Abstract
Ubiquitin-conjugating (UBC) E2 enzyme plays crucial roles in plant growth and development. Limited information can describe the function of UBC enzyme E2 in grapes. A total of 43 UBC enzyme E2 genes with conserved UBC domain were identified in grapes. These genes were divided into five groups based on phylogenetic tree with tomatoes. Sequence analyses indicated that VvUBCs in the same group possessed similar gene structures and conserved motifs. Gene distribution in chromosomes was uneven, and gene duplication existed in 36 VvUBCs. Transcriptome and qRT-PCR analysis indicated that most VvUBCs are involved in ripening and post-harvest stage, and feature functional roles in grape organs. According to the transcriptome and qRT-PCR results, seven and six VvUBCs in grape responded to cold and heat stress, respectively, whereas no remarkable VvUBCs change was noted under salt or water-deficit stress. This study provides new insights to physiological and developmental roles of these enzymes and regulation mechanism of E2 genes in grapes.
Similar content being viewed by others
Introduction
Ubiquitination is an important type of post-translational modification of proteins among all eukaryotes. This important process regulates a wide range of biological processes1, including intracellular translocation of proteins, chromosomal organization, DNA repair, cell cycle control, and apoptosis2,3,4.
Ubiquitin covalently binds with target proteins, causing a series of enzyme catalytic effects. This process requires coordination of three types of enzymes, namely, ubiquitin-activating enzyme (E1), ubiquitin-conjugating (UBC) enzyme (E2), and ubiquitin-ligase enzyme (E3)5. Ubiquitin is activated in an ATP-dependent manner linked with E1; E2 accepts ubiquitin from E1, passes it to active-site cysteine, and then transfers ubiquitin to a targeted protein aided by E35. Additional ubiquitin can be further ligated to initial ubiquitin molecule through sequential ubiquitination cycles, ultimately forming a poly- ubiquitin chain; finally, targeted proteins are modified5. Then, substrates can be degraded to generate other biological effects. E2 plays a crucial role in ubiquitination and is responsible for attachment of ubiquitin to targeted proteins5. E2 protein contains a conserved catalytic domain, called the UBC domain, spanning 140–200 amino acids in length. Various studies indicated that UBC domain mediates the interaction between E2 and E36,7,8,9,10. A special interaction occurs between UBC domain in E2 and RING domain in E311.
E2 genes exist as a multi-gene family and are involved in many plant physiological activities. A total of 14, 50, 41, 39, and 75 E2 genes were identified in Saccharomyces cerevisiae 12, humans13, Arabidopsis 14, rice15, and maize16, respectively. A number of E2 genes are involved in environmental stresses. For example, VrUBC1 of mung bean responded to osmotic17 stress, and E2 genes in soybean and peanut reacted to drought and salt stress in transgenic Arabidopsis 18,19,20. Recently, researchers discovered that fruit-ripening regulator (RIN) can directly bind to the promoter of E2 genes in tomato, pigmentation of fruit was altered at orange ripening by silencing of E2 genes21. E2 genes are also involved in plant disease resistance throng positive plant immune regulation22,23. Some researchers observed association of E2 genes with cryogenic autolysis in Volvariella volvacea 24. GhUNC1/2 is involved in auxin-associated effects and is related to degradation of target proteins, delaying senescence in cotton25.
Grapes (Vitis vinifera) are one of the most important fruit species in the world. Genome sequence of this fruit was released in 2007, it provides foundation for ongoing studies at the genome level26. At present, limited information can describe the role of E2 enzyme in grapes. For example, 45 E2 genes family members were identified in 8× coverage assembly of Vitis vinifera PN40024 genome27. E2-21 is down-regulated at veraison stage in Cabernet Sauvignon (Vitis vinifera)28. Until now, no systematical analysis has been performed on E2 genes family to identify their expression during grapevine development and response during abiotic stress.
The following are objectives of the present study: to identify and clarify members of E2 genes family from the 12× coverage assembly grapevine genome, to characterize their expression pattern during grapevine development and berry ripening, and to explore their functions in abiotic stress. Understanding functions of E2 enzymes bears significance in analyzing regulation mechanism of enzymes in grapevines.
Results
Identification of Vitis vinifera UBC enzyme E2 proteins
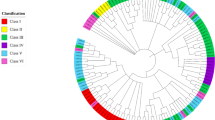
In this study, 43 unique UBC enzyme members were identified using Hidden Markov Model (HMM) and BLAST search methods (Table 1). All these genes contained the UBC domain. A phylogenetic tree was constructed, with 43 VvUBC members in grapes and 52 SlUBC members in tomatoes. VvUBCs showed the relationship between grapes and tomatoes on the phylogenetic tree.
