).
de Lorenzo, L. et al. A novel plant leucine-rich repeat receptor kinase regulates the response of Medicago truncatula roots to salt stress. Plant Cell 21, 668–680. https://doi.org/10.1105/tpc.108.059576 (2009).
Tang, H. et al. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genomics 15, 312. https://doi.org/10.1186/1471-2164-15-312 (2014).
Pecrix, Y. et al. Whole-genome landscape of Medicago truncatula symbiotic genes. Nat. Plants 4, 1017–1025. https://doi.org/10.1038/s41477-018-0286-7 (2018).
El-Gebali, S. et al. The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427-d432. https://doi.org/10.1093/nar/gky995 (2019).
Chen, C. et al. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009 (2020).
Wang, Y. et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49. https://doi.org/10.1093/nar/gkr1293 (2012).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. https://doi.org/10.1093/nar/30.1.325 (2002).
He, J. et al. The Medicago truncatula gene expression atlas web server. BMC Bioinform. 10, 441. https://doi.org/10.1186/1471-2105-10-441 (2009).
Li, F. Q., Chung, T. & Vierstra, R. D. AUTOPHAGY-RELATED11 plays a critical role in general autophagy- and senescence-induced mitophagy in Arabidopsis. Plant Cell 26, 788–807. https://doi.org/10.1105/tpc.113.120014 (2014).
Shin, J. H., Yoshimoto, K., Ohsumi, Y., Jeon, J. S. & An, G. OsATG10b, an autophagosome component, is needed for cell survival against oxidative stresses in rice. Mol. Cells 27, 67–74. https://doi.org/10.1007/s10059-009-0006-2 (2009).
Klionsky, D. J. & Schulman, B. A. Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. Nat. Struct. Mol. Biol. 21, 336–345. https://doi.org/10.1038/nsmb.2787 (2014).
Benedito, V. A. et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 55, 504–513. https://doi.org/10.1111/j.1365-313X.2008.03519.x (2008).
Zhang, J. Y. et al. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 37, 2553–2576. https://doi.org/10.1111/pce.12328 (2014).
Li, D., Su, Z., Dong, J. & Wang, T. An expression database for roots of the model legume Medicago truncatula under salt stress. BMC Genomics 10, 517. https://doi.org/10.1186/1471-2164-10-517 (2009).
Doelling, J. H., Walker, J. M., Friedman, E. M., Thompson, A. R. & Vierstra, R. D. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 277, 33105–33114. https://doi.org/10.1074/jbc.M204630200 (2002).
Niu, D. & He, Y. LEAFY COTYLEDONs: Old genes with new roles beyond seed development. F1000Research https://doi.org/10.12688/f1000research.21180.1 (2019).
Sera, Y. et al. Essential roles of autophagy in metabolic regulation in endosperm development during rice seed maturation. Sci. Rep. 9, 18544. https://doi.org/10.1038/s41598-019-54361-1 (2019).
Minina, E. A. et al. Autophagy and metacaspase determine the mode of cell death in plants. J. Cell Biol. 203, 917–927. https://doi.org/10.1083/jcb.201307082 (2013).
Corral-Martínez, P., Parra-Vega, V. & Seguí-Simarro, J. M. Novel features of Brassica napus embryogenic microspores revealed by high pressure freezing and freeze substitution: evidence for massive autophagy and excretion-based cytoplasmic cleaning. J. Exp. Bot. 64, 3061–3075. https://doi.org/10.1093/jxb/ert151 (2013).
Guiboileau, A. et al. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol. 194, 732–740. https://doi.org/10.1111/j.1469-8137.2012.04084.x (2012).
Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 42, 1045–1053. https://doi.org/10.1111/pce.13404 (2019).
Tang, J. & Bassham, D. C. Autophagy during drought: function, regulation, and potential application. Plant J. https://doi.org/10.1111/tpj.15481 (2021).
Liu, Y. M., Xiong, Y. & Bassham, D. C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5, 954–963. https://doi.org/10.4161/auto.5.7.9290 (2009).
Rana, R. M., Dong, S., Ali, Z., Huang, J. & Zhang, H. S. Regulation of ATG6/Beclin-1 homologs by abiotic stresses and hormones in rice (Oryza sativa L.). Genet. Mol. Res. 11, 3676–3687. https://doi.org/10.4238/2012.August.17.3 (2012).
Zeng, X. W. et al. A barley homolog of yeast ATG6 is involved in multiple abiotic stress responses and stress resistance regulation. Plant Physiol. Biochem. 115, 97–106. https://doi.org/10.1016/j.plaphy.2017.03.013 (2017).
Zhai, Y. et al. Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 7, 131. https://doi.org/10.3389/fpls.2016.00131 (2016).
Wang, P., Sun, X., Jia, X. & Ma, F. Apple autophagy-related protein MdATG3s afford tolerance to multiple abiotic stresses. Plant Sci. 256, 53–64. https://doi.org/10.1016/j.plantsci.2016.12.003 (2017).
Li, B., Liu, G., Wang, Y., Wei, Y. & Shi, H. Overexpression of banana ATG8f modulates drought stress resistance in Arabidopsis. Biomolecules 9, 814. https://doi.org/10.3390/biom9120814 (2019).
Zhou, J. et al. NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 9, e1003196. https://doi.org/10.1371/journal.pgen.1003196 (2013).
Li, Y. B. et al. Autophagic survival precedes programmed cell death in wheat seedlings exposed to drought stress. Int. J. Mol. Sci. 20, 57777. https://doi.org/10.3390/ijms20225777 (2019).
Zhu, T. et al. Mitochondrial alternative oxidase-dependent autophagy involved in ethylene-mediated drought tolerance in Solanum lycopersicum. Plant Biotechnol. J. 16, 2063–2076. https://doi.org/10.1111/pbi.12939 (2018).
Sun, X. et al. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 16, 545–557. https://doi.org/10.1111/pbi.12794 (2018).
Jia, X. et al. MdATG5a induces drought tolerance by improving the antioxidant defenses and promoting starch degradation in apple. Plant Sci. 312, 111052. https://doi.org/10.1016/j.plantsci.2021.111052 (2021).
Li, X. et al. Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 16, 862–877. https://doi.org/10.1080/15548627.2019.1643656 (2020).
Acknowledgements
This work was supported by Natural Science Foundation of Guangdong Province (2021A1515012148), and National Natural Science Foundation of China (31700236).
Author information
Authors and Affiliations
Contributions
L.C. conceived and designed the study. M.Y., L.W., and C.C. performed bioinformatics analysis. X.G. and C.L. prepared all the figures and tables. M.Y., W.H., and L.C. wrote the paper. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, M., Wang, L., Chen, C. et al. Genome-wide analysis of autophagy-related genes in Medicago truncatula highlights their roles in seed development and response to drought stress. Sci Rep 11, 22933 (2021). https://doi.org/10.1038/s41598-021-02239-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02239-6
This article is cited by
-
Bioinformatics and expression analysis of the NRL gene family in Populus trichocarpa
Genetic Resources and Crop Evolution (2024)
-
Systematic analysis and expression of Gossypium ATG8 family reveals the roles of GhATG8f responding to salt stress in cotton
Plant Cell Reports (2024)
-
The role of forkhead-associated (FHA)-domain proteins in plant biology
Plant Molecular Biology (2023)
-
De novo transcriptome assembly and analysis of gene expression in different tissues of moth bean (Vigna aconitifolia) (Jacq.) Marechal
BMC Plant Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.