Abstract
Drosophila models have been instrumental in providing insights into molecular mechanisms of neurodegeneration, with wide application to human disease. The brain degeneration associated with traumatic brain injury (TBI) has been modeled in Drosophila using devices that inflict trauma on multiple parts of the fly body, including the head. However, the injuries produced by these models are not specific in location and are inconsistent between individual animals. We have recently developed a device that can be used to inflict controlled head injury to flies, resulting in physiological responses that are remarkably similar to those observed in humans with TBI. This protocol describes the construction, calibration and use of the Drosophila TBI (dTBI) device, a platform that employs a piezoelectric actuator to reproducibly deliver a force in order to briefly compress the fly head against a metal surface. The extent of head compression can be controlled through an electrical circuit, allowing the operator to set different levels of injury. The entire device can be assembled and calibrated in under a week. The device components and the necessary electrical tools are readily available and cost ~$800. The dTBI device can be used to harness the power of Drosophila genetics and perform large-scale genetic or pharmacological screens, using a 7-d post-injury survival curve to identify modifiers of injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper.
Code availability
The Arduino code used by the device is provided in the Supplementary Software 1.
References
Blennow, K. et al. Traumatic brain injuries. Nat Rev. Dis. Primers 2, 16084 (2016).
Kaur, P. & Sharma, S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 16, 1224–1238 (2018).
Ma, X., Aravind, A., Pfister, B. J., Chandra, N. & Haorah, J. Animal models of traumatic brain injury and assessment of injury severity. Mol. Neurobiol. 56, 5332–5345 (2019).
Dai, J. X., Ma, Y. B., Le, N. Y., Cao, J. & Wang, Y. Large animal models of traumatic brain injury. Int. J. Neurosci. 128, 243–254 (2018).
Angstman, N. B., Frank, H. G. & Schmitz, C. Hypothermia ameliorates blast-related lifespan reduction of C. Elegans. Sci. Rep. 8, 10549 (2018).
Miansari, M. et al. Inducing mild traumatic brain injury in C. Elegans via cavitation-free surface acoustic wave-driven ultrasonic irradiation. Sci. Rep. 9, 12775 (2019).
McCutcheon, V. et al. A novel model of traumatic brain injury in adult zebrafish demonstrates response to injury and treatment comparable with mammalian models. J. Neurotrauma 34, 1382–1393 (2017).
Maheras, A. L. et al. Genetic pathways of neuroregeneration in a novel mild traumatic brain injury model in adult zebrafish. eNeuro 5, 0208-17.2017 (2018).
Katzenberger, R. J. et al. A Drosophila model of closed head traumatic brain injury. Proc. Natl Acad. Sci. USA 110, 9 (2013).
Barekat, A. et al. Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci. Rep. 6, 25252 (2016).
Sun, M. & Chen, L. L. A novel method to model chronic traumatic encephalopathy in Drosophila. J. Vis. Exp. 125, e55602 (2017).
Strange, K. Drug discovery in fish, flies, and worms. ILAR J. 57, 133–143 (2016).
Bellen, H. J., Tong, C. & Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat. Rev. Neurosci. 11, 514–522 (2010).
McGurk, L., Berson, A. & Bonini, N. M. Drosophila as an in vivo model for human neurodegenerative disease. Genetics 201, 377–402 (2015).
Pandey, U. B. & Nichols, C. D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 63, 411–436 (2011).
Reiter, L. T., Potocki, L., Chien, S., Gribskov, M. & Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 11, 1114–1125 (2001).
MacDonald, J. M. et al. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50, 869–881 (2006).
Fang, Y., Soares, L., Teng, X., Geary, M. & Bonini, N. M. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr. Biol. 22, 590–595 (2012).
Neukomm, L. J., Burdett, T. C., Gonzalez, M. A., Züchner, S. & Freeman, M. R. Rapid in vivo forward genetic approach for identifying axon death genes in Drosophila. Proc. Natl Acad. Sci. USA 111, 9965–9970 (2014).
Leyssen, M. et al. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J 24, 2944–2955 (2005).
Katzenberger, R. J. et al. Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife 4, e04790 (2015).
Saikumar, J., Byrns, C. N., Hemphill, M., Meaney, D. F. & Bonini, N. M. Dynamic neural and glial responses of a head-specific model for traumatic brain injury in Drosophila. Proc. Natl Acad. Sci. USA 117, 17269–17277 (2020).
Sunderhaus, E. R. & Kretzschmar, D. Mass histology to quantify neurodegeneration in Drosophila. J. Vis. Exp. 118, e54809 (2016).
