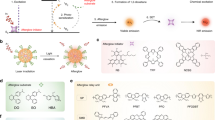
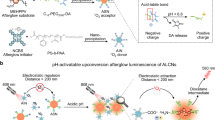
Abstract
Fluorescence imaging represents a vital tool in modern biology, oncology and biomedical applications. Afterglow luminescence (AGL), which circumvents the light scattering and tissue autofluorescence interference associated with real-time excitation source, shows remarkably increased imaging sensitivity and depth. Here we present a protocol for the design and synthesis of AGL nanoprobes with an aggregation-induced emission (AIE) effect to simultaneously red shift and amplify the afterglow signal for tumor imaging and image-guided tumor resection. The nanoprobe (AGL AIE dot) is composed of an enol ether format of Schaap’s agent and a near-infrared AIE fluorogen (AIEgen) (tetraphenylethylene-phenyl-dicyanomethylene-4H-chromene, TPE-Ph-DCM) to suppress the nonradiative dissipation pathway. Pre-irradiating AGL AIE dots with white light could generate singlet oxygen to convert Schaap’s agent to its 1,2-dioxetane format, thus initializing the AGL process. With the aid of AIEgen, the AGL shows simultaneously red shifted emission maximum (from ~540 nm to ~625 nm) and enhanced intensity (by 3.2-fold), facilitating better signal-to-background ratio, imaging sensitivity and depth. Intriguingly, the activated AGL can last for over 10 days. Compared with conventional approaches, our method provides a new solution to concurrently red shift and amplify afterglow signals for better in vivo imaging outcomes. The preparation of AGL AIE dots takes ~2 days, the in vitro characterization takes ~10 days (less than 1 day if not involving afterglow kinetic profile study) and the tumor imaging and image-guided tumor resection takes ~7 days. These procedures can be easily reproduced and amended after standard laboratory training in chemical synthesis and animal handling.
Key points
-
The protocol describes the synthesis and characterization of near-infrared afterglow nanoprobes with an aggregation-induced emission effect to simultaneously red shift and amplify the afterglow signal.
-
Due to the long-lasting and improved signal intensity, these nanoprobes represent a powerful tool for real-time in vivo imaging and have been successfully employed for intraoperative image-guided tumor resection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research paper51. The raw datasets are provided in the Source Data file. The online version also contains a Supplementary Information PDF file. All other data are available for research purposes from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Zhu, S., Tian, R., Antaris, A. L., Chen, X. & Dai, H. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 31, 1900321 (2019).
Smith, A. M., Mancini, M. C. & Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle mediated optogenetics. Science 359, 679–684 (2018).
Kobayashi, H., Ogawa, M., Alford, R., Choyke, P. L. & Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 110, 2620–2640 (2010).
Gao, M., Yu, F., Lv, C., Choo, J. & Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 46, 2237–2271 (2017).
Zhang, Y., Zhang, G., Zeng, Z. & Pu, K. Activatable molecular probes for fluorescence-guided surgery, endoscopy and tissue biopsy. Chem. Soc. Rev. 51, 566–593 (2022).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Li, J. & Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 48, 38–71 (2019).
Kenry, Duan, Y. & Liu, B. Recent advances of optical imaging in the second near-infrared window. Adv. Mater. 30, 1802394 (2018).
Yang, Q. et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. J. Am. Chem. Soc. 140, 1715–1724 (2018).
Wan, H. et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 9, 1171 (2018).
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 10, 507–518 (2013).
Hu, Z. et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 4, 259–271 (2020).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Sun, S. K., Wang, H. F. & Yan, X. P. Engineering persistent luminescence nanoparticles for biological applications: from biosensing/bioimaging to theranostics. Acc. Chem. Res. 51, 1131–1143 (2018).
He, S., Xie, C., Jiang, Y. & Pu, K. An organic afterglow protheranostic nanoassembly. Adv. Mater. 31, 1902672 (2019).
Hananya, N. & Shabat, D. A glowing trajectory between bio- and chemiluminescence: from luciferin-based probes to triggerable dioxetanes. Angew. Chem. Int. Ed. Engl. 56, 16454–16463 (2017).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Yang, M. et al. Chemiluminescence for bioimaging and therapeutics: recent advances and challenges. Chem. Soc. Rev. 49, 6800–6815 (2020).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Yang, Y. & Zhang, F. Activatable chemiluminescent molecular probes for bioimaging and biosensing. Anal. Sens. 1, 75–89 (2021).