Phylogenetic analysis of VvUBC family
Phylogenetic analysis showed that 43 VvUBC members can be classified into five groups (Fig. 1 and Table 1). Groups I to V (Table 1) contained 15, 8, 6, 11, and 3 members, respectively. Compared with grapes, SlUBC members in tomatoes were classified into six groups. Group I, II, and IV each included 12 members. Group III and V contained 9 and 6 members, respectively. SlUBC14 existed in Group VI alone (Fig. 1).
Conserved domain analysis
UBC enzyme E2 gene family possesses a highly conserved UBC domain. Similar to E2 of human, VvUBC members can be divided into four classes according to existence of additional extensions to UBC domain29 (Fig. 2). In the present study, 50 amino acid residues (or less than 50 amino acid but performing other structural domain) beside UBC domain were regarded as additional extension. Most VvUBCs (28 members) possess a single UBC domain and are categorized as Class I. Class II (three members) features an N-terminal extension, Class III (six members) presents a C-terminal extension, and Class IV (six members) exhibits both extensions (see Supplemental Fig. S1). Interestingly, two VvUBCs contain other domains except for UBC, VvUBC27 contains a ubiquitin-associated domain30 (UBA) at C-terminal, and VvUBC12 contains X8 domain31 at N-terminal.
The domain architechture analysis of VvUBC proteins in Vitis vinifera. The UBC domain is indicated as a dark-blue ellipse and extensions as wathet blue blocks. The UBA domain of VvUBC27 is indicated as a green block and the X8 domain of VvUBC12 as a red block. Scale bar indicates protein length (aa).
Conserved motifs, gene structure, and promoter analysis of VvUBCs
Ten motifs were identified to illustrate VvUBC protein structure using MEME program and further annotated by InterPro Scan 5 (Fig. 3 and Fig. S2). Eight of 10 motifs (except motif 5 and motif 7) were localized within the UBC/RWD (RING finger-containing proteins, WD-repeat-containing proteins, and yeast DEAD or DEXD like helicases) domain, which contained an alpha-beta(4)-alpha(3) core fold, and was found in E2 and related proteins32, RING finger and WD repeat-containing proteins33, all VvUBCs contained at least two of them. VvUBC proteins contained 2–6 motifs, and length of motifs ranged from 11–50 amino acids (see Supplemental Fig. S1). Motifs 1 and 5 existed in almost all 43 VvUBCs except for five VvUBCs in Group I; by contrast, motifs 3, 2, and 4 existed in 39, 30, and 25 VvUBCs, respectively. The remaining motifs were detected in less than half of VvUBCs. Motifs 6 and 8 only existed in five VvUBCs in Group IV. Motifs 9 and 10 only existed in three VvUBCs in Group I. Group II and Group V respectively featured the same motifs except for VvUBC12.
The conserved motifs analysis of 43 VvUBC members. The group was indicated by different color. Different motif was represented by box with different color. The legend of each motif were listed in Supplemental Figure S2.
Figure S3 shows gene structure of VvUBC genes. All VvUBC genes contained at least one untranslated region (UTR) in their 5′ or 3′ terminal and 3–11 exons. VvUBCs presented varying gene lengths ranging from 1267 bp (VvUBC11) to 23957 bp (VvUBC44). Length of coding sequence (CDS) averagely accounted for 10.45% of the whole gene length. This length did not relate to gene length.
Locations of promoter region compared to transcriptional initiation site range from −15000 bp (VvUBC15) to −115 bp (VvUBC38). Tween-three VvUBCs are located in the positive strand, whereas 20 VvUBCs are in the negative strand (Fig. S4).
Chromosome localization and gene duplication analysis of VvUBCs
A total of 43 VvUBCs were distributed in all chromosomes except for chromosome 10, and most genes were close to chromosome terminal (Fig. S5). Chromosomes 6 and 8 contained the most VvUBCs (5 members), and other chromosomes contained 1–3 VvUBCs.
According to the whole genome duplication (WGD) related gene duplication analysis, WGD of VvUBCs occurred during grape genome evolution (Fig. 4), and a group of 36 VvUBCs were involved in 71 WGD events. For example, VvUBC26 located in Chromosomes 1 and VvUBC4 in Chromosome 14 are relative genes. These WGD events accounted for 83.72% (36 of 43) of VvUBCs gene expansion.
