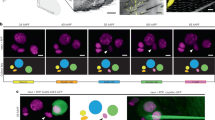
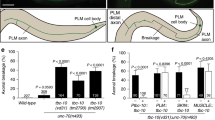
Abstract
The study of how mechanical forces affect biological events in living tissue is important for the understanding of a multitude of physiogical and pathophysiological phenomena. However, these investigations are often impeded by insufficient knowledge about force parameters, inadequate experimental administration of force stimuli and lack of noninvasive means to record their molecular and cellular effects. We therefore introduced a procedure to study the impact of force stimulation on adhesion G-protein-coupled receptor dissociation in mechanosensory neurons. Here, we detail a procedure to harness the mechanical force spectrum that emerges during the natural flexion-extension cycle of the femorotibial joint of adult fruit flies (Drosophila melanogaster). Mechanical load generated during the joint’s motion is transmitted to specialized mechanosensory neurons residing close to the joint axis, which serve as proprioceptive sensors in the peripheral nervous system of the animal. Temporary immobilization of the joint by a restraint made of a human hair allows for the observation of transgenic mechanosensitive reporters by using fluorescent readout in the neurons before, during and after cessation of mechanical stimulation. The assay harnesses physiologically adequate stimuli for joint flexion and extension, can be conducted noninvasively in live specimens and is compatible with various transgenic reporter systems beyond the initially conceived strategy and mechanobiological hypotheses tested. The application of the protocol requires knowledge in Drosophila genetics, husbandry and fluorescence imaging and micromanipulation skills. The experimental procedure can be completed in 10 h and requires an additional 30 min in advance for fly fixation and leg immobilization. The apple agar cooking and heptane glue preparation requires a maximum of 30 min on the day before the experiment is conducted.
Key points
-
This protocol describes an assay to study molecular mechanosensation, in which mechanical forces cause stimulation of mechanosensitive target molecules in proprioceptive neurons. Transgenic reporters of force stimulation or neuronal activity can be coupled to binary expression systems such as the GAL4/upstream activating sequence (UAS), LexA/lexAop and QF/Q upstream activating sequence (QUAS).
-
The approach provides a noninvasive and imaging-based approach to manipulating joint motion (i.e., mechanical force generation) and measuring the molecular effects caused by this motion in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source data for Figs. 1–3 are available in Figshare with the identifier https://doi.org/10.6084/m9.figshare.24024480 (ref. 24).
References
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Hudspeth, A. J. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 15, 600–614 (2014).
Ranade, S. S., Syeda, R. & Patapoutian, A. Mechanically activated ion channels. Neuron 87, 1162–1179 (2015).
Langenhan, T., Piao, X. & Monk, K. R. Adhesion G protein-coupled receptors in nervous system development and disease. Nat. Rev. Neurosci. 17, 550–561 (2016).
Scholz, N., Monk, K. R., Kittel, R. J. & Langenhan, T. Adhesion GPCRs as a putative class of metabotropic mechanosensors. Handb. Exp. Pharmacol. 234, 221–247 (2016).
Wolfenson, H., Yang, B. & Sheetz, M. P. Steps in mechanotransduction pathways that control cell morphology. Annu. Rev. Physiol. 81, 1–21 (2017).
Mammoto, A., Mammoto, T. & Ingber, D. E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 (2012).
Scholz, N. et al. Molecular sensing of mechano- and ligand-dependent adhesion GPCR dissociation. Nature 615, 945–953 (2023).
Scholz, N. et al. The adhesion GPCR Latrophilin/CIRL shapes mechanosensation. Cell Rep. 11, 866–874 (2015).
Dannhäuser, S. et al. Antinociceptive modulation by the adhesion GPCR CIRL promotes mechanosensory signal discrimination. Elife 9, e56738 (2020).
Scholz, N. et al. Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. Elife 6, e28360 (2017).
Desai, B. S., Chadha, A. & Cook, B. The stum gene is essential for mechanical sensing in proprioceptive neurons. Science 343, 1256–1259 (2014).
He, L., Binari, R., Huang, J., Falo-Sanjuan, J. & Perrimon, N. In vivo study of gene expression with an enhanced dual-color fluorescent transcriptional timer. Elife 8, e46181 (2019).
Simpson, J. H. & Looger, L. L. Functional imaging and optogenetics in Drosophila. Genetics 208, 1291–1309 (2018).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018).
Bos, J. L. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4, 733–738 (2003).
Robichaux, W. G. & Cheng, X. Intracellular cAMP sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol. Rev. 98, 919–1053 (2018).
Yang, H. H. & St-Pierre, F. Genetically encoded voltage indicators: opportunities and challenges. J. Neurosci. 36, 9977–9989 (2016).
Bando, Y., Sakamoto, M., Kim, S., Ayzenshtat, I. & Yuste, R. Comparative evaluation of genetically encoded voltage indicators. Cell Rep. 26, 802–813.e4 (2019).
Fosque, B. F. et al. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760 (2015).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Krieg, M., Dunn, A. R. & Goodman, M. B. Mechanical control of the sense of touch by β-spectrin. Nat. Cell Biol. 16, 224–233 (2014).
Schmied, C. & Tomancak, P. Sample reparation and mounting of Drosophila embryos for multiview light sheet microscopy. Methods Mol. Biol. 1478, 189–202 (2016).
Buhlan, M., Ljaschenko, D., Scholz, N. & Langenhan, T. Figshare Raw Image Data. Available at https://doi.org/10.6084/m9.figshare.24024480
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft to N.S. and T.L. through FOR2149, project numbers 265903901 (project P01) and 265996823 (project P03) and through CRC 1423, project number 421152132 (projects A06 and B06), and by a junior research grant from the Faculty of Medicine, Leipzig University to N.S. M.B. is funded by the Studienstiftung des deutschen Volkes. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. The authors thank the Tomancak group at the MPI-CBG Dresden for suggestions regarding fly immobilization.
Author information
Authors and Affiliations
Contributions
M.B. designed, conducted and analyzed the experiments and wrote the manuscript. D.L. initiated and analyzed the experiments. N.S. initiated and designed the experiments and wrote the manuscript. T.L. initiated, designed and analyzed the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Dimitris Placantonakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Scholz, N. et al. Nature 615, 945–953 (2023): https://doi.org/10.1038/s41586-023-05802-5
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buhlan, M., Ljaschenko, D., Scholz, N. et al. Experimental modulation of physiological force application on leg joint neurons in intact Drosophila melanogaster. Nat Protoc 19, 113–126 (2024). https://doi.org/10.1038/s41596-023-00907-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00907-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.