Cao, J., Campbell, J., Liu, L., Mason, R. P. & Lippert, A. R. In vivo chemiluminescent imaging agents for nitroreductase and tissue oxygenation. Anal. Chem. 88, 4995–5002 (2016).
Bruemmer, K. J., Green, O., Su, T. A., Shabat, D. & Chang, C. J. Chemiluminescent probes for activity-based sensing of formaldehyde released from folate degradation in living mice. Angew. Chem. Int. Ed. Engl. 57, 7508–7512 (2018).
Lippert, A. R. Unlocking the potential of chemiluminescence imaging. ACS Cent. Sci. 3, 269–271 (2017).
Hananya, N. & Shabat, D. Recent advances and challenges in luminescent imaging: bright outlook for chemiluminescence of dioxetanes in water. ACS Cent. Sci. 5, 949–959 (2019).
Kagalwala, H. N. & Lippert, A. R. Energy transfer chemiluminescent spiroadamantane 1,2-dioxetane probes for bioanalyte detection and imaging. Angew. Chem. Int. Ed. Engl. 61, e202210057 (2022).
Han, J., Jose, J., Mei, E. & Burgess, K. Chemiluminescent energy-transfer cassettes based on fluorescein and nile red. Angew. Chem. Int. Ed. Engl. 46, 1684–1687 (2007).
Hananya, N., Green, O., Blau, R., Satchi-Fainaro, R. & Shabat, D. A highly efficient chemiluminescence probe for the detection of singlet oxygen in living cells. Angew. Chem. Int. Ed. Engl. 56, 11793–11796 (2017).
Green, O. et al. Near-infrared dioxetane luminophores with direct chemiluminescence emission mode. J. Am. Chem. Soc. 139, 13243–13248 (2017).
Hananya, N., Eldar Boock, A., Bauer, C. R., Satchi-Fainaro, R. & Shabat, D. Remarkable enhancement of chemiluminescent signal by dioxetane–fluorophore conjugates: turn-on chemiluminescence probes with color modulation for sensing and imaging. J. Am. Chem. Soc. 138, 13438–13446 (2016).
Lu, L. et al. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
Chen, Z. et al. Design and synthesis of a small molecular NIR-II chemiluminescence probe for in vivo-activated H2S imaging. Proc. Natl Acad. Sci. USA 120, e2205186120 (2023).
Huang, J., Jiang, Y., Li, J., Huang, J. & Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. Engl. 60, 3999–4003 (2021).
Luo, J. et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 18, 1740–1741 (2001).
Hong, Y., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: phenomenon, mechanism and applications. Chem. Commun. 7, 4332–4353 (2009).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Mei, J. et al. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 26, 5429–5479 (2014).
Peng, Q. & Shuan, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2, e91 (2021).
Feng, G. & Liu, B. Aggregation-induced emission (AIE) dots: emerging theranostic nanolights. Acc. Chem. Res. 51, 1404–1414 (2018).
Ding, D., Li, K., Liu, B. & Tang, B. Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 46, 2441–2453 (2013).
Qian, J. & Tang, B. Z. AIE luminogens for bioimaging and theranostics: from organelles to animals. Chem 3, 56–91 (2017).
Lim, X. The nanolight revolution is coming. Nature 531, 26–28 (2016).
Li, Y. et al. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 11, 1255 (2020).
Feng, G. et al. Ultrabright organic dots with aggregation-induced emission characteristics for cell tracking. Biomaterials 35, 8669–8677 (2014).
Qi, J. et al. Real-time and high-resolution bioimaging with bright aggregation-induced emission dots in short-wave infrared region. Adv. Mater. 30, 1706856 (2018).
Li, D., Qin, W., Xu, B., Qian, J. & Tang, B. Z. AIE nanoparticles with high stimulated emission depletion efficiency and photobleaching resistance for long-term super-resolution bioimaging. Adv. Mater. 29, 1703643 (2017).