Temporal and spatial expression patterns of VvUBCs
A total of 42 VvUBCs (without VvUBC5b) were identified by transcriptome analysis (GSE36128) of 54 organs in Corvina (Vitis vinifera) (Fig. S6). Expression of numerous VvUBCs showed significant changes during grapevine development. VvUBC12 showed decreasing tendency in all organs (Fig. S6). During berry ripening, VvUBC3 increased in three berry tissues (berry pericarp, berry flesh, and berry skin), whereas VvUBC34 decreased, VvUBC7 was up-regulated first and then down-regulated. In post-harvest withering stage, VvUBC3 was rapidly up-regulated in three berry tissues (berry pericarp, berry flesh, and berry skin) and reached the highest level in post-harvest withering-III stage, whereas VvUBC7/29/34 were down-regulated significantly. Several VvUBCs were expressed specially in different organs. For example, VvUBC11 showed low expression level in berry but is highly expressed in leaves, especially in senescent leaves. VvUBC45 featured higher expression level in winter buds than other organs.
To characterize expression pattern of VvUBCs in different genotypes, a transcriptome analysis in five varieties (Sangiovese, Barbera, Negroamaro, Refosco, and Primitivo) was performed using published data (GSE62744). This analysis was performed in four berry developmental stages (pea size, berry tough, soft, and harvest) (Fig. 5). Similar expression patterns of VvUBCs were observed in different varieties. Thirty-seven out of 43 VvUBC genes were expressed in berries. Most genes showed increasing or decreasing expression levels during ripening. Six VvUBCs (VvUBC4/11/12/20/34/44) were remarkably down-regulated in the last two stages of ripening (soft and harvest). VvUBC21 was up-regulated at berry tough stage then down-regulated at soft stage. VvUBC45 was significantly down-regulated after pea size stage. Interestingly, VvUBC51 showed higher expression levels in Sangiovese than other four varieties. The other VvUBCs slightly decreased or increased during grape ripening.
Expression analysis of VvUBCs in different periods among five species using GSE62744. The heatmap was performed by R. Blocks with different colors indicate the expression level relative to the expression average level, original data was normalized by calculate log2 value of the ratio of expression level to expression average level: higher than average(red), equal to average(black), lower than average(green).
To compare the expression patterns in leaves and during fruit ripening, all 43 VvUBCs were performed qRT-PCR using Cabernet Sauvignon. Most VvUBCs (22 of 43) showed down-regulated in three stage34 (EL-33, EL-35, EL-37), especially in VvUBC8 and VvUBC11, which were down-regulated more than 5 fold at EL-37 stage compared to EL-33 stage (Fig. 6). Nine VvUBCs were up-regulated, and three VvUBCs (VvUBC7/23/24) deceased at veraison stage (EL-35) then increased at EL-37 stage. The transcript level of VvUBC3 peaked at veraison stage then declined until EL-37 stage. Eighteen of 43 VvUBCs showed higher expression level in young leaves than that in berries, especially VvUBC30 and VvUBC45, which approximately were 50 and 400 fold in leaves compared to berries, respectively. Eleven VvUBCs showed lower expression level in young leaves than that in berries (Fig. 6).
qRT-PCR results of 43 VvUBCs in young leaves and berries. EL-33, EL-35, EL-37 represent three ripening stage indicating by previous study34. L present young leaves. Data was normalized to VvActin gene expression level. Each VvUBCs at EL-33 stage was normalized as “1”. The mean expression value was calculated from three independent replicates. Vertical bars indicate the standard error of mean. **P < 0.01 and *P < 0.05 compared with berries in EL-33 stage.
Expression analysis of response of VvUBCs to different abiotic stresses
Expression pattern of grape UBC genes under cold and heat stress were investigate using published data SRP018199 and GSE41423 respectively (Fig. S7). Seven (VaUBC3/12/15/17/25d/31/33) and six members (VvUBC9/10/11/20/27/52) responded to cold and heat treatments, respectively. In leaves of Vitis amurensis Rupr., four VaUBCs (VaUBC3/25d/31/33) were significantly down-regulated, and the other three VaUBCs were significantly up-regulated after 4 hours under 4 °C cold treatment. Heat treatment was performed under 45 °C using Cabernet Sauvignon, and then recovery at control condition. Four VvUBCs (VvUBC9/10/20/52) were detected obviously up-regulated compared with control after heat treatment in leaves, whereas VvUBC11/27 was down-regulated slightly. When recovery after heat treatment, VvUBC9/20/52 showed higher expression in control than treatment groups, whereas VvUBC10/11/27 showed lower. Thirty-three VvUBCs were identified in Cabernet Sauvignon from GSE31677, but these genes show no remarkable change during 16 days of salt or water-deficit stress (Fig. S8).