Miller, R. S. & Thomas, J. L. The effects of larval crowding and body size on the longevity of adult Drosophila melanogaster. Ecology 39, 118–125 (1958).
Sorensen, J. G. & Loeschcke, V. Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J. Insect Physiol. 47, 1301–1307 (2001).
Baldal, E. A., van der Linde, K., van Alphen, J. J., Brakefield, P. M. & Zwaan, B. J. The effects of larval density on adult life-history traits in three species of Drosophila. Mech. Ageing Dev. 126, 407–416 (2005).
Tataroglu, O. & Emery, P. Studying circadian rhythms in Drosophila melanogaster. Methods 68, 140–150 (2014).
Blum, J. E., Fischer, C. N., Miles, J. & Handelsman, J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 4, e00860–00913 (2013).
Semenchenko, G. V., Khazaeli, A. A., Curtsinger, J. W. & Yashin, A. I. Stress resistance declines with age: analysis of data from a survival experiment with Drosophila melanogaster. Biogerontology 5, 17–30 (2004).
Heisenberg, M. & Böhl, K. Isolation of anatomical brain mutants of Drosophila by histological means. Z. Naturforsch. 34, 143–147 (1979).
Acknowledgements
We thank M. Suplick and F. Letterio from the University of Pennsylvania Machine Shop for constructing and modifying the Heisenberg fly collars. We thank F. Carranza, A. Srinivasan and A. Perlegos for critical feedback. This work was supported by a predoctoral HHMI fellowship (J.S.), the Vagelos Molecular Life Sciences Scholars Program (J.K.), funding from the NIH R35-NS097275 (N.M.B.), the Paul G Allen Frontiers group and NIH NS088176 (D.F.M.).
Author information
Authors and Affiliations
Contributions
All experiments were performed by J.S. and J.K. under the mentorship of N.M.B. The piezoelectric dTBI device was designed by D.F.M., M.H. and J.S.; M.H. and D.F.M. built the device, and J.S., J.K. and C.N.B. tested it. J.S., D.F.M. and N.M.B. wrote the manuscript, and J.K. and C.N.B. provided critical feedback.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Douglas M. Ruden and David Wassarman for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Saikumar, J. et al. Proc. Natl Acad. Sci. USA 117, 17269–17277 (2020): https://www.pnas.org/content/117/29/17269
Supplementary information
Supplementary Information
Supplementary Videos 1–4 legends, Supplementary Software 1 legend, Supplementary Figs. 1–6 and legends.
Supplementary Video 1
Severe compression in the low-throughput and high-throughput devices.
Supplementary Video 2
Calibration and compression measurements in the low-throughput device: The measurements of % head compression, the gap between the collar surface and piezoelectric actuator (purple), height of uncompressed fly head (green), height of fly head at the point of maximum compression (blue), and the gap between the head and the piezoelectric actuator (red) corresponding in location to the schematic in Fig 5b.
Supplementary Video 3
Collar loading and unloading protocol: A step-by-step guide on using a pair of blunt forceps to load the fly into the collar and move it along the groove with the forceps or a paintbrush.
Supplementary Video 4
dTBI procedure: A step-by-step guide on positioning the collar under the piezoelectric actuator and performing the injury.
Supplementary Software 1
Arduino code to operate dTBI device.
Source data
Source Data Fig. 6
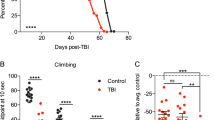
Raw data for regression analyses in Fig 6 a, b and lifespan analyses in Fig. 6 c, d.
Rights and permissions
About this article
Cite this article
Saikumar, J., Kim, J., Byrns, C.N. et al. Inducing different severities of traumatic brain injury in Drosophila using a piezoelectric actuator. Nat Protoc 16, 263–282 (2021). https://doi.org/10.1038/s41596-020-00415-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00415-y
This article is cited by
-
A Hybrid Dynamic Model for a Rotary Piezoelectric Motor Without Sliding Friction
Journal of Vibration Engineering & Technologies (2023)
-
Dietary restriction ameliorates TBI-induced phenotypes in Drosophila melanogaster
Scientific Reports (2022)
-
Origami-inspired folding assembly of dielectric elastomers for programmable soft robots
Microsystems & Nanoengineering (2022)
-
Glial AP1 is activated with aging and accelerated by traumatic brain injury
Nature Aging (2021)
-
Repetitive mild head trauma induces activity mediated lifelong brain deficits in a novel Drosophila model
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.