Ding, D. et al. Ultrabright organic dots with aggregation-induced emission characteristics for real-time two-photon intravital vasculature imaging. Adv. Mater. 25, 6083–6088 (2013).
Wang, Y. et al. Aggregation-induced emission luminogen with deep-red emission for through-skull three-photon fluorescence imaging of mouse. ACS Nano 11, 10452–10461 (2017).
Kang, M. et al. Aggregation-enhanced theranostics: AIE sparkles in the biomedical field. Aggregate 1, 80–106 (2020).
Sheng, Z. et al. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mater. 30, 1800766 (2018).
Ni, X. et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Denk, W., Strickler, J. & Webb, W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Yang, J. et al. Rational design of pyrrole derivatives with aggregation-induced phosphorescence characteristics for time-resolved and two-photon luminescence imaging. Nat. Commun. 12, 4883 (2021).
Xiao, F. et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
Yang, Y. et al. NIR-II chemiluminescence molecular sensor for in vivo high-contrast inflammation imaging. Angew. Chem. Int. Ed. Engl. 59, 18380–18385 (2020).
Chen, C. et al. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Xu, C. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics. Nat. Biomed. Eng. 7, 298–312 (2023).
Jiang, Y. et al. A generic approach toward afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
Kagalwala, H. N., Gerberich, J., Smith, C. J., Mason, R. P. & Lippert, A. R. Chemiluminescent 1,2-dioxetane iridium complexes for near-infrared oxygen sensing. Angew. Chem. Int. Ed. Engl. 61, e202115704 (2022).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. K. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Green, O. et al. Opening a gateway for chemiluminescence cell imaging: distinctive methodology for design of bright chemiluminescent dioxetane probes. ACS Cent. Sci. 3, 349–358 (2017).
Li, K. & Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 43, 6570–6597 (2014).
Jana, D., Boxi, S., Parui, P. P. & Ghorai, B. K. Planar-rotor architecture based pyrene-vinyl-tetraphenylethylene conjugated systems: photophysical properties and aggregation behavior. Org. Biomol. Chem. 13, 10663–10674 (2015).
Rathahao-Paris, E., Alves, S., Junot, C. & Tabet, J. High-resolution mass spectrometry for structural identification of metabolites in metabolomics. Metabolomics 16, 1–15 (2016).
Elyashberg, M. Identification and structure elucidation by NMR spectroscopy. Trends Anal. Chem. 69, 88–97 (2015).
Dickinson, B. C., Lin, V. S. & Chang, C. J. Preparation and use of MitoPY1 for imaging hydrogen peroxide in mitochondria of live cells. Nat. Protoc. 8, 1249–1259 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 51961160730, 52225310, 22205067, 32201178, 32371449), the Fundamental Research Funds for the Central Universities and the Tianjin Science Fund for Distinguished Young Scholars (19JCJQJC61200), Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2023B1212060003), Xi’an Jiaotong University Young Talent Support Grant.
Author information
Authors and Affiliations
Contributions
C.C., G.F. and D.D. conceived the idea of the project, C.C., Z.G. and X.Z. contributed to the experimal work invivoled in this protocol. C.C, Z.G., X.Z., G.F. and D.D. wrote the protocol, D.D. supervised the study and the prepration of the masnucript. All authors contributed to the editing and reviewing of the draft and approved the final manscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Qian Ju, Kanyi Pu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Ni, X. et al. Nano Lett. 19, 318–330 (2019): https://doi.org/10.1021/acs.nanolett.8b03936
Chen, C. et al. J. Am. Chem. Soc. 144, 3429–3441 (2022): https://doi.org/10.1021/jacs.1c11455
Gao, Z. et al. Angew. Chem. Int. Ed. Engl. 61, e202209793 (2022): https://doi.org/10.1002/anie.202209793
Li, J. et al. Adv. Funct. Mater. 33, 2212380 (2023): https://doi.org/10.1002/adfm.202212380
Extended data
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Methods.
Source data
Source Data Fig. 4–7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Zhang, X., Gao, Z. et al. Preparation of AIEgen-based near-infrared afterglow luminescence nanoprobes for tumor imaging and image-guided tumor resection. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00990-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00990-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.