To confirm the transcriptome results, these UBCs of grape were performed qRT-PCR (Fig. 7 and Fig. 8) in Vitis amurensis and Vitis davidii, respectively. Four VaUBCs (VaUBC15/17/25d/33) continuously decreased during cold treatment (4 °C,0–24 h), VaUBC3 and VaUBC31 decreased at 8 h and then increased at 24 h, VaUBC12 showed no significant change at 8 h, but decreased at 24 h (Fig. 7). Six VdUBCs (VdUBC9/10/11/20/27/52) were up-regulated in detached leaves after heat treatment (38 °C 2 h, 47 °C 40 min, Fig. 8).
qRT-PCR results of seven VaUBCs under cold treatment. Data was normalized to VvActin gene expression level. Each VaUBCs at 0 h was normalized as “1”. The mean expression value was calculated from three independent replicates. Vertical bars indicate the standard error of mean. **P < 0.01 and *P < 0.05 compared with 0 h.
qRT-PCR results of six VdUBCs under heat treatment. Data was normalized to VvActin gene expression level. CK and HT represent the control and heat treatment, respectively. Each VdUBCs in CK was normalized as “1”. The mean expression value was calculated from three independent replicates. Vertical bars indicate the standard error of mean. **P < 0.01 and *P < 0.05 compared with CK.
Discussion
In this study, 43 VvUBCs were identified in grape, and this number was higher than the 39 discovered in rice15, 41 in Arabidopsis14, less than 52 in tomato21, 50 in human13, and 75 in maize16. These VvUBCs were divided into five groups based on a phylogenetic tree. VvUBC proteins contained almost similar motifs in one group, especially in Groups II and V. CDS length accounted for 10.45% of the whole gene length, and their promoter location showed a large difference. Chromosome location showed an uneven distribution of VvUBC genes in 19 chromosomes, but no VvUBC gene was found in chromosome 10. Whole genome duplication events played a significant role in evolution of many organisms35, complex WGD events existed in VvUBCs, indicating that VvUBCs perform various functions during grape development. All these analyses showed large differences between VvUBCs in protein structure, gene structure, and promoter location. VvUBCs in one group may exhibit relative functions.
According to domain analysis, conserved domain UBC exists in all VvUBCs. Protein structure of E2 genes is uncommon in plants, but it is widely used in human research. In humans, E2s can be classified based on existence of additional extensions aside from the UBC domain29. These extensions result in functional diversity of E2 genes; this functional diversity is related to subcellular localization and interaction between E2 and E336,37,38,39,40. In this study, fifteen VvUBCs contained extensions with different roles. N-terminal of VvUBC12 contained an X8 domain and a transmembrane domain. This situation indicates that VvUBC12 may contribute to binding of carbohydrates31. Similar to UBE2K in humans29, VvUBC27 contained a UBA domain in C-terminal; this UBA domain might be related to ubiquitin binding30. However, direct role of UBA remains unclear. Conserved motif analysis showed that all VvUBCs contained at least two UBC/RWD domain motifs whereas consist of different motifs, indicating the VvUBCs identified in this study had conserved features of the E2 genes family, and they might play different function in ubiquitination process.
To gain deeper understanding of putative function of VvUBC, temporal and spatial expression profiles were analyzed. In tomato, E2 genes play an important role in regulation of fruit ripening, as determined by virus-induced gene silencing assay21. In grape, most VvUBCs in five Italian varieties change during ripening (Fig. 5), similar expression patterns of VvUBCs were obtained from qRT-PCR in Cabernet Sauvignon (Fig. 6), which indicating that E2 gene family might play extensive roles in grape ripening. VvUBC45 showed different expression profiles in Sangiovese (Fig. 5), indicating its distinct roles in this fruit. Additionally, in Corvina, VvUBC3/7/12/34 were rapidly up-regulated or down-regulated during grape berry development (Fig. S6), indicating involvement of these genes in fruit ripening. Interestingly, VvUBC3 was up-regulated significantly in post-harvest withering stage and VvUBC7/29/34 down-regulated (Fig. S6), they may play significant roles in post-harvest physiology. Aside from berries, E2 genes also played various roles in other organs. In Arabidopsis, AtUBC22 participates in female gametophyte development41. AtUBC1 and AtUBC2 are ubiquitously expressed in roots, leaves, flowers, and seedlings and activation of FLOWERING LOCUS C allow these genes to repress flowering42. In Corvina, VvUBC11 and VvUBC45 exhibited high expression levels in senescing leaves and winter buds, respectively (Fig. S6), VvUBC30 and VvUBC45 showed high expression in young leaves of Cabernet Sauvignon (Fig. 6). These genes may play different roles in grape development compared with other VvUBCs.
E2 genes from both Arabidopsis and rice were not reported to be induced under cold stress43. The present study revealed that ZmUBCs changed significantly under cold conditions16. In Vitis amurensis, seven VaUBCs (VaUBC3/12/15/17/25d/31/33) responded to cold treatment (Fig. S7), and the results were confirmed by qRT-PCR (Fig. 7), but the change tendency of VaUBCs showed a slight difference, which might because of the different cold treatment time. At present, no E2 genes were reported to involve in heat stress. However, expression levels of six UBC genes were obviously changed under heat condition and recovery condition from heat treatment in not only RNA-seq data (Fig. S7) but also qRT-PCR (Fig. 8) in grape in this study. The results indicated that these grape UBC genes might be involved in heat response mechanism in grapes. E2 genes presented different responses to heat and cold stresses. These results indicated that there might be different regulatory mechanisms of ubiquitination in response to heat and cold stresses.
E2 genes in several species were functional under salt or drought. GmUBC2 showed enhanced drought and salt tolerance in soybean18, whereas AtUBC32 was strongly induced by salt stress in Arabidopsis 20. Three genes (OsUBC13/15/45) were also up-regulated under salt and drought stresses in rice15. In peanut plants, the physiological water stress induced by polyethylene glycol, high salinity, abscisic acid, or low temperature, changed the expression levels of AhUBC2 43. Increased transcript levels of CmUBC were observed during drought and salinity stresses in Cucumis melo 44. Based on previous transcriptome resources, VvUBCs showed no significant changes in response to drought and salt stresses in grapes (Fig. S8). This result indicated that E2 genes may play different roles in herbaceous and woody plants.
Conclusion
In this study, 43 VvUBC members were identified and divided into five groups based on their phylogenetic tree. Protein and gene sequences, and duplication events were analyzed to predict functional characteristics of VvUBC genes. Transcriptome data and qRT-PCR results presented significant roles of VvUBCs in grape growth, maturity and post-harvest physiology. Additionally, seven and six VvUBCs showed responses to cold and heat stresses, respectively. These responses may contributed to grape resistance mechanism. These results provide new insights into the E2 genes family in woody plants and a solid foundation for further research on grape breeding.
Materials and Methods
Identification of grape E2 family members
Tomato E2 family members were obtained from a previous research21, which was used in BLAST search to obtain candidate genes of E2 family in grapes. All protein sequences were obtained from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). HMM was constructed using sequence data and was used to search UBC proteins in grapes with a cut-off E-value of 0.001. Then, results of BLAST and HMM searches were merged. Next, candidate UBC protein sequence was scanned again using the domain analysis tool NCBI-Conserved Domain Database (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Finally, 43 UBC proteins were identified in grapes.
Phylogenetic analysis
A phylogenetic tree was generated by MEGA 6.0. Protein sequence was aligned by Clustal W. Then, alignment was imported into the MEGA 6.0 software, and phylogenetic tree was constructed using neighbor-joining statistical method with 1000 bootstrap replication.
Analysis of conserved domain, conserved motif, gene structure and promoters
According to obtained VvUBC protein sequence, domain analysis of proteins was performed by SMART (http://smart.embl-heidelberg.de/). Then, conserved motifs were analyzed by MEME program (http://meme-suite.org/tools/meme). Furthermore, the motifs obtained were annotated using InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan/). Promoter 3.0 was used to annotate grape genome to select the most suitable promoter of VvUBCs, illustrations of promoter and genes were constructed by Gene Structure Display Server (GSDS) software45 (http://gsds.cbi.pku.edu.cn/). Introns and exons of VvUBCs were detected in grape genomic annotation, and the diagram was constructed by GSDS45. Localization of VvUBCs in chromosome was determined according to grape genomic annotation, and diagram was generated by Mapchart 2.3.
Gene duplication
Protein sequence in grape was used for self-BLAST search. Then, BLAST results and documented annotation were combined to analyze duplication of VvUBCs by MCscanX. Finally, a map was drawn by Circos.
Plant growth and Treatments
To analyze expression of VvUBC genes in different tissue and fruit ripening, young leaves and berries were sampled from Cabernet Sauvignon (Vitis vinifera), which planted at the Germplasm Repository for Grapevines in the Institute of Botany of the Chinese Academy of Sciences, Beijing, China (39° 54′N, 116° 23′E). The vines were planted in 2007 in south-to-north oriented rows, trained to a fan-shape trellis with single trunk, and subjected to similar management practices for irrigation, fertilization, soil management, pruning, and disease control. Berries were sampled at three developmental stages (EL-33, EL-35, EL-37) according to EL system34, each sample was collected from nine clusters, and approximately 20 berries from three clusters formed one biological replicate. Sixth leaves were sampled with three biological replicates.
Vitis amurensis were used for cold treatment. Tissue cultured Vitis amurensis were grown on half-strength Murashige and Skoog (1/2 MS, pH 5.8) solid medium 1% sucrose and 0.7% agar in conical flasks (120 mL) in a growth chamber at 26 °C under a 16-h light/8-h dark photoperiod and 100μmol m−2 s−1 light intensity. Six-week-old plantlets were subjected to cold stress, plantlets were transferred to a low-temperature chamber at 4 °C with a 16-h light/8-h darkness cycle. The shoot apex with the first fully expanded leaf was harvested at specific time (0 h, 8 h, and 24 h) after initiating the treatments with three biological replicates.
Spine grape (Vitis davidii) was used to analyze expression of VvUBC genes under heat stress. The vines were planted in the same condition as Cabernet Sauvignon introduced above. Detached leaves of approximately 30 days in age were used for heat treatment according to previous study46. In June of 2017, samples were taken in the morning, placed in the dark with the petiole in water, and then treated by heat stress. The heat stress process was as follows: leaf discs (5.5 cm in diameter) were cut from the detached sample leaves, wrapped in a wet paper towel and placed in a small vessel made of aluminum foil. The vessels were then floated on water in a temperature-controlled water bath, 38 °C 2 h and then 47 °C 40 minutes. Leaves samples were collected with three biological replicates at this time. The control was the same condition as heat treatment except temperature-controlled water bath in 25 °C, and the leaves samples were collected at the same time with three biological replicates.
RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
All samples were immediately obtained frozen in liquid nitrogen and stored at −80 °C for RNA extraction. Total RNA was extracted from collected samples using RNAprep Pure Plant Kit (TIANGEN, Beijing, China) following the manufacturer’s procedure. A maximum of 1 μg total RNA was used for synthesizing cDNA by HiScript Q RT SuperMix (Vazyme, Nanjing, China), and the product was subjected to qRT-PCR with an Opticon thermocycler (CFX Connect Real-Time System; Bio-Rad, Hercules, CA) using SYBR Green PCR master mix (Vazyme, Nanjing, China) according to the manufacturer’s instructions. The PCR cycling conditions were as follows: 95 °C for 10 min, 40 cycles of 95 °C for 10 s, 60 °C for 30 s; a 65–95 °C melt curve was analyzed to detect possible primer dimers or nonspecific amplification. VvActin (Accession number: EC969944) was used as stable reference genes. Gene specific primer pairs for qRT-PCR (listed in Table S1) were designed by NCBI Primer BLAST. The specificity of the primers was further verified through gel electrophoresis and reaction product sequencing. Three biological replicates were performed to ensure the accuracy of results. The relative expression of the target genes was determined using the 2−ΔΔCt method47. All experiments were performed with three biological replicates and three technical replicates. Statistical difference were performed by t-test (**P < 0.01, *P < 0.05, n = 3) using R software.
Transcriptomic resources
Transcriptomic data used in this study were obtained from previous research48,49,50,51,52. Expression levels in different organs were analyzed using GSE3612848. A total of 54 organs were collected from grapevines Corvina (Vitis vinifera) for RNA extraction. The entire list of 54 organs can be found as Supplementary Table S2. Three biological replicates were obtained for each sample. Data of four stage of berries in five varieties analysis were obtained from GSE6274449. Grape berries were collected from five red-skin grapevine (Vitis vinifera) cultivars (Sangiovese, Barbera, Negro amaro, Refosco, and Primitivo) at four phenological stages (pea size, berry tough, soft, and harvest), with three biological replicates acquired for each sample.
Cold treatment in SRP018199 was performed as follows50: Vitis amurensis seedlings were grown in 16 h light/8 h dark photoperiod at 26 °C. These seedlings were then transferred into a chamber at 24 °C under 16 h light at 6:00 am. Cold treatment was started at 9:00 am with constant light. During the first four hours, temperature dropped to 5 °C per hour and was held at 4 °C for an additional four hours. Seedlings used for control were also transferred to growth chambers but without cold treatment. Shoot apices with one well-developed leaf were harvested from three independent replicates. RNAs were isolated for digital expression library construction.
Heat treatment in GSE41423 was conducted as follows51: Cabernet Sauvignon (Vitis vinifera) was grown in 25/18 °C day/night condition before treatment. Then, the experimental group was treated at 45 °C from 9:00 to 14:30. Next, leaf samples were obtained and recovered rapidly at 25 °C for 15 min. Leaf samples were collected the following morning at 9:00. Control group was grown in 25/18 °C day/night condition. Leaf samples were collected from the experimental group.
Cabernet Sauvignon (Vitis vinifera) were treated under water-deficit and salinity stress conditions (GSE31677)52. This process is listed in Supplemental Table S3.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Dye, B. T. & Schulman, B. A. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu Rev Biophys Biomol Struct. 36, 131 (2007).
Finley, D. U. Annu Rev Cell Biol. 7, 25–69 (1990).
Hershko, A. & Ciechanover, A. The ubiquitin system for protein degradation. Annu Re Biochem. 61, 761–807 (1992).
Hochstrasser, M. Ubiquitin-dependent protein degradation. Annu Re Biochem. 30, 405 (2003).
Hershko, A., Heller, H., Elias, S. & Ciechanover, A. Components of ubiquitin-protein ligase system. resolution, affinity purification, and role in protein breakdown. J Biol Chem. 258, 8206–8214 (1983).
Huang, L. et al. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade. Science. 286, 1321–1326 (1999).
Schulman, B. A. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 408, 381 (2000).
Zheng, N., Wang, P., Jeffrey, P. D. & Pavletich, N. P. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 102, 533–539 (2000).
Christensen, D. E., Brzovic, P. S. & Klevit, R. E. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 14, 941 (2007).
Poyurovsky, M. V. et al. The Mdm2 RING domain C-terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J. 26, 90–101 (2007).
Lorick, K. L. & J. P, J. S. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. PNAS. 96, 11364–11369 (1999).
Michelle, C., Vourc, H.,P., Mignon, L. & Andres, C. R. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J Mol Evol. 68, 616–628 (2009).
Jiang, Y. H. & Beaudet, A. L. Human disorders of ubiquitination and proteasomal degradation. Curr Opin Pediatr. 16, 419–426 (2004).
Kraft, E. et al. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis, [w]. Plant Physiol. 139, 1597 (2005).
Zhiguo, E., Zhang, Y., Li, T., Wang, L. & Zhao, H. Characterization of the ubiquitin-conjugating enzyme gene family in rice and evaluation of expression profiles under abiotic stresses and hormone treatments. PLoS One. 10, e122621 (2015).
Jue, D. et al. Genome-wide identification, phylogenetic and expression analyses of the ubiquitin-conjugating enzyme gene family in maize. PLoS One. 10, e143488 (2015).
Eunsook Chung, C. C. H. S. Overexpression of VrUBC1, a mung bean E2 ubiquitin-conjugating enzyme, enhances osmotic stress tolerance in Arabidopsis. PLoS One. 8, e66056 (2013).
Zhou, G. A., Chang, R. Z. & Qiu, L. J. Overexpression of soybean ubiquitin-conjugating enzyme gene GmUBC2 confers enhanced drought and salt tolerance through modulating abiotic stress-responsive gene expression in Arabidopsis. Plant Mol Biol. 72, 357 (2010).
Wan, X., Mo, A., Liu, S., Yang, L. & Li, L. Constitutive expression of a peanut ubiquitin-conjugating enzyme gene in Arabidopsis confers improved water-stress tolerance through regulation of stress-responsive gene expression. Biosci. Bioeng. 111, 478 (2011).
Cui, F. et al. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell. 24, 233–244 (2012).
Wang, Y. et al. Tomato nuclear proteome reveals the involvement of specific E2 ubiquitin-conjugating enzymes in fruit ripening. Genome Biol. 15, 548 (2014).
Mural, R. V. et al. The tomato Fni3 lysine-63-specific ubiquitin-conjugating enzyme and suv ubiquitin E2 variant positively regulate plant immunity. Plant Cell. 25, 3615–3631 (2013).
Unver, T., Turktas, M. & Budak, H. In planta evidence for the involvement of a ubiquitin conjugating enzyme (UBC E2 clade) in negative regulation of disease resistance. Plant Mol Biol Rep. 31, 323–334 (2013).
Gong, M. et al. A newly discovered ubiquitin-conjugating enzyme E2 correlated with the cryogenic autolysis of Volvariella volvacea. Gene. 583, 58–63 (2016).
Zhang, X. D. et al. Molecular cloning, differential expression, and functional characterization of a family of class I ubiquitin-conjugating enzyme (E2) genes in cotton (Gossypium). Biochim Biophys Acta Gene Struct. 1625, 269–279 (2003).
Jaillon, O. et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 449, 463 (2007).
Zhou, D., Xin, Z., Li, L. & Zhen, S. Plantsups: a database of plants’ ubiquitin proteasome system. BMC Genomics. 10, 227 (2009).
Zhang, J. et al. Grape berry plasma membrane proteome analysis and its differential expression during ripening. J Exp Bot. 59, 2979–2990 (2008).
van Wijk, S. J. & Timmers, H. T. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 24, 981–993 (2010).
Wilkinson, C. R. et al. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol. 3, 939–943 (2001).
Henrissat, B. & Davies, G. J. Glycoside hydrolases and glycosyltransferases. families, modules, and implications for genomics. Plant Physiol. 124, 1515–1519 (2001).
Miura, T., Klaus, W., Ross, A., Güntert, P. & Senn, H. The NMR structure of the class I human ubiquitin-conjugating enzyme 2b. J Biomol NMR. 22, 89–92 (2002).
Nameki, N. et al. Solution structure of the RWD domain of the mouse GCN2 protein. Protein Sci. 13, 2089–2100 (2004).
Coombe, B. G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust J Grape Wine R. 1, 104–110 (1995).
Xu, G., Guo, C., Shan, H. & Kong, H. Divergence of duplicate genes in exon-intron structure. PNAS. 109, 1187–1192 (2012).
Goebl, M. G. et al. The yeast cell cycle gene Cdc34 encodes a ubiquitin-conjugating enzyme. Science. 241, 1331–1335 (1988).
Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 91, 209–219 (1997).
Sadowski, M., Mawson, A., Baker, R. & Sarcevic, B. Cdc34 C-terminal tail phosphorylation regulates Skp1/cullin/F-box (SCF)-mediated ubiquitination and cell cycle progression. Biochem J. 405, 569–581 (2007).
Coccetti, P. et al. The CK2 phosphorylation of catalytic domain of Cdc34 modulates its activity at the G1 to s transition in Saccharomyces cerevisiae. Cell Cycle. 7, 1391–1401 (2008).
Summers, M. K., Pan, B., Mukhyala, K. & Jackson, P. K. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell. 31, 544–556 (2008).
Wang, S., Cao, L. & Wang, H. Arabidopsis ubiquitin-conjugating enzyme UBC22 is required for female gametophyte development and likely involved in Lys11-linked ubiquitination. J Exp Bot. 67, 3277 (2016).
Lin, X. et al. The E2 ubiquitin-conjugating enzymes, AtUBC1 and AtUBC2, play redundant roles and are involved in activation of FLC expression and repression of flowering in Arabidopsis thaliana. Plant J. 57, 279–288 (2009).
Wan, X., Mo, A., Liu, S., Yang, L. & Li, L. Constitutive expression of a peanut ubiquitin-conjugating enzyme gene in Arabidopsis confers improved water-stress tolerance through regulation of stress-responsive gene expression. J Biosci Bioeng. 111, 478–484 (2010).
Baloglu, M. C. & Patir, M. G. Molecular characterization, 3D model analysis, and expression pattern of the CmUBC gene encoding the melon ubiquitin-conjugating enzyme under drought and salt stress conditions. Biochem Genet. 52, 90 (2013).
Hu, B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 31, 1296 (2015).
Xu, H. et al. Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol. 14, 156 (2014).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods. 25, 402–408 (2001).
Fasoli, M. et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell. 24, 3489–3505 (2012).
Palumbo, M. C. et al. Integrated network analysis identifies fight-club nodes as a class of hubs encompassing key putative switch genes that induce major transcriptome reprogramming during grapevine development. Plant cell. 26, 4617–4635 (2014).
Xin, H. et al. Genome wide transcriptional profile analysis of Vitis amurensis and Vitis vinifera in response to cold stress. Plos one. 8, e58740 (2013).
Liu, G. T. et al. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol. 12, 174 (2012).
Cramer, G. R. et al. Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics. 7, 111–134 (2007).
Acknowledgements
We thank Erpeng Zhang and Cheng Cheng for revising the manuscript, and Xinna Liu for help with the R software. This work was supported by the grants from the National Science Foundation of China (31572090), Agricultural Breeding Project of Ningxia Hui Autonomous Region (NXNYYZ20150203) and Hundred Talents of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Z.L. and S.L. designed the research. Y.G. and Y.W. performed the experiments. Y.G., Y.W., and H.X. analyzed data. Y.G., Z.L., S.L. and H.X. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, Y., Wang, Y., Xin, H. et al. Involvement of Ubiquitin-Conjugating Enzyme (E2 Gene Family) in Ripening Process and Response to Cold and Heat Stress of Vitis vinifera . Sci Rep 7, 13290 (2017). https://doi.org/10.1038/s41598-017-13513-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13513-x
This article is cited by
-
Characterization and regulation mechanism analysis of ubiquitin-conjugating family genes in strawberry reveals a potential role in fruit ripening
BMC Plant Biology (2022)
-
Functional characterization of the pUceS8.3 promoter and its potential use for ectopic gene overexpression
Planta (2022)
-
Transcriptomic analysis of grapevine Dof transcription factor gene family in response to cold stress and functional analyses of the VaDof17d gene
Planta (2021)
-
Global profiling of alternative splicing landscape responsive to salt stress in wheat (Triticum aestivum L.)
Plant Growth Regulation (2020)
-
Genome-wide identification and expression analysis of the E2 gene family in potato
Molecular Biology Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.